Abstract
Transient Receptor Potential Melastatin–8 (TRPM8), a recently identified member of the transient receptor potential (TRP) family of ion channels, is activated by mild cooling and by chemical compounds such as the supercooling agent, icilin. Since cooling, possibly involving TRPM8 stimulation, diminishes injury-induced peripheral inflammation, we hypothesized that TRPM8 activation may also attenuate systemic inflammation. We thus studied the involvement of TRPM8 in regulating colonic inflammation using two mouse models of chemically induced colitis. TRPM8 expression, localized immunohistochemically in transgenic TRPM8GFP mouse colon, was up-regulated in both human- and murine-inflamed colon samples, as measured by real-time PCR. Wild-type mice (but not TRPM8-nulls) treated systemically with the TRPM8 agonist, icilin showed an attenuation of chemically induced colitis, as reflected by a decrease in macroscopic and microscopic damage scores, bowel thickness, and myeloperoxidase activity compared with untreated animals. Furthermore, icilin treatment reduced the 2,4,6-trinitrobenzenesulfonic acid–induced increase in levels of inflammatory cytokines and chemokines in the colon. In comparison with wild-type mice, Dextran Sodium Sulfate (DSS)-treated TRPM8 knockout mice showed elevated colonic levels of the inflammatory neuropeptide calcitonin-gene–related peptide, although inflammatory indices were equivalent for both groups. Further, TRPM8 activation by icilin blocked capsaicin-triggered calcitonin-gene–related peptide release from colon tissue ex vivo and blocked capsaicin-triggered calcium signaling in Transient Receptor Potential Vaniloid-1 (TRPV1) and TRPM8 transfected HEK cells. Our data document an anti-inflammatory role for TRPM8 activation, in part due to an inhibiton of neuropeptide release, pointing to a novel therapeutic target for colitis and other inflammatory diseases.
Keywords: CGRP, TRPV1, IBD, Crohn's disease
Nonresolving inflammation is a major pathological component of a number of diseases including inflammatory bowel diseases (IBDs) (1, 2). IBDs, which include Crohn's disease and ulcerative colitis (UC), are chronic and relapsing inflammatory disorders (3, 4) that are characterized by proinflammatory cytokine production, leukocyte infiltration, and consequent structural and functional damage to the gut (3, 5, 6). Despite significant advances in our understanding of the pathological basis of these diseases, there exists an important unmet need in the treatment of these chronic inflammatory conditions.
Controlled tissue cooling or hypothermia is widely used to suppress tissue damage resulting from trauma, ischemia, and surgery (7) and is known to result in a reduction in inflammation (8, 9) and severity of peripheral nerve injury (10). Recently, Transient Receptor Potential Melastatin–8 (TRPM8) was identified as a temperature-sensitive ion channel activated by mild cooling (11–14). TRPM8-deficient mice show no preference for warm temperatures over cold temperatures and have impaired cold avoidance behavior (12–14). In addition to activation by environmental cold, TRPM8 is activated by chemical stimuli such as menthol and icilin (11, 15) that elicit the sensation of coolness. Apart from its role in thermosensation, acute activation or inhibition of TRPM8 can have analgesic effects either to diminish neuropathic and visceral pain (16–18) or to attenuate cold hypersensitivity in inflammatory and nerve-injury pain models (19), suggesting that neuronal TRPM8 may play a neurogenic anti-inflammatory role in certain settings.
In this study we hypothesized that TRPM8 activation, which very likely mediates some of the anti-inflammatory effects of mild cooling for trauma-induced peripheral inflammation, in addition to its neuronal sensory function, might also attenuate tissue inflammation in the setting of experimental colitis. We report here the detection of TRPM8 expression in the colon and localization of TRPM8 expression using a TRPM8GFP transgenic mouse. TRPM8 mRNA was up-regulated in inflamed human and murine colon tissue. We also show that in two models [2,4,6-trinitrobenzenesulfonic acid (TNBS)/Dextran Sodium Sulfate (DSS)] of murine colitis, TRPM8 activation with icilin has potent anti-inflammatory and disease-attenuating effects. This anti-inflammatory effect of icilin-stimulated TRPM8 activation occurs, at least in part, through the suppression of Transient Receptor Potential Vanilloid-1 (TRPV1)-dependent calcitonin-gene–related peptide (CGRP) release in the colon. Thus, in addition to its antinociceptive action, our work defines an anti-inflammatory role for TRPM8, thus identifying this channel as a promising therapeutic target for treating inflammatory diseases such as colitis/IBD.
Results
TRPM8 Expression in the Colon.
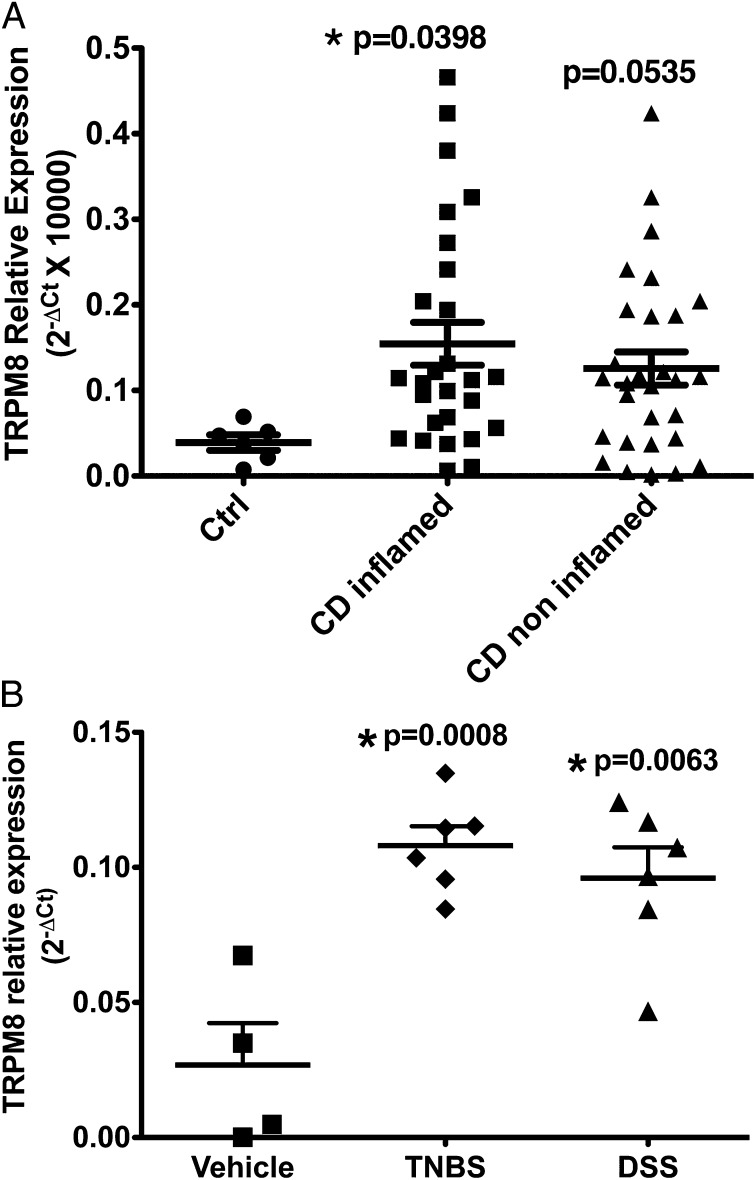
TRPM8 mRNA was detected by real-time PCR in both human and mouse colon tissue. A significant increase in TRPM8 expression was observed in both inflamed human colonic tissue from patients with IBD (Fig. 1A) as well as in samples from TNBS- or DSS-treated mice (Fig. 1B). Noninflamed colonic tissues from IBD patients showed an elevated but slightly lower level of TRPM8 expression than in the inflamed tissue.
Fig. 1.
TRPM8 expression in human and mouse colon. (A) Real-time PCR detection of TRPM8 expression in colonic biopsies from control (closed circle), noninflamed Crohn's disease patient colons (closed squares), or inflamed Crohn's disease patient colons (closed triangles). Data are shown as mean ± SEM. *P < 0.05. n = 5–29. (B) TRPM8 expression in the colon of mice treated with vehicle, TNBS, or DSS. A significant increase in TRPM8 mRNA levels is seen in colonic tissue from TNBS- or DSS-treated mice compared with vehicle-treated mice. Data are shown as mean ± SEM. *P < 0.05. n = 4–6.
To determine the anatomical location of TRPM8 in the mouse colon, we monitored TRPM8 expression using a transgenic mouse in which GFP reporter expression is driven by the TRPM8 transcriptional promoter (TRPM8GFP). Confocal imaging of GFP-stained colonic sections derived from TRPM8GFP mice (Fig. S1 C and D) revealed abundant TRPM8 expression in the luminal epithelial cells (Fig. S1D, red arrows), and in neuronal-like structures in the myenteric plexus (Fig. S1D, white arrow). Costaining with CGRP revealed that TRPM8 expression closely associated with peptidergic neurons but did not colocalize (Fig. S1 E–G). Abundant colocalization was observed between the epithelial cell marker Zona Occludens Protein-1 (ZO-1) and TRPM8-expressing cells in the mucosa (Fig. S1 H–J).
Attenuation of TNBS–DSS Colitis and Inflammatory Cytokine/Chemokine Release by Icilin.
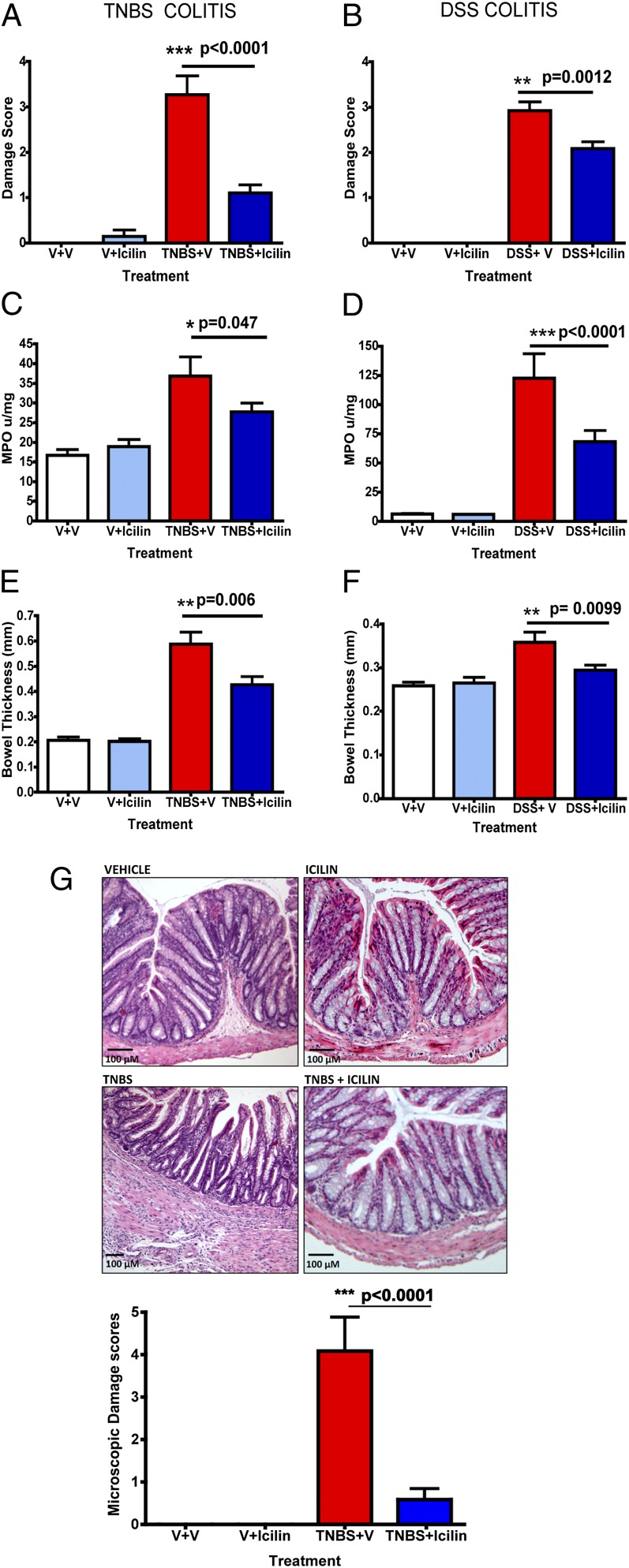
Colitis was induced in mice by intracolonic administration of TNBS or by administration of DSS in the drinking water for 7 d. Male C57BL/6 mice treated with TNBS (Fig. 2 A, C, and E) or DSS (Fig. 2 B, D, and F) developed all of the hallmarks of colitis described for these models including weight loss (Fig. S2), bloody diarrhea, and increases in histochemical–biochemical indices of inflammation. Upon sacrifice on day 7 these animals showed significant macroscopic damage to the colon (Fig. 2 A and B), with erythema, edema, ulceration, and strictures. The colons were thickened (Fig. 2 E and F) and myeloperoxidase (MPO) activity, indicative of granulocyte infiltration, was significantly increased (Fig. 2 C and D). Histological assessment of the TNBS-treated colons showed transmural inflammation with thickening of the muscularis, an influx of inflammatory cells, absence of goblet cells, and gland disorganization (Fig. 2G, Lower). In contrast, mice treated with icilin (i.p. daily) (Fig. 2), but not menthol (Fig. S3), in conjunction with TNBS or DSS administration, showed significantly attenuated macroscopic damage scores, bowel thickness, and colonic MPO levels (Fig. 2 E and F). Furthermore, icilin-treated mice showed substantially diminished histological damage (Fig. 2G) as quantified by a histological scoring system (20) (Fig. 2G, Lower). Little to no damage was observed in mice treated with icilin alone or vehicle.
Fig. 2.
Icilin attenuates colonic inflammation in mice. Assessment of intestinal damage scores, colonic MPO levels, and bowel thickness in mice treated with vehicle, icilin, TNBS or DSS, and TNBS or DSS plus icilin or vehicle. Mice treated with icilin exhibit reduced intestinal damage scores, colonic MPO levels, and bowel thickness compared with vehicle-treated animals in both TNBS- (A, C, and E) and DSS- (B, D, and F) induced colitis. No significant differences are observed between vehicle- and icilin-treated groups. (G) Representative pictograms of H&E-stained colon sections from mice treated with vehicle, icilin, TNBS, or TNBS plus icilin. Histogram depicts the microscopic damage scores in mice from each of the four groups. *P < 0.05, **P < 0.005, and ***P < 0.0005. n = 8 animals per group.
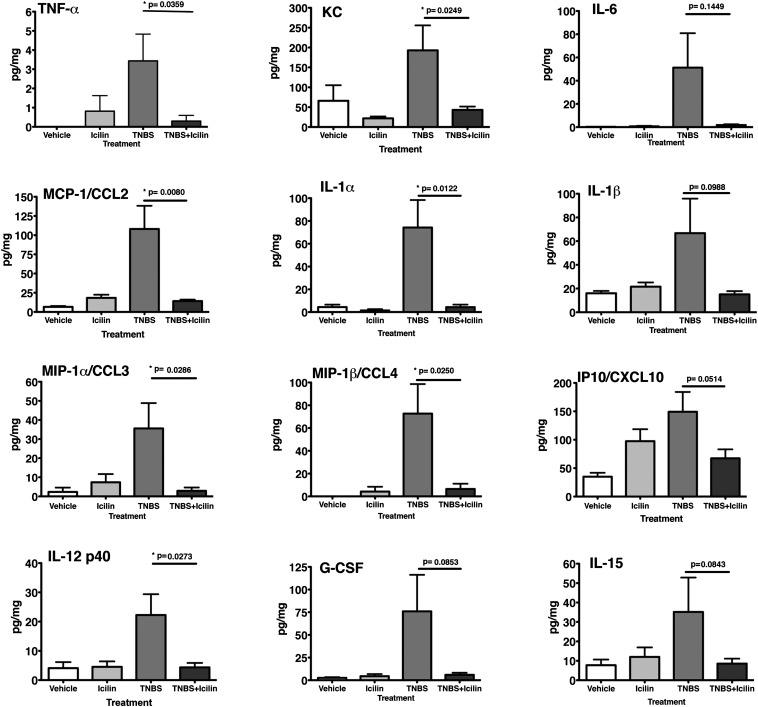
The abundance of a number of proinflammatory cytokines and chemokines in mouse colonic tissue was also profiled using the MILLIPLEX MAP Mouse Cytokine/Chemokine assay (Millipore). As expected, TNBS-treated mouse colons exhibited elevated levels of a number of proinflammatory cytokines and chemokines. Strikingly, icilin treatment significantly attenuated the levels of a wide spectrum of inflammatory markers including TNF-α, keratinocyte-derived chemokine (KC), IL-6, monocyte chemoattractant protein-1 (MCP-1) [chemokine (C-C) ligand (CCL) 2], IL-1α, macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), and IL-12 p40 (Fig. 3). The levels of some cytokines (IL-1β, CXC chemokine ligand (CXCL) 10 (CXCL10), granulocyte colony stimulating factor (GCSF), and IL-15), which showed quite variable increases in the TNBS colitis mice did not rise significantly above baseline levels in icilin-treated TNBS-inflamed animals. Compared with controls, icilin treatment alone did not significantly change the levels of any of the cytokines/chemokines tested. Icilin also significantly attenuated leukocyte adherence in colonic venules of mice treated with TNF-α (Fig. S4A). No significant differences in leukocyte rolling or vessel diameter were observed in any of the treatment groups (Fig. S4 B and C).
Fig. 3.
Icilin reduces inflammatory cytokine and chemokine levels in mice with TNBS-induced colitis. Cytokine and chemokine profiling in the colons of mice treated with vehicle, icilin, TNBS, or TNBS plus icilin. Mice treated with icilin during the course of TNBS-induced colitis show significantly reduced cytokine/chemokine levels compared with mice with TNBS-induced colitis. *P < 0.05. n = 8 animals per group.
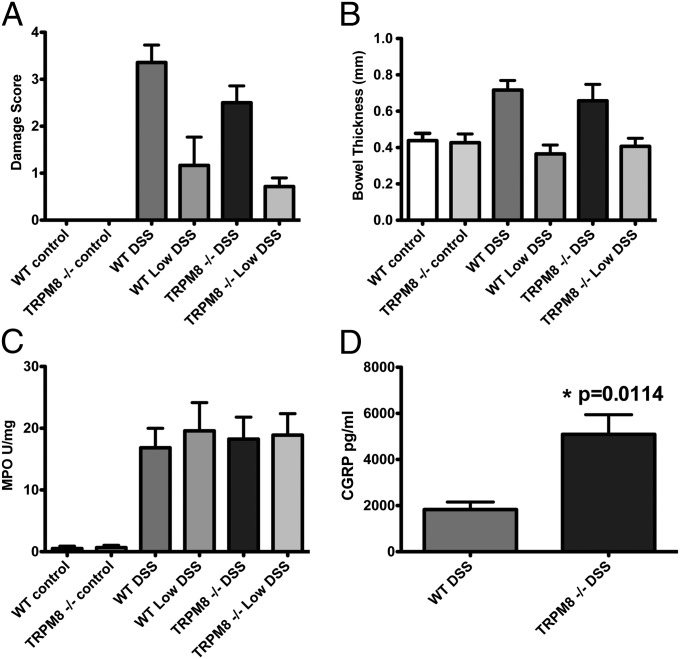
The anti-inflammatory effect of icilin was not observed in TRPM8-null mice (Fig. S5). While TRPM8-deficient mice showed similar DSS (Fig. 4 A–C) or TNBS (Fig. S6) colitis disease parameters compared with wild-type mice, significantly higher levels of the inflammatory neuropeptideCGRP were detected in the colons of DSS-treated TRPM8-null mice compared with DSS-treated wild-type mice (Fig. 4D).
Fig. 4.
TRPM8−/− mice do not show enhanced DSS-induced colonic inflammation but have elevated levels of CGRP. Assessment of intestinal damage scores, colonic MPO levels, and bowel thickness in WT and TRPM8−/− mice treated with vehicle, DSS (2.5% wt/vol), or low-dose DSS (1% wt/vol:Low DSS). TRPM8−/− mice do not show significantly different (A) damage scores, (B) bowel thickness, or (C) MPO levels compared with WT mice. (D) Comparison of CGRP levels in colonic tissue from WT and TRPM8−/− mice after 7 d of DSS administration. Data are shown as mean ± SEM. n = 8 animals per group. *P < 0.05.
TRPM8 Inhibits TRPV1 Activation to Regulate CGRP Release in the Colon.
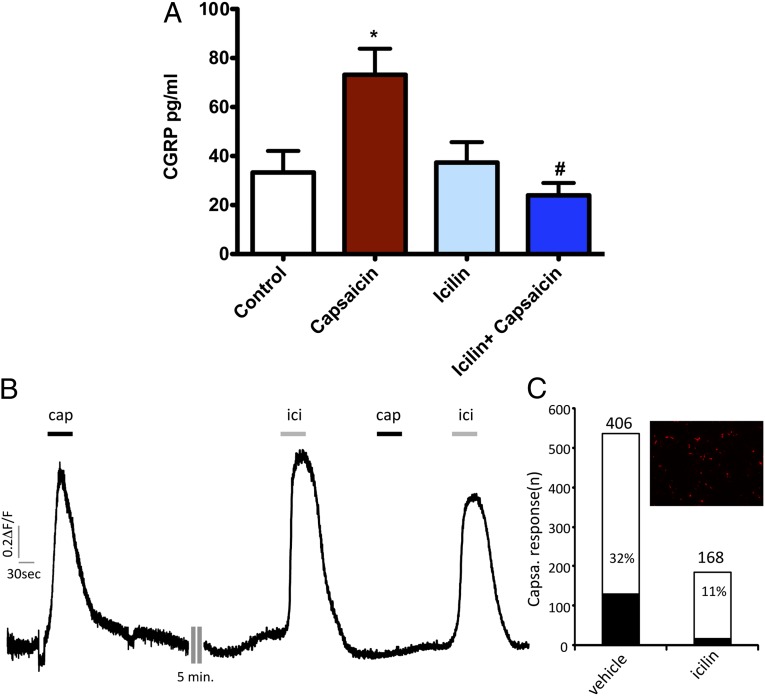
Since we found that CGRP was elevated in DSS-treated TRPM8-null versus wild-type mice, we suspected that part of the anti-inflammatory action of icilin might be to diminish the release of inflammatory neuropeptides. We thus investigated the ability of TRPM8 activation to reduce TRPV1-stimulated neuropeptide release from colonic tissue ex vivo. As expected, exposure of colon tissue to the TRPV1 agonist capsaicin significantly elevated CGRP release (Fig. 5A). Icilin treatment alone did not cause CGRP release. However, pretreatment of the colon tissue with icilin before capsaicin exposure significantly reduced colonic CGRP release (Fig. 5A). This ability of icilin-induced TRPM8 activation to block TRPV1-mediated neuropeptide release from intact tissue was mirrored by its ability to block capsaicin-stimulated TRPV1 calcium signaling (Fig. 5B).
Fig. 5.
Inhibition of TRPV1-dependent CGRP release and calcium signaling by TRPM8 activation. (A) TRPV1 activation by capsaicin triggers CGRP release from distal colon tissue. TRPM8 activation by icilin treatment does not stimulate CGRP release from the colon. Pretreatment of colonic tissue with icilin inhibits TRPV1-stimulated CGRP release. * indicates significant elevation of CGRP release compared with control. # indicates significant attenuation compared with capsaicin-stimulated CGRP release. Data are expressed as mean ± SEM. n = 3 animals per group. *P < 0.05. (B) Calcium signaling was monitored in TRPM8- and TRPV1-transfected HEK cell monolayers. Representative trace showing fluorescence intensity (0.2ΔF/F) elicited by capsaicin (1 μM) and icilin (30 μM) in human embryonic kidney-tsA-201 cells transfected with TRPV1, TRPM8, and mCherry (to visualize transfected cells). (C) Percentage of transfected cells responding to capsaicin following vehicle or icilin application. (Inset) Representative image of mCherry-transfected tsA-201cells. Cells responding to capsaicin and icilin were mCherry-positive. Capsaicin responses were considered positive when ΔF/F was >10% of the initial response before vehicle or icilin challenge.
Discussion
We report here that activation of TRPM8 by its potent and selective agonist, icilin, was able to attenuate inflammation in two murine models of colitis. This inhibition of the inflammatory response by icilin, attributed to TRPM8 activation, since no such effect was observed in TRPM8-null mice, was characterized not only by attenuation of all of the tissue indices of inflammation known for the TNBS/DSS colitis models, but also by marked reductions in the TNBS-triggered inflammatory tissue cytokines/chemokine levels. We also observe an increase in TRPM8 expression in inflamed mouse and human colon tissues. Following the development of DSS colitis, TRPM8-null mice showed significantly higher levels of the neuroinflammatory peptide, CGRP, compared with wild-type mice, but this elevation was evidently not sufficient to cause an enhanced inflammation in the TRPM8-deficient animals, compared with wild-type mice. However, icilin treatment was able to abolish CGRP release caused by capsaicin-induced TRPV1 activation in colonic tissue ex vivo. Thus, the counterregulatory anti-inflammatory role of TRPM8 in vivo due to its activation by endogenous agonists may depend on the abundance of these mediators, which remain to be identified. Notwithstanding, the exogenous activation of TRPM8 by icilin is clearly able to diminish inflammation, acting in part by reducing inflammatory neuropeptide release and attenuating proinflammatory cytokines/chemokine release and suppressing leukocyte recruitment to the colon. Thus, our work extends the impact of TRPM8 activation from its currently recognized antinociceptive and thermosensing role to an additional anti-inflammatory role.
One stimulus for the work we report here was our knowledge of the accepted use of hypothermia for the treatment of soft tissue injuries or to mitigate the inflammatory response following surgery (7). Cooling is established as an effective means of reducing neuronal damage in a number of clinical conditions including anoxic brain injury following cardiac arrest (21) and hypoxic ischemia-induced neonatal encephalopathy (22). In the brain, therapeutic hypothermia results in a number of neuroprotective responses (23) including a reduction in leukocyte infiltration as well as a decrease in the levels of adhesion molecules (24) and proinflammatory cytokines (25). Based on our findings, we suggest that TRPM8 might be mediating these accepted therapeutic modalities involving cooling.
Although TRPM8 was first discovered as a prostate-expressed protein (26), the main emphasis since that time on the function of this channel has been as a “cold-sensing” nonselective cation channel in neuronal cells (11, 14). While widely expressed in neuronal cells, TRPM8 is also found in other sites, including the genitourinary tract (27, 28), lung (29, 30), liver (31), blood vessels (32, 33), and sperm (34). Like the colon, inflammation in these tissues may also be blocked by icilin stimulation of TRPM8. However, the endogenous activators of TRPM8 that might mimic the actions of icilin in an inflammatory setting in these tissues remain to be determined.
Endogenous regulators of TRPM8 that have been identified include phosphatidylinositol 4,5-bisphosphate (PIP2) (35–37) (also a negative regulator of TRPV1) (38), endovanniloids–endocannabinoids (39, 40), and Phospholipase A2 (PLA2)–derived lysophospholipids (lysophosphatidylcholine, lysophosphatidylinositol, and lysophosphatidylserine), all of which can enhance the thermal sensitivity of TRPM8, leading to its activation at physiological body temperatures (41). Of note, levels of PLA2 are elevated in Crohn's disease and UC patients (42), while levels of lysophospholipids are significantly decreased (43). These changes may affect the function of TRPM8 during colitis. In this regard, we found that the inflammatory response to colitic agents in the TRPM8−/− mice was not greater than in wild-type controls, even though levels of the inflammatory neuropeptide CGRP were elevated in the knockout mice. This is likely due to compensatory protective mechanisms in TRPM8-deficient mice that attenuate the CGRP-mediated inflammatory response. These results also argue in favor of a counterregulatory anti-inflammatory role for TRPM8 that would depend on the local production of endogenous TRPM8 regulators, rather than on its role as an active participant in the inflammatory response. In addition, the increase in TRPM8 expression seen in inflamed human or mouse colons suggests that channel up-regulation, as opposed to increased levels of a channel activator, might be responsible for the anti-inflammatory responses. Identification of the endogenous agonist(s) of TRPM8, particularly in the setting of colitis, could provide further mechanistic insights into the role of TRPM8 in regulating the inflammatory response.
Previous studies on TRPM8 function in the colon have focused on the regulation of visceral hypersensitivity (16). TRPM8 is co-expressed with TRPV1 in a subset of colonic sensory neurons where its activation with icilin leads to antinociceptive responses that occur through suppression of TRPV1 activity (16). This in vivo antinociceptive action relates directly to our in vitro data obtained using a heterologous HEK cell expression system in which TRPM8 activation inhibits TRPV1 calcium signaling (Fig. 4B). This inhibitory mechanism can account for our results obtained with the colonic tissue ex vivo, where icilin-dependent TRPM8 activation blocked the release of CGRP following TRPV1 activation with capsaicin (Fig. 4A). Thus, we suggest that the anti-inflammatory action of icilin is due in part to its ability to block inflammatory neuropeptide release. Since TRPV1 expression is increased in IBDs (44–46) and its activation leads to the release of inflammatory neuropeptides (substance-P and CGRP), triggering neurogenic inflammation, we predict that an icilin-mediated block of TRPV1 function might prove of value for human colitis therapy.
Icilin has been well characterized as a potent and selective TRPM8 agonist (14, 47). However, in therapeutically targeting TRPM8, it is important to note that the activation of TRPM8 by icilin differs mechanistically from its activation by menthol or cold (48, 49). This distinction suggests that different agonists may act as allosteric modulators of TRPM8 to elicit distinct responses (50). Indeed in our study we see potent anti-inflammatory responses with icilin but not with menthol. While this result could be due to the greater potency of icilin, it is also possible that icilin modulates TRPM8 in a manner that favors anti-inflammatory signaling over other responses. Importantly, our work used icilin at doses selective for this channel (51) and lower than those that could also activate the related Transient Receptor Potential A1 (TRPA1) channel (52, 53). In contrast with TRPM8, TRPA1 activation appears to be proinflammatory and proalgesic (54). Thus, TRPA1 antagonists (not agonists, as for TRPM8) are suggested for blocking colitis-induced chemo-nociceptive signals (55, 56).
In addition to the colonic sensory neurons, we observed abundant expression of TRPM8 in the colonic epithelial cells. It will be important to better understand the contribution of the different cellular locales of TRPM8 to its anti-inflammatory actions. Of note, lung epithelial cells can express a TRPM8 variant that paradoxically increases proinflammatory cytokine production upon stimulation with cold air or menthol (29, 30). The expression of these inhibitory variant TRPM8 channels has also been demonstrated in the prostate, where they act as negative regulators of the full-length channel (57). Future studies are clearly warranted to identify the specific TRPM8 isoforms and their regulation in inflammatory settings like colitis.
In summary, we show that TRPM8 activation by icilin leads to potent anti-inflammatory effects that are not observed in TRPM8-null mice. Our work therefore defines TRPM8 as an “anti-inflammatory” target, which adds to its recognized role as a “thermosensor.” The data point to an integration of sensory signaling and innate immune responses in the body’s reaction to inflammation. Further, our work suggests that selective TRPM8-targeted compounds may have therapeutic utility in the treatment of colitis as well as other inflammatory diseases.
Materials and Methods
Detailed methods, including reagents, human biopsy, mice strains, induction of colitis, study design, mRNA extraction, real-time PCR detection of TRPM8, immunocytochemical detection of TRPM8 expression in the mouse colon, measurements of MPO activity, measurement of cytokine levels, intravital microscopy, CGRP Enzyme Immunometric Assay, and statistical analyses, appear in SI Materials and Methods. Human intestinal biopsy samples were collected from participants consented through the Intestinal Inflammation Tissue Bank under an ethics protocol approved by the Conjoint Health Research Ethics Board at the University of Calgary. All animal experiments were approved by the University of Calgary Animal Care Committee and were performed in accordance with the international guidelines for the ethical use of animals in research and guidelines of the Canadian Council on Animal Care.
Supplementary Material
Acknowledgments
These studies were supported in large part by grants from the Canadian Institutes of Health Research (CIHR) (to M.D.H., C.A., N.V., and P.L.B.). Some aspects of the study were also funded by grants from Crohn's and Colitis Foundation of Canada (CCFC) and Alberta Innovates-Health Solutions (AI-HS) (to P.L.B.). R.R. received support from a Canadian Association of Gastroenterology/CIHR/Ortho-Jensen postdoctoral fellowship and from an AI-HS postdoctoral fellowship. T.K.L. is supported by an AI-HS postdoctoral fellowship. C.L.H. received support from an AI-HS/CCFC postdoctoral fellowship. TRPM8 knockout mice were kindly provided by Dr. Ardem Patapoutian (the Scripps Research Institute). Human biopsy tissue was obtained through the Intestinal Inflammation Tissue Bank at the University of Calgary with support from the Alberta Inflammatory Bowel Disease Consortium, which is funded by an Alberta Heritage Foundation for Medical Research (AHFMR) Interdisciplinary Team Grant (AHFMR is now AI-HS). The microscopy work was supported by the Live Cell Imaging Facility funded by an equipment and infrastructure grant from the Canadian Foundation Innovation and the Alberta Science and Research Authority. Colitis studies were supported by the Snyder Institute/Centre for Advanced Technologies/Health Sciences Animal Resource Centre Behavior Core Facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217431110/-/DCSupplemental.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartor RB. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 6.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 7.Finley DS. Basis for the use of localized hypothermia during radical pelvic surgery. Nat Rev Urol. 2011;8(6):345–350. doi: 10.1038/nrurol.2011.65. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, et al. Induction of profound hypothermia modulates the immune/inflammatory response in a swine model of lethal hemorrhage. Resuscitation. 2005;66(2):209–216. doi: 10.1016/j.resuscitation.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto K, et al. Early induction of moderate hypothermia suppresses systemic inflammatory cytokines and intracellular adhesion molecule-1 in rats with caerulein-induced pancreatitis and endotoxemia. Pancreas. 2008;37(2):176–181. doi: 10.1097/MPA.0b013e318162cb26. [DOI] [PubMed] [Google Scholar]
- 10.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371(9628):1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 11.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 12.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 13.Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54(3):379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 16.Harrington AM, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152(7):1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Brignell JL, Chapman V, Kendall DA. Comparison of icilin- and cold-evoked responses of spinal neurones, and their modulation of mechanical activity, in a model of neuropathic pain. Brain Res. 2008;1215:87–96. doi: 10.1016/j.brainres.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 18.Proudfoot CJ, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16(16):1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 19.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS ONE. 2011;6(9):e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N. Protease-activated receptor-2 activation: A major actor in intestinal inflammation. Gut. 2008;57(9):1222–1229. doi: 10.1136/gut.2008.150722. [DOI] [PubMed] [Google Scholar]
- 21.Holzer M. Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 22.Gluckman PD, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 23.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 24.Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003;34(10):2495–2501. doi: 10.1161/01.STR.0000091269.67384.E7. [DOI] [PubMed] [Google Scholar]
- 25.Aslami H, et al. Mild hypothermia reduces ventilator-induced lung injury, irrespective of reducing respiratory rate. Transl Res. 2012;159(2):110–117. doi: 10.1016/j.trsl.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61(9):3760–3769. [PubMed] [Google Scholar]
- 27.Thebault S, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2005;280(47):39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 28.Stein RJ, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004;172(3):1175–1178. doi: 10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- 29.Sabnis AS, Shadid M, Yost GS, Reilly CA. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am J Respir Cell Mol Biol. 2008;39(4):466–474. doi: 10.1165/rcmb.2007-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabnis AS, Reilly CA, Veranth JM, Yost GS. Increased transcription of cytokine genes in human lung epithelial cells through activation of a TRPM8 variant by cold temperatures. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L194–L200. doi: 10.1152/ajplung.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonfria E, et al. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26(3):159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 32.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1267–L1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, et al. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;296(6):H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Blas GA, et al. TRPM8, a versatile channel in human sperm. PLoS ONE. 2009;4(6):e6095. doi: 10.1371/journal.pone.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brauchi S, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104(24):10246–10251. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284(3):1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25(7):1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 39.De Petrocellis L, et al. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): Effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313(9):1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 40.De Petrocellis L, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325(3):1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 41.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27(12):3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minami T, et al. Elevation of phospholipase A2 protein in sera of patients with Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 1993;88(7):1076–1080. [PubMed] [Google Scholar]
- 43.Braun A, et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: A clue to pathogenesis. Inflamm Bowel Dis. 2009;15(11):1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 44.Yiangou Y, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357(9265):1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- 45.Akbar A, et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59(6):767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- 46.Akbar A, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150(2):340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandell M, et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9(4):493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 49.Kühn FJ, Kühn C, Lückhoff A. Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives. J Biol Chem. 2009;284(7):4102–4111. doi: 10.1074/jbc.M806651200. [DOI] [PubMed] [Google Scholar]
- 50.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42(4-5):427–438. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Bödding M, Wissenbach U, Flockerzi V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium. 2007;42(6):618–628. doi: 10.1016/j.ceca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Ma S, G G, Ak VE, Jf D, H H. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci. 2008;21(4):370–378. [PubMed] [Google Scholar]
- 53.Rawls SM, Gomez T, Ding Z, Raffa RB. Differential behavioral effect of the TRPM8/TRPA1 channel agonist icilin (AG-3-5) Eur J Pharmacol. 2007;575(1-3):103–104. doi: 10.1016/j.ejphar.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 54.Trevisani M, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitrovic M, Shahbazian A, Bock E, Pabst MA, Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br J Pharmacol. 2010;160(6):1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, et al. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat Cell Biol. 2012;14(8):851–858. doi: 10.1038/ncb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bidaux G, et al. Regulation of activity of transient receptor potential melastatin 8 (TRPM8) channel by its short isoforms. J Biol Chem. 2012;287(5):2948–2962. doi: 10.1074/jbc.M111.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.