A recent issue of PNAS opened with an unfortunate Core Concepts article titled “Epigenetics” (1). The author, a science writer, began promisingly enough:
Despite the fact that every cell in a human body contains the same genetic material, not every cell looks or behaves the same….How does each cell retain its unique properties when, in its DNA-containing nucleus, it has the same master set of genes as every other cell?
Indeed understanding this problem has been an overarching goal of research in molecular, developmental, and, increasingly,evolutionary biology. And over the past 50 years a compelling answer has emerged from studies in a wide array of organisms. Curiously, the article ignores this body of knowledge, and substitutes for it misguided musings presented as facts.
Let me begin with a very brief overview of what drives development, a process that unfolds with essentially no changes in DNA sequence. Development of an organism from a fertilized egg is driven primarily by the actions of regulatory proteins called transcription factors. In sequential waves and combinations, these proteins bind to specific DNA sequences—called cis-regulatory sequences—associated with specific genes, and encourage (activate) or discourage (repress) transcription into mRNA of those genes (2–4).
The process is started by transcription factors, contributed mainly by the mother, found in the fertilized egg. In the first cell division these transcription factors are distributed asymmetrically to the daughter cells. Then, and in subsequent cell generations, new patterns of gene expression arise in two ways: as a matter of course (some transcription factors activating expression of genes encoding other regulatory proteins, etc.), and in response to signals sent by other cells. Signaling used in development affects expression of genes encoding yet more transcription factors—it does so by changing activities of transcription factors, already present, which target those genes (2). Retinoic acid (a small molecule) and growth hormone (a protein) are examples of such signaling molecules.
The determinative power of transcription factors is illustrated by a 2012 Nobel Prize–winning experiment: Forced expression of four specific DNA binding regulatory proteins can “reprogram” fibroblasts to stem cells (5). Many cell-reprogramming experiments have been done in recent years, all of which reflect the action of ectopically expressed transcription factors (6).
Patterns of gene expression underlying development can be very complex indeed. But the underlying mechanism by which, for example, a transcription activator activates transcription of a gene is well understood: only simple binding interactions are required. These binding interactions position the regulator near the gene to be regulated, and in a second binding reaction, the relevant enzymes, etc., are brought to the gene. The process is called recruitment (3, 4). Two aspects are especially important in the current context: specificity and memory.
Specificity.
Transcription regulators work specifically—activating one gene or set of genes and not another, for example—as defined by the location(s) of their affined cis-regulatory sequences. Thus, for many regulators and genes, any regulator will work on any gene as determined by the locations of its affined cis-regulatory sequence. Evolution can thus bring any gene under control of any regulator by apposing the proper cis-regulatory site with the gene. A recruiter (e.g., a transcription activator) is in effect a specificity determinant. In the absence of such a specificity determinant, the relevant enzymes work as background functions, that is, at low levels across the genome.
Memory (or Lack Thereof).
Genes do not automatically stay “on” or “off” once activated or repressed. Rather, those states of gene expression require the continual activities of the specific regulators to maintain that state of expression. Put another way, continual recruitment of the relevant enzymes is required to maintain the imposed state of gene regulation (7, 8). Nevertheless, memory effects are important for development, and the question will arise as to how they are achieved.
The Core Concepts Misconception
Curiously, the picture I have just sketched is absent from the Core Concepts article. Rather, it is said, chemical modifications to DNA (e.g., methylation) and to histones—the components of nucleosomes around which DNA is wrapped in higher organisms—drive gene regulation. This obviously cannot be true because the enzymes that impose such modifications lack the essential specificity: All nucleosomes, for example, “look alike,” and so these enzymes would have no way, on their own, of specifying which genes to regulate under any given set of conditions. I ignore DNA methylation in the remainder of this article because its possible role in development remains unclear, and it does not exist in, for example, flies and worms—model organisms the study of which has taught us much of what we know about development.
Histone modifications are called “epigenetic” in the Core Concepts article, a word that for years has implied memory (see Epigenetic). This is odd: It is true that some of these modifications are involved in the process of transcription per se—facilitating removal and replacement of nucleosomes as the gene is transcribed, for example (9, 10). And some are needed for certain forms of repression (11). But all attempts to show that such modifications are “copied along with the DNA,” as the article states, have, to my knowledge, failed (12–14). Just as transcription per se is not “remembered” without continual recruitment, so nucleosome modifications decay as enzymes remove them (the way phosphatases remove phosphates put in place on proteins by kinases), or as nucleosomes, which turn over rapidly compared with the duration of a cell cycle, are replaced (15). For example, it is simply not true that once put in place such modifications can, as stated in the Core Concepts article, “lock down forever” expression of a gene (7).
It is true, however, that during the course of development as well as at its final stages, certain genes, once “turned on” by a transiently acting transcription factor, stay on even in the absence of the original activator that triggered expression. But I noted above that activation of transcription quickly decays once the activator is removed or inhibited. And so whence this critical memory effect?
Positive Feedback
The answer—where we know it—is positive feedback, a process understood for years for bacterial regulatory systems (4). Thus, a transcriptional activator will maintain (self-perpetuate) transcription of its own gene if a copy of its affined cis-regulatory sequence is placed in front of its own gene (Fig. 1). Any other specified activator, working only transiently, can trigger the feedback loop, provided the cis-regulatory sequence recognized by that other activator is properly positioned. There are increasingly many illustrations of such systems at work in biological systems. For example, positive feedback loops facilitate the reprogramming of fibroblasts to stem cells referred to above. In that case, transient expression of the added transcription factors triggers feedback loops that maintain expression of the endogenous genes encoding those transcription factors. The differentiated functions of a nematode sensory neuron are activated and maintained in that way (16) (Fig. 2), and positive feedback loops are found widely in other developmental gene regulatory networks as well (17, 18).
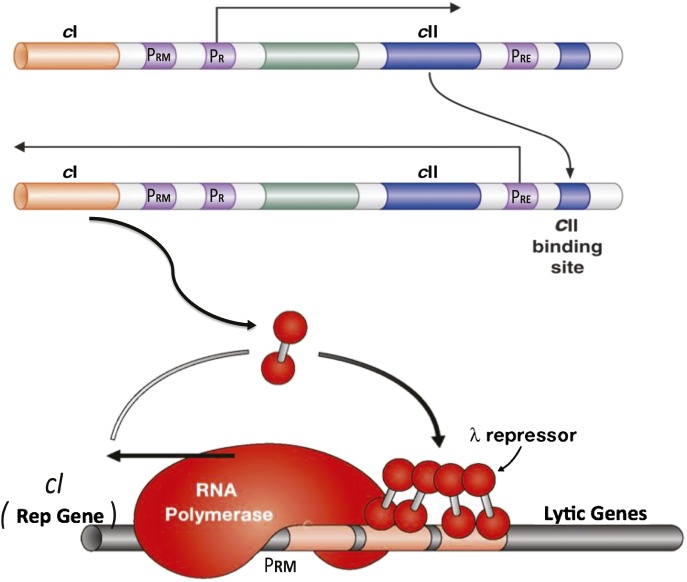
Fig. 1.
Establishment and maintenance of a positive feedback loop in a bacterium. Represented here are a few of the ca 50 phage genes of the bacteriophage lambda. The initial rightwards transcript extends through gene cII. The CII protein, a transcription activator, then activates a leftwards transcript that includes the gene cI. The cI protein, another transcription activator, activates expression of its own gene (a positive feedback reaction) as it represses rightwards transcription, including that of cII. This feedback loop, once established, is stable for very many cell divisions as the continuously produced CI protein (called the lambda repressor) is distributed to daughter cells. The bacteria are “lysogenic:”exposure to a signal (e.g., UV light) inactivates the repressor, previously silent phage genes areexpressed, and the cell lyses with the production of many new phage particles. PRE, PRM, and PR designate the “establishment,” “maintenance,” and “rightwards” promoters, respectively. (Reproduced with permission from refs. 3 and 4.)
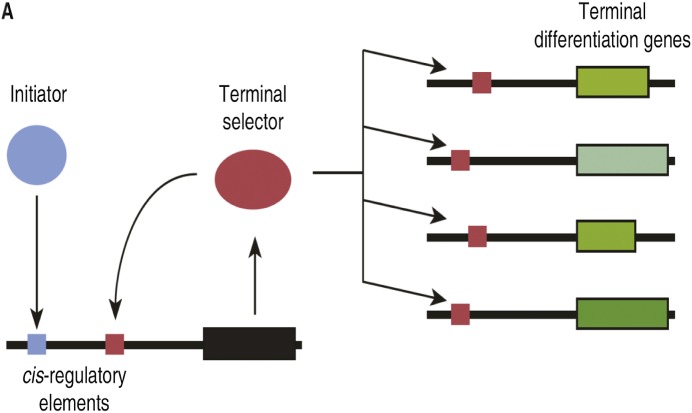
Fig. 2.
Establishment and maintenance of expression of terminal differentiation genes required to define a specific type of sensory neuron in a nematode. A series of gene regulatory events (not shown) produces, transiently, an activator depicted as a blue ball. That activator binds to the establishment cis-regulatory sequence (blue cube), and activates transcription of a terminal selector gene. The latter, another transcription activator, then maintains transcription of its own gene as it activates genes required for specific functions of the neuron. (Reprinted from ref. 16, Copyright 2011, with permission from Elsevier.)
There are mechanisms for self-perpetuating effects other than the one I described. For example, immunity to a virus evoked in a nematode by infection with a virus can be transmitted to subsequent generations. The mechanism turns out to be the transmission of small bits of RNA carried in the sperm. In worms these RNA molecules are automatically amplified by an enzymatic function not present in humans, and are readily passed along as the worms mate (19). RNA molecules share with specific DNA binding proteins a critical aspect lacking from histone modifying enzymes: They bear specificities in their base sequences, and so whatever effect they have (repressive in this case) can be directed to specific targets. In other words, RNA molecules, like regulatory proteins, can act specifically (20). For an example in which a transient inflammatory stimulus activates a chronic inflammatory loop involving transcription factors and small RNAs (called micro-RNAs) see ref. 21.
Epigenetic
And finally to that dreaded word “epigenetic.” For as long as I can remember, the word has been used to imply memory: A transient signal or event triggers a response that is then perpetuated in the absence of the original signal. I have mentioned important examples of such epigenetic effects, and the figures explain two examples in more detail. The bizarre situation we find ourselves in is that, as in the Core Concepts article, histone modifications are often called “epigenetic.” One can only wonder why (22, 23). As mentioned above, the enzymes that impose such modifications lack requisite specificity; the modified states are not self-perpetuating; and the roles played by the modifications remain for the most part obscure (10, 24). As pointed out by others (25–27), the ENCODE project got itself into problems by assuming that every espied event or modification is functional without subjecting that surmise to further testing. In our case, only by studying the effects of removing recruiters can one tell whether histone modifications are self-perpetuating, or whether (as is evidently the case) their continuing presence requires maintenance.
Scholars can be excused for taking the word “epigenetic” to have a broader meaning than the one I have so far described. For example, in 1952 the developmental biologist C. H. Waddington used the term to refer in general to what we now know to be changing patterns of gene expression that underlie development and that are often triggered by signals sent from other cells. Expanding on the subtitle of his book, Epigenetics: The science concerned with the causal analysis of development, Waddington, while mentioning memory (self-perpetuating) effects as important, was largely trying to describe the effects of one part of the embryo (the Spemann organizer, for example) on another. An overarching theme was to distinguish epigenetic development from the alternative Russian doll model, wherein fully formed adults were present in fertilized eggs and merely had to be revealed (28).
And so, in the modern parlance we have epigenetic changes as a subset of gene regulatory changes (i.e., self-perpetuating changes); and in the older Waddington sense we could refer to all developmental gene regulation (including signaling) as “epigenetic.” But in neither case is it correct to refer to nucleosome modifiers as “epigenetic”—they, like the very many proteins recruited to genes by specific transcription regulators, are parts of a response, not a cause, and there is no convincing evidence they are self-perpetuating. Labeling histone modifications “epigenetic,” moreover, obscures what we know. The Core Concepts article discussed here (1) is a good example, further muddying the waters. The important point is to attend to how things actually work.
Acknowledgments
I thank Bob Kingston, Thoru Pederson, Eric Davidson, Gene Bryant, Oliver Hobert, Kevin Struhl, Ann Hochschild, Lucy Gordon, Alex Gann, and Xin Wang for helpful comments.
Footnotes
The author declares no conflict of interest.
References
- 1.Williams SCP. Epigenetics. Proc Natl Acad Sci USA. 2013;110(9):3209. doi: 10.1073/pnas.1302488110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson EH. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. Burlington, MA: Academic; 2006. [Google Scholar]
- 3.Ptashne M, Gann A. Genes and Signals. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2002. [Google Scholar]
- 4.Ptashne M. A Genetic Switch (Third Edition): Phage Lambda Revisited. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2004. [Google Scholar]
- 5.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462(7273):587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 7.Cheng TH, Gartenberg MR. Yeast heterochromatin is a dynamic structure that requires silencers continuously. Genes Dev. 2000;14(4):452–463. [PMC free article] [PubMed] [Google Scholar]
- 8.Ho SN, Biggar SR, Spencer DM, Schreiber SL, Crabtree GR. Dimeric Ligands define a role for transcriptional activation domains in reinitiation. Nature. 1996;382(6594):822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 9.Smolle M, Workman JL, Venkatesh S. reSETing chromatin during transcription elongation. Epigenetics. 2013;8(1):10–15. doi: 10.4161/epi.23333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends Genet. 2011;27(10):389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Grunstein M. Histone function in transcription. Annu Rev Cell Biol. 1990;6:643–678. doi: 10.1146/annurev.cb.06.110190.003235. [DOI] [PubMed] [Google Scholar]
- 12.Katan-Khaykovich Y, Struhl K. Dynamics of global histone acetylation and deacetylation in vivo: rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes Dev. 2002;16(6):743–752. doi: 10.1101/gad.967302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radman-Livaja M, Liu CL, Friedman N, Schreiber SL, Rando OJ. Replication and active demethylation represent partially overlapping mechanisms for erasure of H3K4me3 in budding yeast. PLoS Genet. 2010;6(2):e1000837. doi: 10.1371/journal.pgen.1000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringrose L, Paro R. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development. 2007;134(2):223–232. doi: 10.1242/dev.02723. [DOI] [PubMed] [Google Scholar]
- 15.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328(5982):1161–1164. doi: 10.1126/science.1186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobert O. Maintaining a memory by transcriptional autoregulation. Curr Biol. 2011;21(4):R146–147. doi: 10.1016/j.cub.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Peter IS, Davidson EH. Handbook of Systems Biology: Concepts and Insights. San Diego: Academic; 2013. Transcriptional network logic: The systems biology of development; pp. 211–228. [Google Scholar]
- 18.Holmberg J, Perlmann T. Maintaining differentiated cellular identity. Nat Rev Genet. 2012;13:429–439. doi: 10.1038/nrg3209. [DOI] [PubMed] [Google Scholar]
- 19.Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248–1256. doi: 10.1016/j.cell.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moazed D. Small RNAs in transcriptional gene silencing and genome defense. Nature. 2009;457(7228):413–420. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ptashne M. On the use of the word ‘epigenetic’. Curr Biol. 2007;17(7):R233–R236. doi: 10.1016/j.cub.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Ptashne M. Faddish stuff: epigenetics and the inheritance of acquired characteristics. FASEB J. 2013;27(1):1–2. doi: 10.1096/fj.13-0101ufm. [DOI] [PubMed] [Google Scholar]
- 24.Rando OJ. Combinatorial complexity in chromatin structure and function: revisiting the histone code. Curr Opin Genet Dev. 2012;22(2):148–155. doi: 10.1016/j.gde.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graur D, et al. On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biol Evol. 2013 doi: 10.1093/gbe/evt028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddy SR. The ENCODE project: missteps overshadowing a success. Curr Biol. 2013 doi: 10.1016/j.cub.2013.03.023. in press. [DOI] [PubMed] [Google Scholar]
- 27.Doolittle WF. Is junk DNA bunk? A critique of ENCODE. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1221376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waddington CH. The Epigenetics of Birds. Cambridge, UK: Cambridge University Press; 1952. [Google Scholar]