Abstract
Prior studies with carbonic anhydrase (CA) inhibitors implicated mitochondrial CA in ureagenesis and gluconeogenesis. Subsequent studies identified two mitochondrial CAs. To distinguish the contribution of each enzyme, we studied the effects of targeted disruption of the murine CA genes, called Car5A and Car5B. The Car5A mutation had several deleterious consequences. Car5A null mice were smaller than wild-type littermates and bred poorly. However, on sodium–potassium citrate-supplemented water, they produced offspring in expected numbers. Their blood ammonia concentrations were markedly elevated, but their fasting blood sugars were normal. By contrast, Car5B null mice showed normal growth and normal blood ammonia levels. They too had normal fasting blood sugars. Car5A/B double-knockout (DKO) mice showed additional abnormalities. Impaired growth was more severe than for Car5A null mice. Hyperammonemia was even greater as well. Although fertile, DKO animals were produced in less-than-predicted numbers even when supplemented with sodium-potassium citrate in their drinking water. Survival after weaning was also reduced, especially for males. In addition, fasting blood glucose levels for DKO mice were significantly lower than for controls (153 ± 33 vs. 230 ± 24 mg/dL). The enhanced hyperammonemia and lower fasting blood sugar, which are both seen in the DKO mice, indicate that both Car5A and Car5B contribute to both ammonia detoxification (ureagenesis) and regulation of fasting blood sugar (gluconeogenesis). Car5A, which is expressed mainly in liver, clearly has the predominant role in ammonia detoxification. The contribution of Car5B to ureagenesis and gluconeogenesis was evident only on a Car5A null background.
Keywords: carbonic anhydrase V, gene targeting, hypoglycemia, mouse model, anti-obesity
Carbonic anhydrases (CAs) are zinc-containing metalloenzymes that catalyze the reversible hydration of carbon dioxide according to the following reaction: CO2 + H2O ⇆ HCO3− + H+ (1). This reaction contributes to regulation of acid–base balance in most organisms. In addition, CAs participate in a number of other physiological processes, such as CO2 and HCO3− transport, bone resorption, production of body fluids, gluconeogenesis, ureagenesis, and lipogenesis (2, 3). During evolution, at least 13 active CA isoenzymes have emerged in rodents and 12 in humans, as the human gene, CA XV, is a pseudogene. These isoenzymes have differences in their tissue distribution, kinetic properties, and subcellular localizations: CAs I, II, III, VII, and XIII are cytoplasmic (4, 5); CAs IV, IX, XII, XIV, and XV are membrane-bound (6–10); CA VI is secreted (11); and CA VA and VB are located in mitochondria (12).
A role for a CA in the mitochondria was initially suggested when it was realized that mitochondria are impermeant to HCO3− ions and that HCO3− is required in mitochondria by several enzymes, including pyruvate carboxylase for gluconeogenesis and carbamoyl phosphate synthetase I for ureagenesis (reviewed in refs. 13 and 14). Inhibition of both processes by CA inhibitors provided pharmacological evidence that mitochondria require a CA for these metabolic processes (15–19).
A mitochondrial CA was first isolated from guinea pig liver and named CA V (16). Subsequently, mouse, rat, and human cDNAs for CA V were cloned (20–22). The murine CA genes are called Car5A and Car5B. The murine Car5A transcript was identified by Northern blots only in mRNA from mouse liver (20). Western blot data (21) and immunohistochemical results (23) suggested a wider distribution in rat tissues. A possible solution to the apparent discrepancies in tissue distribution in different studies was suggested by GenBank expressed sequence tag (EST) data indicating that there are at least two mitochondrial CAs with differing tissue distributions. David Hewett-Emmett discovered an EST from mouse kidney (AA123271) that encodes a CA with the greatest sequence identity to CA V that also contains a mitochondrial leader sequence (12). EST databases showed identical ESTs to be present in libraries from kidney and male mammary gland. Together, we reported that this second mitochondrial CA has a wider tissue distribution than that originally described for human CA V and that this mitochondrial CA was even more highly conserved than Car5A (12). The Human Genome Organization Nomenclature Committee referred to the new mitochondrial CA V as CA VB and to the original CA V as CA VA. The genes in mice are referred to as Car5A and Car5B and in humans as CA5A and CA5B.
To clarify the individual contributions of CA VA and CA VB in physiological systems, we characterized Car5A and Car5B knockout (KO) mouse strains produced by targeted mutagenesis. From these individual Car5A KO and Car5B KO mice, we produced the doubly deficient Car5A/Car5B double-knockout (DKO) mice by intercrossing. In this article, we describe the production and characterization of these strains and present data that indicate that, although both CA VA and CA VB contribute to gluconeogenesis and ammonia detoxification, CA VA plays the predominant role in ammonia detoxification (ureagenesis).
Results
Generation of Car5A KO Mice.
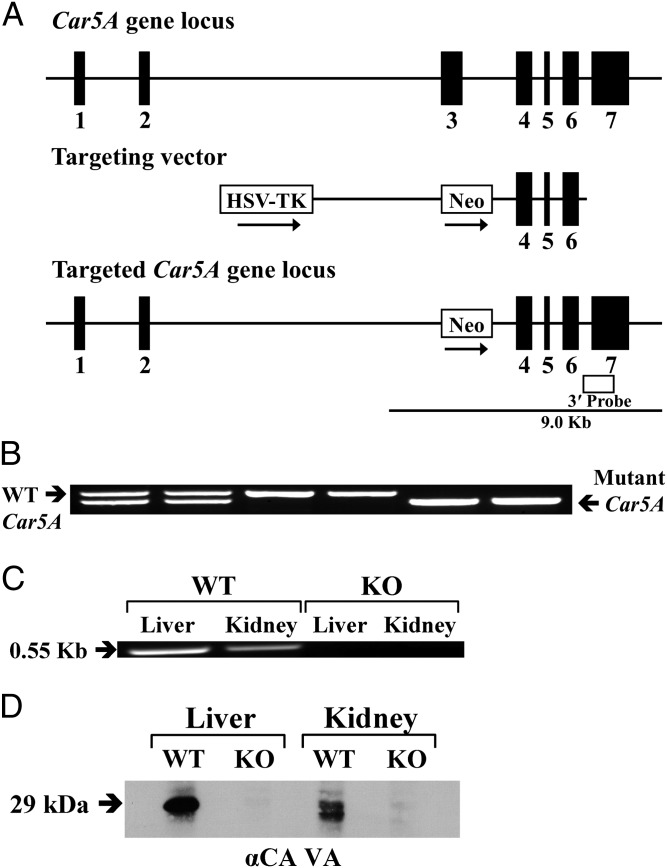
The Car5A gene was targeted in ES cells, and the mutant allele was identified by Southern blot using an external probe (Fig. 1A). Tail-positive chimeric mice produced from blastocyst injections confirmed germ-line transmission of the mutant allele. F1 and F2 offspring produced by mating heterozygotes were genotyped by PCR from genomic DNA, and homozygotes were identified (Fig. 1B). Homozygous Car5A KO mice were produced by heterozygote or homozygote matings. The numbers and genders of WT, heterozygous, and homozygous mice were nearly those expected from Mendelian ratios. The Car5A KO mice appeared healthy, grew, and were fertile. However, both female and male KO mice were smaller than WT controls (described below).
Fig. 1.
Generation, genotyping and characterization of Car5A KO mice. (A) Structure of the Car5A gene (Top), the targeting construct (Middle), and the predicted structure of the disrupted Car5A gene after homologous recombination and excision of the neomycin-resistance (Neo) cassette (Bottom). The open box indicates the position of the external probe. Only the relevant restriction sites are shown. The numbered solid boxes represent exons. Neo and TK (thymidine kinase) refer to positive and negative selection markers, respectively. (B) Genotyping of Car5A KO colony. Genotyping by PCR was done on mouse-tail genomic DNA. PCR products present in WT (0.6 kb) and mutant (0.55 kb) mice were present in the heterozygotes. (C) Car5A transcripts in liver and kidney from WT and KO mice. Total RNA (≤1 µg) was reverse-transcribed and amplified by PCR. A 0.35-kb band was amplified from both tissues from the WT animals but not from the KO mice. (D) Western blot analysis. The membrane proteins (50 μg) from liver and kidney of CA VA WT and KO mice were electrophoresed on a 12% (wt/vol) SDS-PAGE gel. After blotting, the membranes were probed with rabbit anti-mouse CA VA antibodies. A 29-kDa band present in both liver and kidney from the WT animals was absent in the same tissues from KO animals.
Analysis of KO Mice for Car5A Transcripts and CA VA Protein.
The presence of Car5A transcripts was ascertained by RT-PCR on total RNA from liver and kidney of either WT or the putative KO mice (Fig. 1C). The expected 0.35-kb fragment was amplified from total RNA from both liver and kidney from the WT mouse, but not from RNA from the same tissues from homozygous KO mice. CA VA protein was analyzed by Western blot on membrane preparations from liver and kidney from either WT or KO animals (Fig. 1D). A 29-kDa band was present in extracts of both tissues examined from WT animals. No CA VA protein was detectable in KO mice in the same tissues.
Generation of Car5B KO Mice.
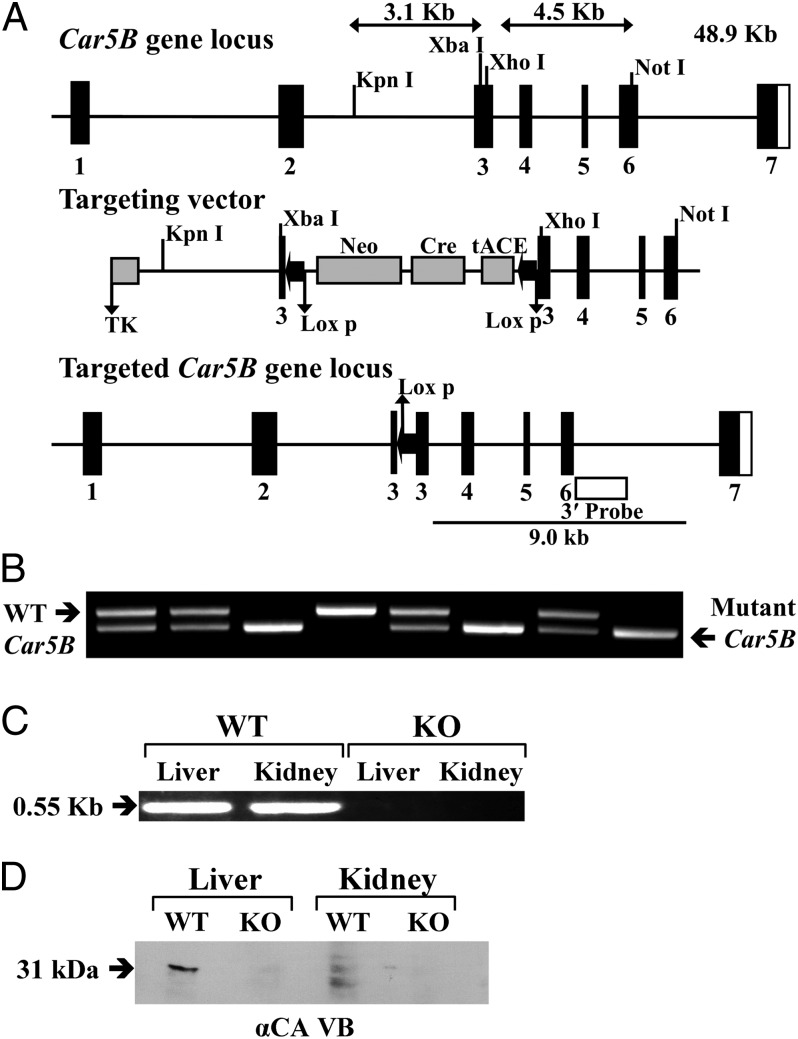
The Car5B gene, which is on the mouse X chromosome, was targeted in ES cells, and the mutant allele was identified by Southern blot using an external probe (Fig. 2A). Tail-positive chimeric mice produced from blastocyst injections confirmed germ-line transmission of the mutant allele. F1 and F2 offspring produced by mating hemizygous males with heterozygous females were genotyped by PCR from genomic DNA, and male and female Car5B null mice were identified (Fig. 2B). The WT, hemizygous male, and heterozygous and homozygous female mice were produced in expected Mendelian ratios. The Car5B KO mice appeared to be healthy, grew normally, and were fertile.
Fig. 2.
Generation, genotyping, and characterization of Car5B KO mice. (A) Structure of the Car5B gene (Top), the targeting construct (Middle), and the predicted structure of the disrupted Car5A gene after homologous recombination and excision of the neomycin-resistance (Neo) cassette (Bottom). The open box indicates the position of the external probe. Only the relevant restriction sites are shown. The numbered solid boxes represent exons. Neo and TK (thymidine kinase) refer to positive and negative selection markers, respectively. (B) Genotyping of the Car5B KO colony. Genotyping by PCR was done on mouse-tail genomic DNA. PCR products present in WT (0.52 kb) and mutant (0.44 kb) mice were present in the heterozygotes. (C) Car5B transcripts in liver and kidney amplified from WT mice but not from KO mice. (D) Western blot analysis. The membrane proteins (50 μg) from liver and kidney of CA VB WT and KO mice were electrophoresed on a 12% (wt/vol) SDS-PAGE gel. After blotting, the membranes were probed with rabbit anti-mouse CA VB antibodies. A 31-kDa band present in both liver and kidney from the WT animals was absent in the same tissues from KO animals.
Analysis of KO Mice for Car5B Transcripts and CA VB Protein.
The presence of Car5B transcripts was ascertained by RT-PCR on total RNA from liver and kidney of either WT or putative KO mice, using a forward primer from coding exon 3 and a reverse primer from exon 7 (Fig. 2C). The expected 0.55-kb fragment was amplified from total RNA from both liver and kidney from the WT mouse, but not from RNA from the same tissues from hemizygous male or homozygous female KO mice. However, using forward and reverse primers in exons 2 and 4, respectively, we found a transcript in mRNA from Car5B KO mice that was 98 bp shorter and much less abundant than the transcript in the WT or Car5A KO mice. Upon sequencing, this low-abundancy transcript proved to be the product of skipping exon 3 and is out of frame from the start of exon 4.
RT-PCR was used to estimate the relative abundance of Car5A and Car5B transcripts in liver and kidney. Car5A has been reported to be expressed mainly in liver with few other tissues being positive by Northern blot analysis. Using RT-PCR, we found Car5A transcripts to be about 20 times as abundant as those for Car5B in wild-type liver mRNA. In the kidney, the reverse was true, with Car5B transcripts being 18- to 20-fold more abundant than Car5A transcripts. Neither Car5 transcript was up-regulated in mouse null for the other Car5 allele. In fact, each was less than 50% the level of wild-type in the mouse null for the other Car5 gene.
CA VB protein was analyzed by Western blot on homogenates of liver and kidney from either WT or KO animals (Fig. 2D). A 31-kDa fragment was present in extracts of both tissues examined from WT animals. A smaller fragment was also seen in extracts from both tissues. No CA VB protein was detectable in KO mice in the same tissues.
Generation of Car5A/Car5B DKO Mice.
To produce mice lacking both Car5A and Car5B, we bred Car5A null males with Car5B null females and backcrossed to obtain breeding pairs of Car5A+/−, Car5B−/− females and Car5A+/−, Car5B-/y males. These breeding pairs produced live-born pups. However, the number of Car5A/Car5B DKO mice was only 45% of the number expected (38 instead of 84 males and 11 of 17 females). The DKO mice were significantly smaller than WT (see below).
Comparisons of Weight Gain and Mortality of WT, KO, and DKO Mice.
Mice were maintained on chow diets with sodium–potassium citrate-supplemented water. The basis for maintaining the mice on citrate salt-supplemented water was the report that mortality of premature newborn infants treated with the carbonic anhydrase inhibitor acetazolamide to reduce hemorrhage-associated brain swelling was improved in infants who received blood transfusions (24). In this report, they presented experiments in newborn swine that suggested that the protective factor was the citrate used as an anticoagulant in the packed red blood cells and not the erythrocytes (24). Many earlier experiments had documented the marked inhibition of urine citrate excretion in the rat by acetazolamide (25). We indeed found that citrate salt supplementation improved breeding and reduced the neonatal mortality of the Car5A null and DKO mice, but we did not observe that it improved growth or reduced mortality after weaning.
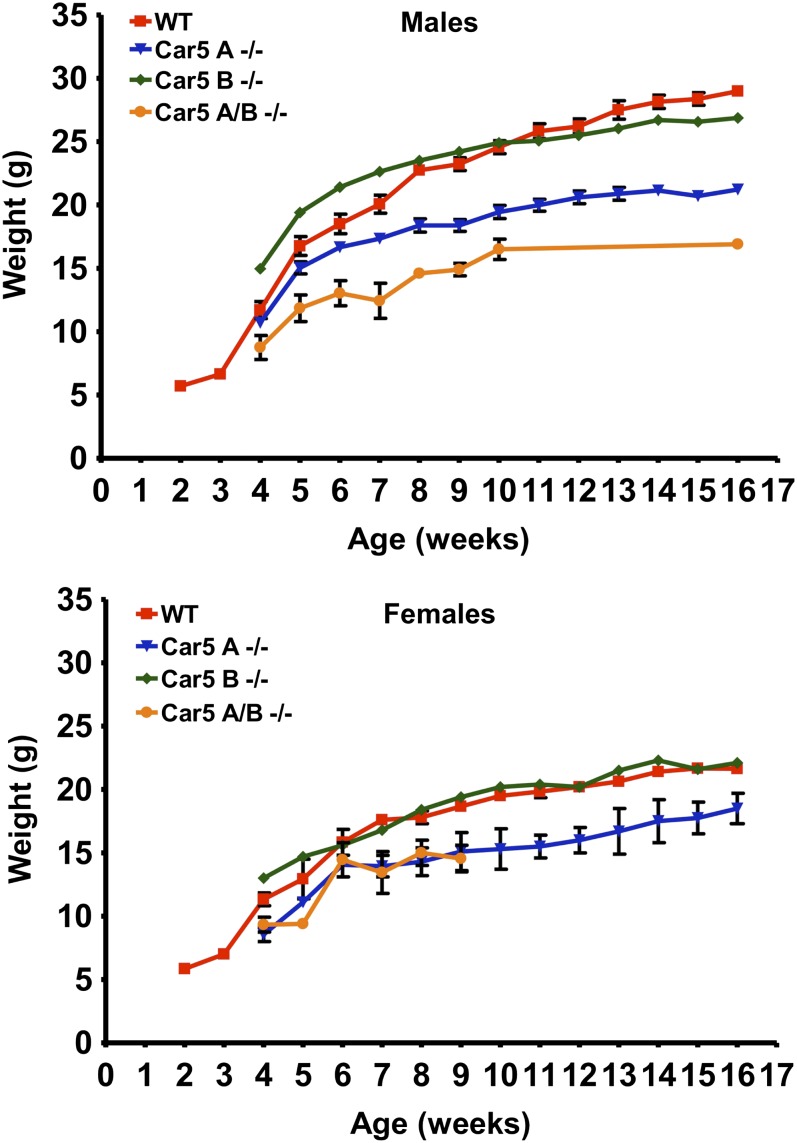
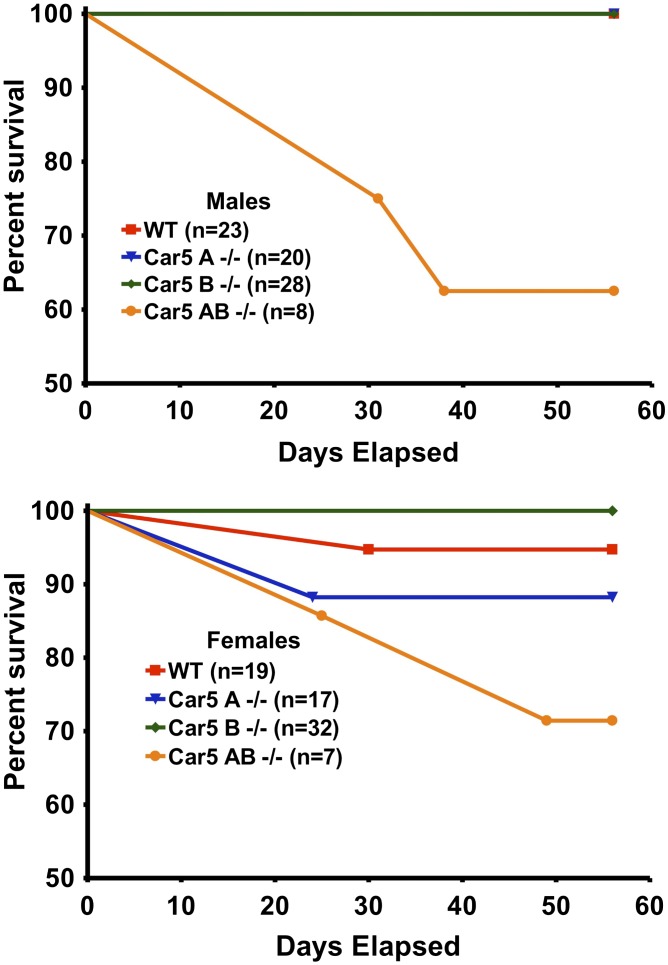
Fig. 3 presents data on growth of single and DKO mice compared with B6 controls. Both male and female Car5A null mice were smaller than either WT or Car5B null mice, which were not different from each other. However, the Car5A/Car5B DKO mice were even smaller than the Car5A mice. Fig. 4 presents data on mortality of single and DKO mice vs. controls over 60 d following birth. Neither Car5A nor Car5B single KO mice showed increased mortality. However, Car5A/Car5B DKO mice died prematurely with losses starting soon after weaning, even though all of these mice were maintained on citrate salt-supplemented water. Males were lost faster than females. The cause of death is uncertain but could be related to extreme hyperammonemia, which is described below.
Fig. 3.
Comparison of weights of Car5A/B DKO female and male mice with WT controls of the same sex. All mice were fed normal rat chow ad libitum and given citrate-supplemented water.
Fig. 4.
Mortality. Percentage survival was plotted from birth to age 56 d. Initial numbers in each group are shown.
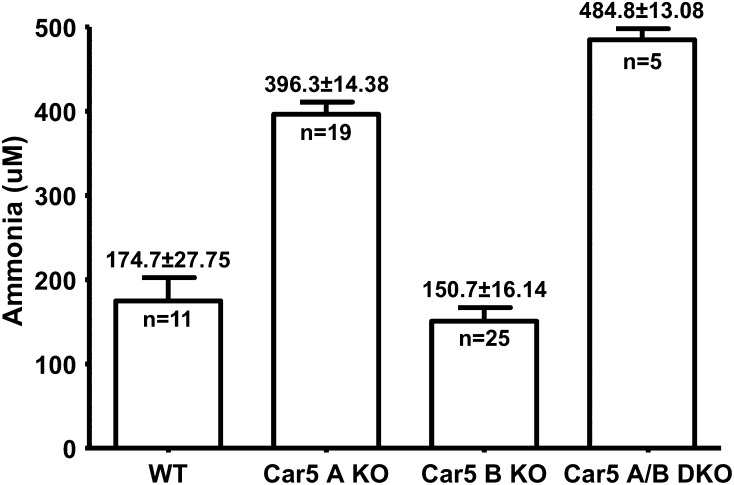
Blood Ammonia Levels.
Blood ammonia levels in the Car5B mice were no different from those of littermate controls (Fig. 5). By contrast, blood ammonia concentrations in plasma of the Car5A KO mice were significantly higher than the littermate WT controls (P = 0.00001). Blood ammonia levels in the DKO mice were even higher than in the Car5A KO mice (P = 0.006), indicating that Car5B may also contribute to ammonia detoxification, although its contribution can be demonstrated only on the Car5A KO background.
Fig. 5.
Ammonia concentration in the blood of WT, Car5A, and Car5B null mice and the DKO animals. Plasma ammonia levels were measured in the fed state for each of the genotypes shown. The statistically significant elevations in the Car5A mice are described in Results, as is the even greater elevation seen in the DKO mice.
Blood Glucose Levels.
The fasting blood sugar levels in the DKO animals (n = 8) were significantly lower than in the WT controls (n = 9) (153 ± 33 deficient vs. 230 ± 24.3 mg/dL, P = 0.008). No discernible differences were observed between the DKO and the WT control animals under fed conditions. Likewise, there were no differences either in fed or fasted conditions between the single KO animals and the WT littermate controls.
Urinary Metabolites.
Table S1 presents data on urinary metabolites from wild-type and Car5A and Car5B null mice. Abnormalities in the urine of Car5A null mice include significant elevations of α-ketoglutarate, fumarate, and malate and somewhat lesser but still significant elevations of acetoacetate, subaric acid, and sebacic acids. The metabolic basis for these changes is not yet clear. The tricarboxylic acid (TCA) cycle intermediates may be downstream products of the renal glutaminase reaction in response to acidosis (26) or may accumulate as a secondary effect of hyperammonemia, which is known to inhibit several enzymes in the TCA cycle (27). The other changes are seen in one or more of the individual carboxylase deficiencies and in multiple carboxylase deficiency (28).
Discussion
Pharmacological studies with CA inhibitors have implicated mitochondrial CAs in several metabolic pathways, including gluconeogenesis, ureagenesis, and lipogenesis (14–17). The common thread is the requirement in mitochondria for HCO3−, which does not freely cross the inner mitochondrial membrane (29), in contrast to CO2, which is freely permeable. Mitochondrial CAs appear to be required to convert CO2 to HCO3− at a rate sufficient to support the metabolic needs for these three essential pathways.
Four mitochondrial enzymes require HCO3− and could be limited by deficiency of mitochondrial CA. The most limited by CA V deficiency appears to be carbamoyl phosphate synthase 1, which catalyzes the synthesis of carbamoyl phosphate from NH3 and HCO3−, the first committed step in ureagenesis. The second enzyme is pyruvate carboxylase, which generates oxaloacetate from pyruvate and HCO3− for gluconeogenesis, oxidative metabolism of glucose (30), and anapleurosis of the Kreb’s cycle (31). The third enzyme is propiony CoA carboxylase, which catalyzes an important step in the breakdown of branched chain amino acids and provides succinate for the TCA cycle. The fourth enzyme is methylcrotonyl CoA carboxylase, which participates in leucine degradation. Complete deficiency of any of these four enzymes can produce a severe, often lethal, illness in newborn infants. Deficiency of the three carboxylases, all of which are biotin-dependent, in holocarboxylase deficiency produces metabolic ketoacidosis, hyperammonemia, organic acidemia, seizures, and coma (28). The effects of complete deficiency of mitochondrial CA in the DKO mice are less severe, indicating that the mitochondrial CAs are not strictly essential for survival. Presumably, the nonenzymatic hydration of CO2 in mitochondria can provide enough HCO3− to these four enzymes to prevent uniform lethality. However, fewer DKO mice are produced than expected, and the physiological importance of these CAs is manifested by the growth deficiency, impaired fertility, and reduced longevity of the DKO mice.
Comparison of the phenotypes of the individual mitochondrial CA deficiencies reveals the relative greater importance of CA VA. The absence of CA VA alone produces impressive hyperammonemia and growth deficiency, although less extreme than that seen in the DKO mice. The absence of CA VB in the Car5B null produces no obvious phenotype, but its role in metabolism can be appreciated on a Car5A null background.
Ureagenesis appears to be the pathway most sensitive to CA VA deficiency. The small amount of CA VB in liver appears to be insufficient to compensate for the absence of CA VA and provide enough HCO3− for carbamoyl phosphate synthetase 1 to prevent hyperammonemia. However, the higher NH3 levels in the DKO show that CA VB does make a modest contribution to ureagenesis. Similarly, the more severe growth deficiency and increased mortality seen in the DKO mice, as opposed to the Car5A null alone, indicate a compensatory role of CA VB in contributing to growth and survival. Thus, although the deficiency of CA VB alone produces no phenotype, its contribution to metabolism could be shown on the Car5A null background where the presence of CA VB lessened hyperammonemia and improved growth and survival.
The marked hyperammonemia in the DKO mice is a plausible cause of the reduced number of DKO offspring and their reduced survival after weaning. Neurotoxicity leading to coma and death is the most urgent issue in hyperammonemia from human urea cycle defects and other causes of severe hyperammonemia (27, 32).
Whether the hyperammonemia itself is the cause of the growth deficiency by virtue of its effect on appetite and its secondary effects on multiple metabolic pathways (27) remains to be established. Trials with ammonia scavengers and/or carglumic acid [an activator of carbamoyl phosphate synthase 1 shown effective in human newborns with hyperammonemia (33)] to determine their effectiveness in improving hyperammonemia, growth deficiency, and survival in these mice could help answer this question.
Studies in these mice might also answer some other open questions. Price et al. (34) used these mice to show the importance of mitochondrial CAs in producing the metabolites that lead to oxidative stress. Also, CA inhibitors have been shown to inhibit lipogenesis in hepatocytes (35) and adipocytes (17), and the effects are suggested to be due to inhibition of CA V. The presence of other CA isoforms in these tissues, notably CA II and CA III among others, has made it difficult to pinpoint the critical CA targeted by the inhibitors. Studies in tissues from the CA V-deficient mice reported here could help resolve this question. This issue is especially interesting because CA inhibitors have attracted great interest as antiobesity drugs (36). Is CA V their critical target? Similarly, several in vitro studies have provided support for the idea that CA V is important in gluconeogenesis (37). Indeed, we show here that elimination of both enzymes in the DKO mice reduced fasting blood sugar, providing strong support for their role in gluconeogenesis. Could this effect be related to the growth deficiency of CA V-deficient mice and to the antiobesity properties of CA inhibitors? These are among the several important questions remaining that these mouse models may help address.
A related question of considerable clinical interest is whether human CA VA deficiency is a yet-undiscovered form of hyperammonemia of the newborn. The findings reported here suggest that human infants exhibiting hyperammonemia in the newborn period that cannot be ascribed to defects in other enzymes in the urea cycle should be screened for mutations in the Car5A gene. If a CA VA deficiency syndrome exists in humans, as seems likely, the mouse model may provide a useful tool to study ways to optimize therapeutic interventions and improve patient outcomes.
Materials and Methods
Gene Targeting.
Techniques and constructs used to produce the CA VA- and CA VB-deficient mice are described in SI Materials and Methods. All experiments and procedures involving live animals were conducted in compliance with approved Saint Louis University Institutional Animal Care and Use Committee protocols. In accordance with Guidelines for Nomenclature of Genes, Genetic Markers, Alleles, and Mutations in Mouse and Rat from The Jackson Laboratories (www.informatics.jax.org/mgihome/nomen/gene.shtml), the Car5A KO mouse line was designated Car5Adl1Sws. The Car5B KO mouse line was designated Car5Bdl1Sws.
RT-PCR of Car5A and Car5B Transcripts.
One microgram of total RNA isolated from mouse tissues with a RNeasy kit (Qiagen) was reverse-transcribed with oligo(dT) primer in a 20-μL final volume and used as template for PCR amplification. The Car5A primers were 5′-TCAGAGCACGCAGTGGACGGCCATACC as forward primer and 5′-ACCATCACATCCTCTTCCTCACCTCGC as reverse primer. The PCR conditions included 10 cycles at 92 °C for 15 s, 63 °C for 30 s, and 68 °C for 1 min and 20 cycles of 92 °C for 15 s, 63 °C for 30 s, 68 °C for 1 min with 10-s autoextension. The reverse transcription conditions for Car5B transcripts were as described above. The primers used were 5′-TGATGCTTGGGGCTCTGAGC as forward primer and 5′-TGTCTTAGGGGGTGACTTCG as reverse primer. The PCR conditions were 10 cycles at 92 °C for 15 s, 60 °C for 30 s, and 68 °C for 1 min and 20 cycles of 92 °C for 15 s, 60 °C for 30 s, and 68 °C for 1 min with 10-s autoextension.
Immunochemical Methods.
Rabbit antibodies against mouse CA VA C-tail peptides were the same as described (21). Affinity-pure first antibodies against mouse CA VA C-tail were purified using a peptide containing the last 8-aa C-tail peptide-Affigel 10 column. First antibodies against the carboxyl terminal 17-aa CA VB peptide were produced by injecting the C-terminal peptide of mouse CA VB conjugated to thyroglobulin into rabbits in complete Freund’s adjuvant (21). The titer and specificity of the antiserum were checked by Western blot. Affinity-pure anti-CA VB C-tail–specific IgG was purified using CA VB C-tail peptide-Affigel-10 column (12).
Western Blot Analyses Using the Above Antibodies.
SDS-PAGE was performed under reducing conditions according to Laemmli (38). After electrophoretic transfer of the polypeptides from the gel to the Immobilon-P membranes, the membranes were incubated with first antibodies followed by peroxidase-conjugated goat anti-mouse IgG second antibodies as described. The polypeptides were visualized by using chemiluminescence substrate.
Determination of Plasma Ammonia Concentrations.
Blood was drawn by cardiac puncture from WT, Car5A−/−, Car5B−/−, and from Car5A/Car5B DKO mice. The blood was collected in heparinized tubes, which were filled completely to prevent air exposure. Cells were removed by centrifugation, and plasma ammonia analyses were done by dry-slide technology on an Ortho Clinical Diagnostics Fusion chemistry analyzer in the clinical chemistry laboratory of Cardinal Glennon Children’s Hospital of St. Louis (Ortho Clinical Diagnostics, Johnson and Johnson Inc., Rochester, NY).
Determination of Blood Glucose Levels.
Blood was collected in heparinized hematocrit tubes by retro-orbital bleed from WT and mutant mice. Plasma glucose levels were determined by i-STAT Portable Clinical Analyzer (Abbott Laboratories).
Diet and Citrate Supplementation.
Mice were fed standard chow ad libitum. After some initial observations of perinatal death, we observed that breeding and survival of Car 5A null mice, and even more so, Car5A/Car5B doubly deficient mice, were improved when mice were placed on citrate-supplemented water. Accordingly, all Car5A, Car5B, and DKO breeding pairs were maintained on citrate-supplemented water. The citrate-supplemented water contains 1.9 mM sodium citrate tribasic dihydrate (Sigma-Aldrich) and 1.74 mM potassium citrate tribasic monohydrate (Sigma-Aldrich) in Millipore water.
Urine Organic Compound Analysis.
Urine samples were collected manually from each mouse onto Parafilm at 1700 hours daily for 3 consecutive days, transferred by micropipette to microcentrifuge tubes, and frozen. The pooled samples from each mouse were processed for gas chromatography–mass spectrometry as described (39), and the metabolites were expressed as mmol/mol creatinine.
Supplementary Material
Acknowledgments
We acknowledge Tracey Baird for editorial assistance. This work was supported by National Institutes of Health Grants DK040163 (to W.S.S.) and DK083485 (to G.N.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305805110/-/DCSupplemental.
References
- 1.Lindskog S, Silverman DN. In: The Carbonic Anhydrases: New Horizons. Chegwidden WR, Carter ND, Edwards YH, editors. Basel, Switzerland: Birkhauser; 2000. pp. 175–195. [Google Scholar]
- 2.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 3.Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 4.Hewett-Emmett D. In: The Carbonic Anhydrases: New Horizons. Chegwidden WR, Carter ND, Edwards YH, editors. Basel, Switzerland: Birkhauser; 2000. pp. 29–76. [Google Scholar]
- 5.Lehtonen J, et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem. 2004;279(4):2719–2727. doi: 10.1074/jbc.M308984200. [DOI] [PubMed] [Google Scholar]
- 6.Fleming RE, et al. Carbonic anhydrase IV expression in rat and human gastrointestinal tract regional, cellular, and subcellular localization. J Clin Invest. 1995;96(6):2907–2913. doi: 10.1172/JCI118362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastoreková S, et al. Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112(2):398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 8.Kyllönen MS, et al. Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney. J Histochem Cytochem. 2003;51(9):1217–1224. doi: 10.1177/002215540305100912. [DOI] [PubMed] [Google Scholar]
- 9.Parkkila S, et al. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA. 2001;98(4):1918–1923. doi: 10.1073/pnas.98.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilvo M, et al. Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J. 2005;392(Pt 1):83–92. doi: 10.1042/BJ20051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen J, Parkkila S, Kaunisto K, Koivunen P, Rajaniemi H. Secretion of carbonic anhydrase isoenzyme VI (CA VI) from human and rat lingual serous von Ebner’s glands. J Histochem Cytochem. 2001;49(5):657–662. doi: 10.1177/002215540104900513. [DOI] [PubMed] [Google Scholar]
- 12.Shah GN, et al. Mitochondrial carbonic anhydrase CA VB: Differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci USA. 2000;97(4):1677–1682. doi: 10.1073/pnas.97.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster RE, II, Dodgson SJ, Storey BT, Lin L. Measurement of carbonic anhydrase activity inside cells and subcellular particles. Ann N Y Acad Sci. 1984;429:415–429. doi: 10.1111/j.1749-6632.1984.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 14.Dodgson SJ, Forster RE, II, Storey BT. The role of carbonic anhydrase in hepatocyte metabolism. Ann N Y Acad Sci. 1984;429:516–524. doi: 10.1111/j.1749-6632.1984.tb12380.x. [DOI] [PubMed] [Google Scholar]
- 15.Dodgson SJ, Forster RE., II Inhibition of CA V decreases glucose synthesis from pyruvate. Arch Biochem Biophys. 1986;251(1):198–204. doi: 10.1016/0003-9861(86)90066-4. [DOI] [PubMed] [Google Scholar]
- 16.Dodgson SJ. Inhibition of mitochondrial carbonic anhydrase and ureagenesis: A discrepancy examined. J Appl Physiol. 1987;63(5):2134–2141. doi: 10.1152/jappl.1987.63.5.2134. [DOI] [PubMed] [Google Scholar]
- 17.Hazen SA, Waheed A, Sly WS, LaNoue KF, Lynch CJ. Differentiation-dependent expression of CA V and the role of carbonic anhydrase isozymes in pyruvate carboxylation in adipocytes. FASEB J. 1996;10(4):481–490. doi: 10.1096/fasebj.10.4.8647347. [DOI] [PubMed] [Google Scholar]
- 18.Parkkila AK, et al. Expression of carbonic anhydrase V in pancreatic beta cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J Biol Chem. 1998;273(38):24620–24623. doi: 10.1074/jbc.273.38.24620. [DOI] [PubMed] [Google Scholar]
- 19.Arechederra RL, Waheed A, Sly WS, Supuran CT, Minteer SD. Effect of sulfonamides as carbonic anhydrase VA and VB inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem. 2013;21(6):1544–1548. doi: 10.1016/j.bmc.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Amor-Gueret M, Levi-Strauss M. Nucleotide and derived amino-acid sequence of a cDNA encoding a new mouse carbonic anhydrase. Nucleic Acids Res. 1990;18(6):1646. doi: 10.1093/nar/18.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagao Y, et al. Mitochondrial carbonic anhydrase (isozyme V) in mouse and rat: cDNA cloning, expression, subcellular localization, processing, and tissue distribution. Proc Natl Acad Sci USA. 1994;91(22):10330–10334. doi: 10.1073/pnas.91.22.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagao Y, Batanian JR, Clemente MF, Sly WS. Genomic organization of the human gene (CA5) and pseudogene for mitochondrial carbonic anhydrase V and their localization to chromosomes 16q and 16p. Genomics. 1995;28(3):477–484. doi: 10.1006/geno.1995.1177. [DOI] [PubMed] [Google Scholar]
- 23.Väänänen HK, Carter ND, Dodgson SJ. Immunocytochemical localization of mitochondrial carbonic anhydrase in rat tissues. J Histochem Cytochem. 1991;39(4):451–459. doi: 10.1177/39.4.1900871. [DOI] [PubMed] [Google Scholar]
- 24.Harrison HE, Harrison HC. Inhibition of urine citrate excretion and the production of renal calcinosis in the rat by acetazoleamide (diamox) administration. J Clin Invest. 1955;34(11):1662–1670. doi: 10.1172/JCI103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippi L, et al. Pathogenic mechanism, prophylaxis, and therapy of symptomatic acidosis induced by acetazolamide. J Investig Med. 2002;50(2):125–132. doi: 10.2310/6650.2002.31297. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim H, Lee YJ, Curthoys NP. Renal response to metabolic acidosis: Role of mRNA stabilization. Kidney Int. 2008;73(1):11–18. doi: 10.1038/sj.ki.5002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braissant O, McLin VA, Cudalbu C. Ammonia toxicity to the brain. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-012-9546-2. 10.1007/s10545-012-9546-2. [DOI] [PubMed] [Google Scholar]
- 28.Wolf B, et al. Multiple carboxylase deficiency: Clinical and biochemical improvement following neonatal biotin treatment. Pediatrics. 1981;68(1):113–118. [PubMed] [Google Scholar]
- 29.Lehninger AL, Carafoli E, Rossi CS. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- 30.Shah GN, et al. Pharmacological inhibition of mitochondrial carbonic anhydrases protects mouse cerebral pericytes from high glucose-induced oxidative stress and apoptosis. J Pharmacol Exp Ther. 2013;344(3):637–645. doi: 10.1124/jpet.112.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazen SA, Waheed A, Sly WS, LaNoue KF, Lynch CJ. Effect of carbonic anhydrase inhibition and acetoacetate on anaplerotic pyruvate carboxylase activity in cultured rat astrocytes. Dev Neurosci. 1997;19(2):162–171. doi: 10.1159/000111202. [DOI] [PubMed] [Google Scholar]
- 32.Bega D, Vaitkevicius H, Boland TA, Murray M, Chou SH. Fatal hyperammonemic brain injury from valproic acid exposure. Case Rep Neurol. 2012;4(3):224–230. doi: 10.1159/000345226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniotti M, la Marca G, Fiorini P, Filippi L. 2011. New developments in the treatment of hyperammonemia: Emerging use of carglumic acid. Int J Gen Med 4:21–28. [DOI] [PMC free article] [PubMed]
- 34.Price TO, Eranki V, Banks WA, Ercal N, Shah GN. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology. 2012;153(1):362–372. doi: 10.1210/en.2011-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch CJ, et al. Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochem J. 1995;310(Pt 1):197–202. doi: 10.1042/bj3100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supuran CT. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs. 2012;17(1):11–15. doi: 10.1517/14728214.2012.664132. [DOI] [PubMed] [Google Scholar]
- 37.Dodgson SJ, Cherian K. Mitochondrial carbonic anhydrase is involved in rat renal glucose synthesis. Am J Physiol. 1989;257(6 Pt 1):E791–E796. doi: 10.1152/ajpendo.1989.257.6.E791. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Shoemaker JD. One-step metabolomics: Carbohydrates, organic and amino acids quantified in a single procedure. J Vis Exp. 2010;(40) doi: 10.3791/2014. pii:2014. 10.3791/2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.