Abstract
A 25-year-old Caucasian heterosexual man with a recent history of unprotected sex in Vietnam while on a holiday was prescribed HIV postexposure prophylaxis by a local doctor; nevirapine, stavudine and lamivudine. He was subsequently admitted to a UK hospital with sore throat, bilateral conjunctivitis, genital ulceration and severe widespread maculo-papular rash. Extensive investigations for infective causes were negative and he was subsequently recovered with conservative therapy.
Background
Nevirapine was the first non-nucleoside reverse transcriptase (RT) inhibitor (NNRTI) to be approved for use in HIV-infected individuals. It is highly specific for HIV1-RT, but has no activity against HIV2. It is approved for use as part of combination therapy for HIV-infected patients,1 and has a well-established role in preventing perinatal transmission in resource-limited settings, but it is not recommended for HIV postexposure prophylaxis.2
Nevirapine monotherapy rapidly selects for high-level resistance by a single amino acid substitution in the HIV-RT gene. This pattern of resistance mutation overlaps with those of other NNRTIs, but is distinct from those of nucleoside analogue RT inhibitors and protease inhibitors (PIs). Nevirapine has excellent oral bioavailability and tissue distribution with a long half-life. It is extensively metabolised by the liver via CYP3A4, a reason to be cautious when coadministering medications including other antiretrovirals (ARVs).
Nevirapine-induced rash is a very well-documented adverse effect with several reports of life-threatening presentations. The incidence of rash has been linked to several risk factors including: a history of drug allergy, lower body weight and higher CD4 cell counts.3 There is evidence to support the use a 2-week lead-in dose and only to escalate the dose if no adverse events occur during this period. Owing to the significant risk of hepatotoxicity, neivrapine is not recommended in patients with high CD4 counts (women>250, men>400).
Case presentation
We present a case of a 25-year-old heterosexual man, originally from the UK, but resident in Australia for the 2 years prior to presentation. He travelled to the UK via Vietnam where he reported having unprotected sexual intercourse with a local Vietnamese woman. The following day he sought medical advice from a local clinic where he tested negative for HIV. He was prescribed a 4-week course of postexposure prophylaxis comprising of nevirapine (without a lead-in period), stavudine and lamivudine, which he continued to take for the 2 weeks prior to presentation.
Upon return to the UK, he presented with a three day history of malaise, sore throat; red, gritty eyes; and a non-itchy rash. The rash began on the arms then progressed to cover the whole body. The patient did not have a regular sexual partner and had undergone a negative sexual health screen in the UK 6 months prior. He also did not have a sexual encounter subsequently until his trip to Vietnam. He was otherwise healthy without comorbidity or allergies, and he did not take any regular medications. Other than the postexposure prophylaxis, he had not taken other prescribed or over-the-counter medications recently. He smoked 20 cigarettes per day, took recreational drugs occasionally (none recently) and did not drink alcohol to excess.
On examination he looked ill and had a tachycardia at 105 beats per minute. He was apyrexial and vital signs were otherwise within normal ranges. There was evidence of bilateral conjunctival injection (figure 1). Additionally, he had severe mucosal involvement of the lips and palate with erythema, mucosal sloughing and ulceration and pseudo-membrane formation (figure 2). There was widespread maculo-papular rash all over the body, including the palmar surfaces, upper arms, chest and scrotum (figures 3–6), respectively. Close examination revealed scrotal and penile ulceration.
Figure 1.
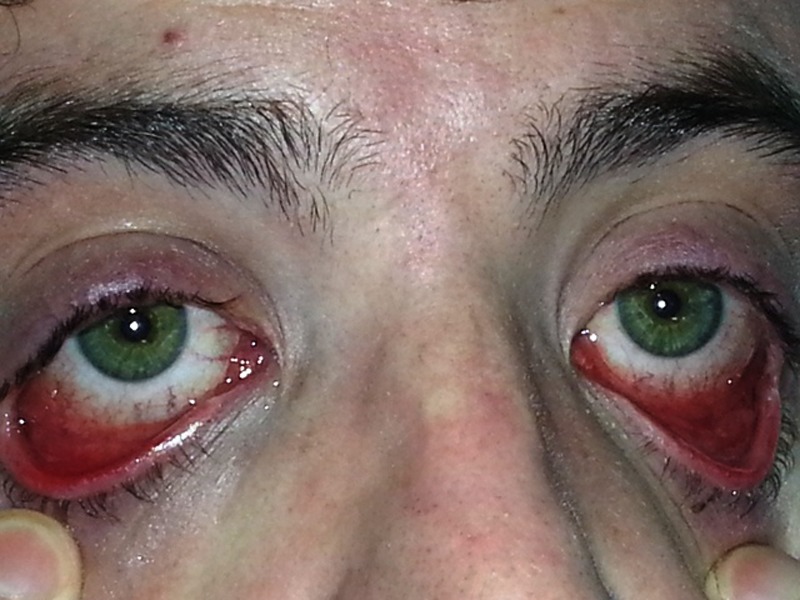
Bilateral conjunctivitis.
Figure 2.
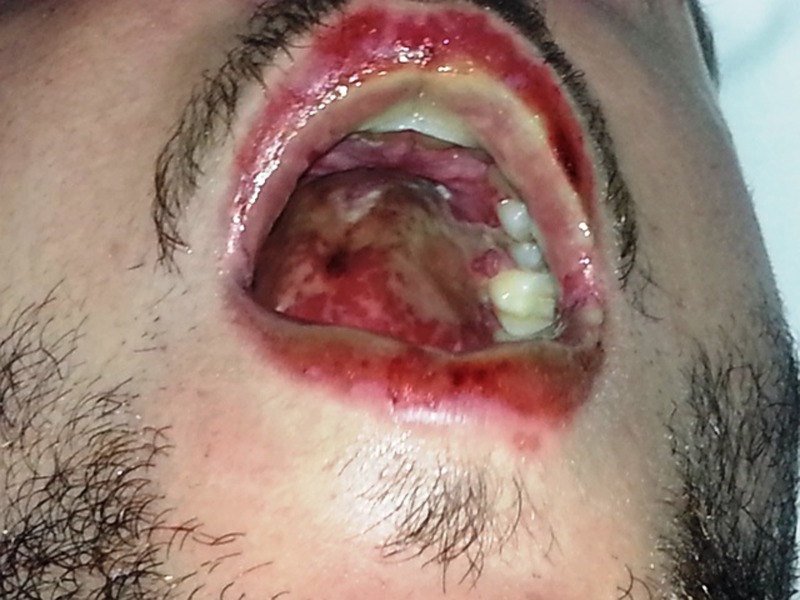
Mucosal and palate involvement in form of oedema and sloughing.
Figure 3.
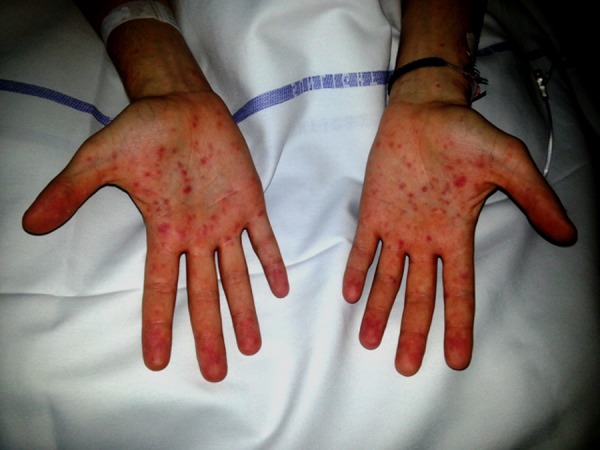
Palmar macular rash.
Figure 4.
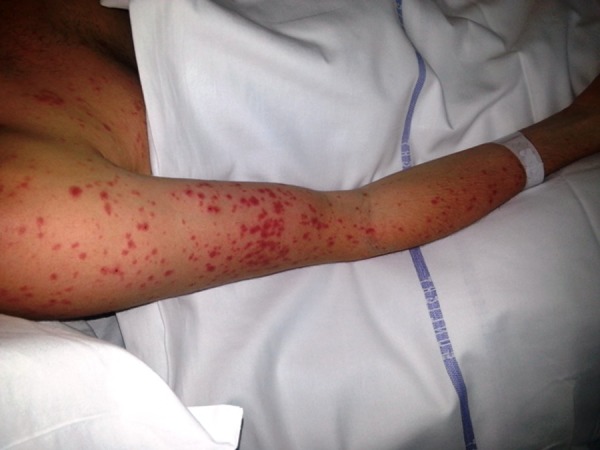
Macular rash on the right upper arm and forearm.
Figure 5.
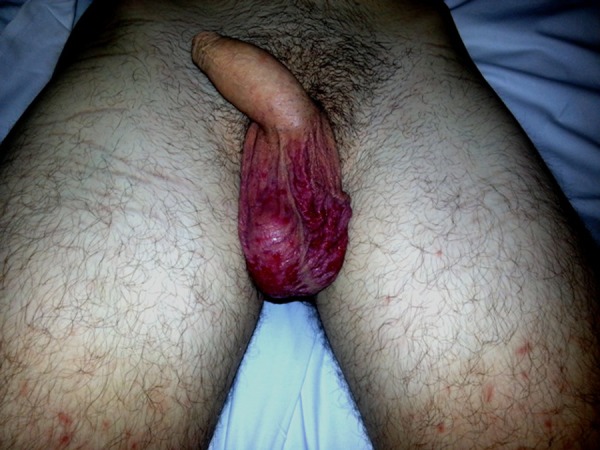
Macular rash on the scrotum and thighs.
Figure 6.
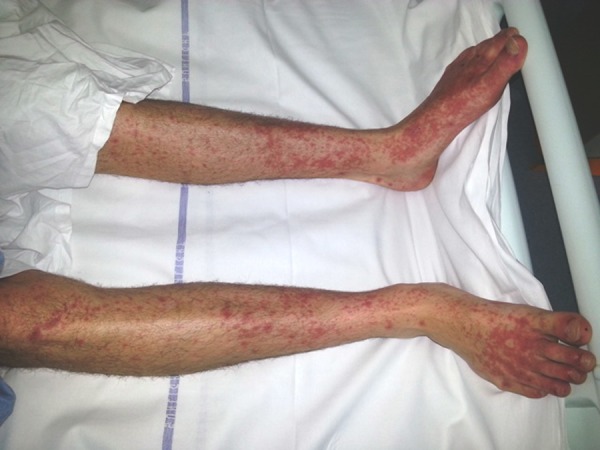
Macular rash on the lower limbs and urticaritic plaques on the dorsum of the feet.
Investigations
Blood tests, including liver enzymes, were normal apart from an elevated C reactive protein (160 mg/l) and below normal lymphocyte count (0.47×109/l). Extensive serological tests for HIV, HBV, HCV, EBV, CMV, parvovirus B 19, measles, syphilis and rubella were negative. A full sexual health screen, including HIV PCR and urine for chlamydia and gonococci was negative. Blood, urine and throat swab cultures (bacterial and viral) cultures/PCR were negative. His antistreptolysin O titre was normal. Other serological tests were performed for dengue fever and rickettsia, which were negative. The patient tested weakly positive for Japanese B encephalitis and this was secondary to immunisation. The PCR tests were carried out for HSV, Influenza A and B, RSV, metapneumovirus, parainfluenzavirus types 1–4, adenovirus, enterocirus, parechovirus, swine influenza virus (H1N1) and rhinovirus, which were all negative. A chest radiograph was reported as being normal by a consultant radiologist. Blood films for malaria were negative.
Differential diagnosis
The clinical picture was suggestive of Stevens-Johnson syndrome (SJS), probably triggered by one of the antiretroviral agents. Given the well-known association between starting of nevirapine and skin hypersensitivity reactions, including SJS,1 nevirapine would seem to be the most likely causative agent. The sexual history and presentation mandated the need for investigations to exclude an infective aetiology, including sexually transmitted infections. Rash is well described in acute HIV infection, which was high on the list of differential diagnoses;4 the palmar distribution of the rash5 required the exclusion of secondary syphilis, although the time from exposure to illness did not support this. The clinical context of the case supported by the extensive negative infection screen made the diagnosis of the SJS secondary to nevirapine the more likely diagnosis in this patient.
Treatment
His symptoms and muco-cutaneous manifestations markedly improved after stopping antiretroviral therapy and supportive treatment consisting of: (1) intravenous fluids; (2) mouth-wash; (3) oral fluconazole 50 mg once daily for 7 days and (4) chloramphenicol eye ointment.
Outcome and follow-up
He was discharged after 5 days from the hospital with safe sex advice and a planned outpatient review in 2–3 months time. Patient attended the clinic and was retested again for HIV and syphilis and they were negative and was discharged from outpatient clinic.
Discussion
The term postexposure prophylaxis (PEP) is generally understood to mean the medical response given to prevent the transmission of blood-borne pathogens following a potential exposure to HIV. The use of PEP against HIV infection dates back to the early 1990s after exposure to HIV; antiretroviral therapy may also be administered for prophylaxis against infection, although no efficacy data are available for this strategy, but substantial safety and feasibility data have led to its widespread of acceptance.6
SJS is a hypersensitivity reaction that typically involves the skin and mucous membranes and has an incidence of 2.6 cases per million population per year.7 SJS and severe drug eruptions have been reported following treatment with nevirapine in HIV-infected patients. Rash (incidence 15–20%) is one of the most frequent adverse events of nevirapine and is described as serious in 9% of patients.8 Warren et al9 reported 20 cases of nevirapine-associated drug eruptions requiring hospital admission, of which 3 were fatal while Jao et al10 reported nevirapine to cause SJS and fulminant hepatic failure requiring liver transplantation.
In this case it seems that the risk of developing a potentially life-threatening adverse effect was not adequately considered in clinical decision making, which appeared to focus on the potential risk of acquiring HIV infection. The decision to administer PEP is clinically indicated with regard to the individual risk of HIV acquisition, accounting for the exposure history, and the potential adverse effects of the planned prophylactic regimen.2 There is need to mention that in this case PEP was administered within the correct window; which is up to 72 h after the sexual exposure.11 12 The joint WHO and Iinternational Labour Organisation guidelines on PEP administration emphasises on the eligibility prior to prescribing PEP drug regimen; these eligibility criteria include that the exposure occurring in high prevalence area of HIV.11 The prevalence of HIV among female sex workers in Vietnam varies geographically, but, for example, has been reported to be as high as 34% in Can Tho in 2006.13 The decision to prescribe PEP was therefore reasonable in this patient but the regimen was contraindicated because nevirapine is no longer recommended in the setting of PEP because of hepatic and cutaneous toxicity14 and this is also supported by the current guidelines on PEP.2 11 12
Zidovudine (an NRTI) is the only drug to date, which has been shown to reduce the risk of HIV transmission. However, it is frequently poorly tolerated, which is likely to contribute to non-adherence. Other studies suggest that other NRTIs including stavudine, tenofovir and emtricitabine, either given in combination with PIs or given as triple nucleoside/nucleotide analogue regimens, are better tolerated.2 11 12
Although it was sensible of our patient to seek advice following sexual exposure, this case clearly demonstrates the need for at-risk travellers, travel medicine practitioners and prescribing clinicians to be aware of the potentially serious adverse effects of PEP and, if taken, the need for follow-up on return. If that had occurred in this case, it is possible that the serious adverse effect and admission to hospital would have been avoided by switching him to an equally effective, but lower risk regimen.
Learning points.
Stevens-Johnson syndrome is a serious drug reaction.
Nevirapine is contraindicated in HIV postexposure prophylaxis.
Adhere to the UK guidelines for the use of HIV postexposure prophylaxis.
As well as being aware of what to do if HIV exposure occurs, at-risk travellers should be educated about the potential risks of PEP and the need for early follow-up on return.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Deeks S. Antiretroviral treatment of HIV infected adults. BMJ 2006;2013:1489–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benn P, Fisher M, Kulasegaram R. UK guideline for the use of post-exposure prophylaxis for HIV following sexual exposure. Int J STD AIDS 2011;2013:695–708 [DOI] [PubMed] [Google Scholar]
- 3.Kiertiburanakul S, Sungkanuparph S, Charoenyingwattana A, et al. Risk factors for nevirapine-associated rash among HIV-infected patients with low CD4 cell counts in resource-limited settings. Curr HIV Res 2008;2013:65–9 [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Segawa S, Kawashima M, et al. A case of symptomatic primary HIV infection. J Dermatol 2005;2013:137–42 [DOI] [PubMed] [Google Scholar]
- 5.Badri T, Ben Jennet S. Rash associated with secondary syphilis. N Engl J Med 2011;2013:71. [DOI] [PubMed] [Google Scholar]
- 6.Landovitz R, Currie J. Postexposure prophylaxis for HIV infection. N Eng J Med 2009;2013:1768–75 [DOI] [PubMed] [Google Scholar]
- 7.Strom B, Carson J, Halpern A, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;2013:831–8 [PubMed] [Google Scholar]
- 8.Cohn J. Recent advances in HIV infection. BMJ 1997;2013:487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren K, Boxwell D, Kim N, et al. Nevirapine associated Stevens-Johnson syndrome. Lancet 1998;2013:567. [DOI] [PubMed] [Google Scholar]
- 10.Jao J, Sturdevant M, del Rio Martin J, et al. Nevirapine induced Stevens-Johnson syndrome and fulminant hepatic failure requiring liver transplantation. Am J Transplant 2010;2013:1713–16 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. 2007. WHO: Publications: Guidelines: Post-exposure prophylaxis to prevent HIV infection: joint WHO/ILO guidelines on post-exposure prophylaxis (PEP) to prevent HIV infection. http://www.who.int (Accessed 15 Jan 2013)
- 12.Rey D. Post-exposure prophylaxis for HIV infection. Anti Infec Ther 2011;2013:431–42 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T, Khuu N, Truong P, et al. Correlation between HIV and sexual behavior, drug use, trichomoniasis and candidiasis among female sex workers in a Mekong Delta province of Vietnam. AIDS Behav 2009;2013:873–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S, Johnson S, Belknap S, et al. Serious adverse cutaneous and hepatic toxicities associated with nevirapine use by non-HIV-infected individuals. J Acquir Immune Defic Syndr 2004;2013:120–5 [DOI] [PubMed] [Google Scholar]


