Abstract
Since the discovery of neural stem cells in the mammalian brain, there has been significant interest in understanding their contribution to tissue homeostasis at both the cellular and molecular level. Wnt/β-catenin signaling is crucial for development of the central nervous system and has been implicated in stem cell maintenance in multiple tissues. Based on this, we hypothesized that the Wnt pathway likely controls neural stem cell maintenance and differentiation along the entire developmental continuum. To test this, we performed lineage tracing experiments using the recently developed tamoxifen-inducible Cre at Axin2 mouse strain to follow the developmental fate of Wnt/β-catenin–responsive cells in both the embryonic and postnatal mouse brain. From as early as embryonic day 8.5 onwards, Axin2+ cells can give rise to spatially and functionally restricted populations of adult neural stem cells in the subventricular zone. Similarly, progeny from Axin2+ cells labeled from E12.5 contribute to both the subventricular zone and the dentate gyrus of the hippocampus. Labeling in the postnatal brain, in turn, demonstrates the persistence of long-lived, Wnt/β-catenin–responsive stem cells in both of these sites. These results demonstrate the continued importance of Wnt/β-catenin signaling for neural stem and progenitor cell formation and function throughout developmental time.
Keywords: radial glia cell, astrocyte, forebrain
In the embryonic brain, radial glia cells (RGCs) function as the earliest stem cells committed to the neural lineage, as demonstrated by their capacity to generate all neuronal and glial lineages of the adult central nervous system (1, 2). These cells first arise around embryonic day 10.5 (E10.5), when cells that make up the single layered neuroepithelium begin to take on the typical morphological characteristics of RGCs, including a tissue-spanning radial fiber, a cell body that remains in the ventricular zone, and the expression of glial-lineage markers, among others. Forebrain RGCs divide to generate neuron-committed progenitor cells starting at E11.5. These progenitors can differentiate directly into neurons or may undergo additional rounds of amplifying divisions before exiting the cell cycle. As development progresses, RGCs begin to produce glial-restricted progeny. Whereas neurogenesis is virtually complete at birth, gliogenesis continues through the end of gestation and well into postnatal life (1–3).
Most RGCs terminally differentiate into mature astrocytes (4) but some are thought to persist in adulthood as specialized neural stem cells (NSCs). Similar to RGCs in development, the adult NSCs are most abundant in the subventricular zone (SVZ) of the lateral ventricle and display a radial morphology and express glial markers (1, 2, 5). Similar cells are also found in a specialized subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) (3, 6). Although glia are continuously generated throughout the brain, the SVZ and SGZ are unique in their ability to generate adult-born neurons throughout life.
The stem cells of the SVZ divide to produce transient amplifying progenitors, which in turn give rise to migratory neuroblasts (4, 7), forming chains of cells that leave the ventricle and migrate long distances in the rostral migratory stream (RMS) to the olfactory bulb (8, 9). Multiple signaling pathways, including Notch, Hedgehog, and Wnt, control distinct aspects of NSC behavior (4, 10–12), but little is known about the lineal relationship between developmental and adult neural stem cells or the signaling mechanisms that are involved in stem cell maintenance during the transition from development to adulthood.
Wnt signaling is instrumental in the maintenance and differentiation of many developmental and adult stem cells, including those of the intestinal epithelium and hair follicle (13, 14). In the central nervous system, Wnt/β-catenin signaling is critical for proper patterning during development (15) and for instructing cell fate choices both embryonically and postnatally (12, 16, 17). To address the role of the Wnt/β-catenin in neural stem and progenitor cell development and function in vivo, we performed lineage tracing studies with the recently generated tamoxifen-inducible Cre at Axin2 (Axin2CreERT2) mouse strain (18).
Results
Lineage Tracing of Wnt Responding Cells.
To track the developmental fate of Wnt-responsive cell populations in the developing central nervous system, we used a lineage tracing mouse, Axin2CreERT2, which expresses a tamoxifen-inducible Cre protein from the endogenous Axin2 locus (18). When crossed to the Rosa26 membrane tomato/membrane green fluorescent protein (Rosa26-mT/mG; R26RmTmG) reporter strain, tamoxifen-induced Axin2CreERT2-expressing cells express membrane-bound green fluorescent protein (GFP) (19). To examine Wnt/β-catenin responsiveness during early neuroectodermal development, we administered tamoxifen to pregnant females bearing Axin2CreERT2/+; R26RmTmG/+ embryos at gestational day E6.5. One day later (E7.5), embryos were examined for the presence of GFP+ cells. In addition to GFP+ cells in the mesoderm (Fig. S1A), we could detect GFP+ cells in the ectoderm, the germ layer from which the nervous system will ultimately arise (Fig. 1A).
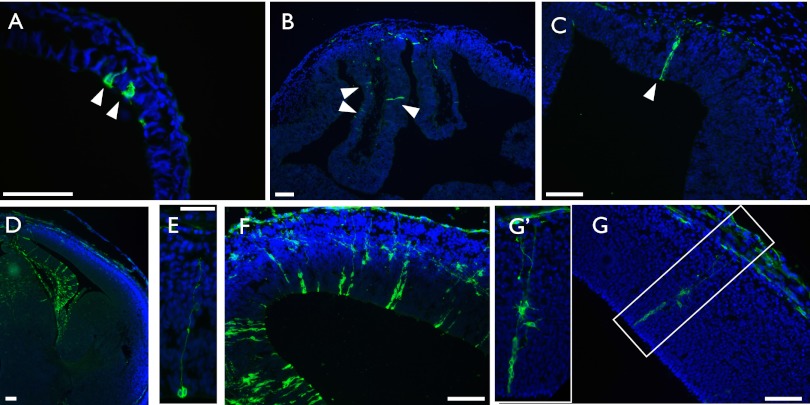
Fig. 1.
Axin2CreERT2 marks cells of the neural lineage at multiple developmental time points. Pregnant females carrying Axin2CreERT2/+; R26RmTmG/+ embryos were injected with tamoxifen at various developmental time points and embryos examined 24–48 h later. (A) E6.5–E7.5; (B and C) E8.5–E10.5; (D and E) E12.5–E13.5; and (F and G) E12.5–E14.5. (A) DAPI-stained tissue section of an Axin2CreERT2/+; R26RmTmG/+ embryo at E7.5, 24 h after administration of tamoxifen shows rare GFP+ cells in the ectoderm. (B) An E8.5–E10.5 trace shows GFP+ cells in the pallium and midline structures (C), which are beginning to take on elongated radial glial cell morphology. (D) In an E12.5–E13.5 traced forebrain, many GFP+ cells are present in the cortical hem and septum, and fewer in the pallium, and none in the ganglionic eminences. (E) Pallial GFP+ cell with a long radial fiber undergoing cell division at the ventricular surface, traced E12.5–E13.5. (F) After an E12.5–E14.5 trace, there is an increase in the total number of GFP+ cells in a given clone compared with E12.5–E13.5, although overall regional distributions remain the same. (G) E12.5–E14.5, a single clone of GFP+ cells containing at least three radial glial cells and multiple differentiated progeny. Boxed region is magnified in the Inset (G′). (A) Transverse section, (B–G) coronal section. DAPI staining is shown in blue, GFP staining is shown in green. (Scale bar, 50 μm, except in E and Inset in G, where it represents 25 μm.)
As development proceeds, the brain is first composed of a single layer of neuroepithelial cells, which begin to take on RGC characteristics around E10.5. When tamoxifen was administered at E8.5, when the neuroepithelium is fully specified, and embryos were examined at E10.5, we could detect GFP+ cells in the pallium and midline structures of the forebrain (Fig. 1B). As expected, these cells were beginning to elongate and take on the morphology of RGCs (Fig. 1C). Thus, Axin2CreERT2 marks both early ectodermal and neuroepithelial cells, the embryonic precursors that will ultimately generate the brain.
Next, we administered tamoxifen at E12.5, a period of active neurogenesis, and examined the embryos 24 h later. In the forebrain, sporadic GFP+ RGCs could be found in the pallium (Fig. 1D). Following this short trace, the majority of GFP+ cells were RGCs (84.2 ± 4%). Occasionally, we observed pallial GFP+ cells with long radial fibers that were undergoing division at the ventricular surface, a characteristic of RGCs (Fig. 1E). Many GFP+ cells were also detected in the cortical hem and ventral midline structures of the developing septal area and thalamus, the former of which is critical for proper patterning and development of the hippocampus (Fig. 1D), in agreement with the known role of Wnt/β-catenin signaling in hippocampal development (20–22). Upon tracing cells for 48 rather than 24 h (Fig. 1F), we found an increase in the total number of GFP+ cells per clone (3.6 ± 0.3 cells vs. 1.7 ± 0.1 cells, Student’s t test, P < 0.0001), as well as discrete pallial clones containing multiple RGCs and differentiated progeny (Fig. 1 F and G). Of note, we did not find labeling in the lateral or medial ganglionic eminences from which the basal ganglia arise (Fig. 1D), suggesting that Wnt/β-catenin responsiveness could be differentially localized or temporally regulated in specific neurogenic zones. These results are consistent with other reports, which demonstrate a restriction of Wnt signaling to the dorsal forebrain (23, 24), but are in contrast to findings of Gulacsi and Anderson (25) using a transgenic Wnt reporter, a difference likely inherent to the various reporter lines (26).
Embryonic Wnt-Responsive Cells Give Rise to Spatially Restricted Adult SVZ Stem Cells.
Most RGCs are lost around birth in differentiative divisions (4). However, retrovirus-based labeling studies demonstrated that some early postnatal RGCs can give rise to the adult NSCs of the lateral ventricle (27, 28). Because of these observations, we wondered if Wnt-responsive cells in the ventricular zone of the embryo were lineally related to adult SVZ NSCs. To test this, Axin2CreERT2/+; R26RmTmG/+ mice were exposed to tamoxifen in utero at embryonic day 8.5 (E8.5) or E12.5 and pups were allowed to develop until postnatal day 21 (P21) or adulthood (≥8 wk).
Progeny from Wnt-responding cells indelibly marked at E8.5 were present in the adult SVZ (Fig. 2A), and GFP+ cells with migratory morphology were also observed in the RMS (Fig. 2B), as well as in the olfactory bulb, with morphologies consistent with olfactory bulb neurons (Fig. 2C). Although rare, there were also GFP+ subgranular and granular cells in the dentate gyrus of the hippocampus, when mice were traced from E8.5 (Fig. S1B).
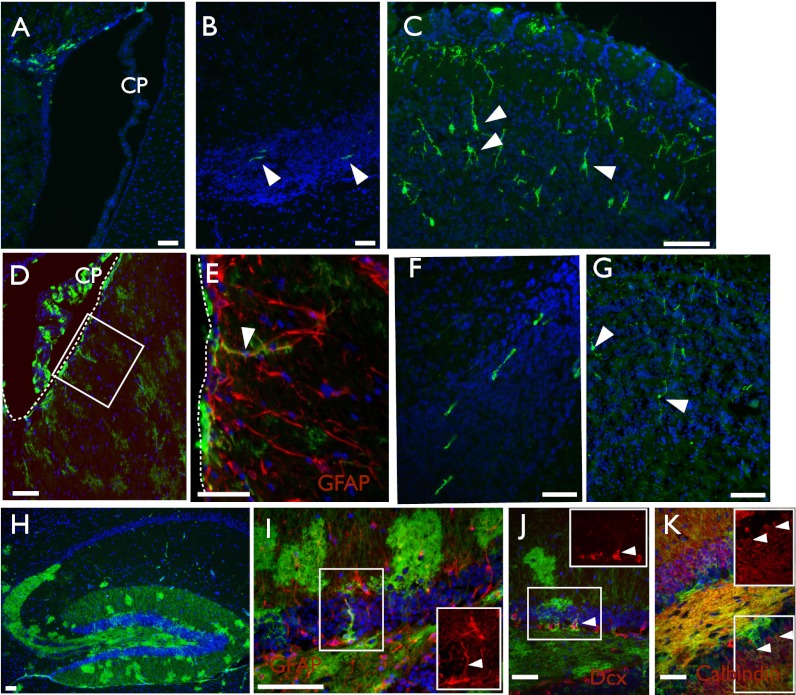
Fig. 2.
Descendants of embryonically labeled Axin2CreERT2 cells become functional adult SVZ and DG stem cells. Representative images of E8.5 or E12.5 tamoxifen-induced Axin2CreERT2/+; R26RmTmG/+ embryos. (A–C) E8.5–P21; (D–K) E12.5–P56. (A, D, and E) Lateral ventricle; (B and F) rostral migratory stream; (C and G) olfactory bulb; and (H and K) hippocampus. (A) E8.5-labeled Axin2+ RGCs produce GFP+ progeny with morphologies consistent with ependymal and subependymal cells, (B) migratory neuroblasts, and (C) olfactory neurons. (D) Progeny of Axin2+ cells labeled at E12.5 also generate GFP+ subependymal cells, some of which also stain for GFAP (red, E). (F) GFP+ neuroblasts and (G) olfactory neurons are also present. (H) Progeny of Axin2+ cells traced from E12.5–P56 make significant contributions to multiple structures in the hippocampus. (I) Some Axin2+ descendants are radial cells that coexpress GFAP; Inset shows GFAP only. (J) Clusters of GFP+ Dcx+ cells are also present (Inset shows Dcx only), as well as many GFP+ calbindin+ neurons (Inset shows calbindin only) (K). (Scale bar, 50 μm.) CP, choroid plexus. Boxed region in D is magnified in E. Arrows point to cells of interest and double positive cells; dashed lines in D and E mark the edge of the ventricle. DAPI staining is shown in blue, GFP staining in green, and all other markers in red.
Progeny from Axin2CreERT2+ RGCs labeled at E12.5 were also abundant in the adult SVZ. From these later traces, we observed subependymal GFP+ cells underlying the lateral ventricle with long parenchymal processes, the typical morphology of adult NSCs (Fig. 2D). A subset of these GFP+ cells also expressed GFAP, a marker of adult NSCs (Fig. 2E). As further demonstration of their maintained function as NSCs, we could observe GFP+ neuroblasts within the RMS (Fig. 2F), as well as differentiated neurons within the olfactory bulb (Fig. 2G).
Other areas of the brain also contained GFP+ progeny, including an abundant population of cells with mature astrocyte-like arborization domains within the parenchyma and various populations in multiple aspects of the hippocampus (Fig. 2H). Within the dentate gyrus of the hippocampus, the other neurogenic zone of the adult, we could detect GFP+ GFAP+ radial-like cells (Fig. 2I), GFP+ Dcx+ immature precursor cells (Fig. 2J), and granule layer neurons (Fig. 2K).
Interestingly, similarly to the Axin2CreERT2+ RGCs themselves, the adult SVZ NSCs derived from them were spatially restricted, occupying only distinct regions around the lateral ventricle. When RGC progeny were traced from E12.5 into adulthood, we only rarely detected GFP+ cells along the dorsal cortical wall (Fig. 3 A and B), whereas GFP+ cells were abundant along the dorsal medial wall (Fig. 3A). In contrast, we failed to detect any labeled cells on the lateral wall or ventral portion of the subventricular zone (Fig. 3A). This observation is in agreement with the fact that the latter two regions are derived from the embryonic ganglionic eminences (29), where Wnt/β-catenin responsive RGCs were absent (Fig. 1D).
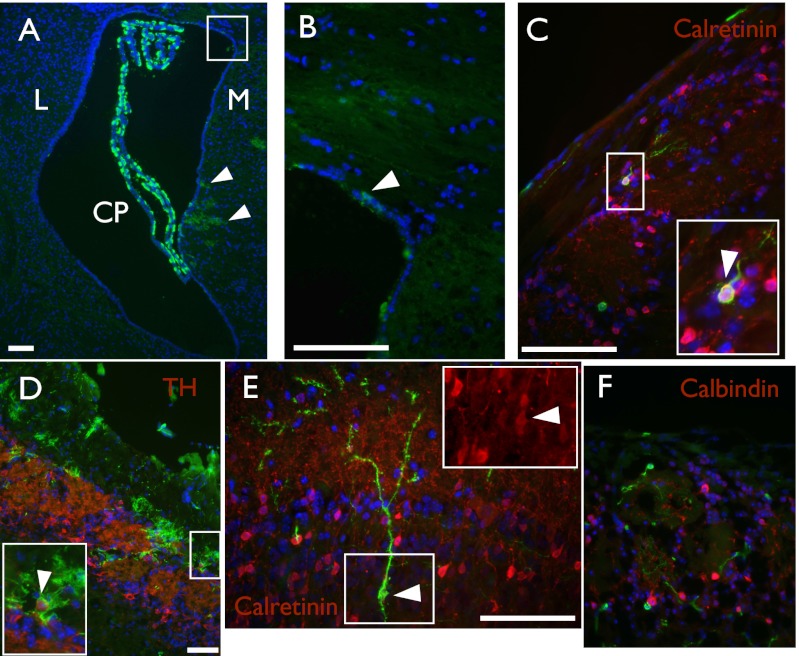
Fig. 3.
Descendants of embryonically labeled Axin2CreERT2 cells are regionally and developmentally restricted adult SVZ stem cells. Coronal sections of Axin2CreERT2/+; R26RmTmG/+ embryos traced from E12.5–P56. (A and B) Lateral ventricle; (C–F) olfactory bulb. (A and B) E12.5–P56 traces reveal that the progeny of Axin2+ cells are regionalized postnatally and are only found on the medial and dorsal walls (Inset) of the lateral ventricle. (C–F) In the olfactory bulb, adult SVZ descendants of E12.5 Axin2+ cells generate (C and E) calretinin+ GFP+, and (D) tyrosine hydroxylase+ GFP+ olfactory neurons, but not (F) calbindin+ GFP+ olfactory neurons. CP, choroid plexus; L, lateral; M, medial. Boxed region in A is magnified in B; Insets in C–E magnify double positive cells. Arrowheads point to cells of interest. GFP is shown in green, DAPI is shown in blue, and all other markers are shown in red. (Scale bar, 50 μm.)
It was recently described that the SVZ NSCs of the adult mouse brain are regionally specified with regards to their progeny (29, 30). To determine if adult NSCs derived from embryonically labeled Axin2CreERT2+ RGCs are similarly restricted, we examined the olfactory bulbs of these embryonically labeled mice. Similar to previous findings, we could detect large numbers of GFP+ granule cells (GCs) and calretininR+, periglomerlar cells (CalR+ PGCs) (Fig. 3 C and E), and a more limited overlap between GFP and tyrosine hydroxylase+ PGCs (Fig. 3D). Conversely, we could not detect GFP+ calbindin+ PGCs (Fig. 3F), which are largely generated from the lateral and ventral walls, in agreement with the NSC labeling pattern around the ventricle. Whereas these regional NSC restrictions have been broadly described (29, 30), a role for Wnt signaling in specification of these compartments has not been recognized.
Postnatal SVZ Stem Cells Are Persistently Wnt/β-Catenin Responsive.
Static Wnt/β-catenin reporters have demonstrated Wnt-pathway activity in the SVZ, but have not allowed an analysis of the long-term fate or differentiation capacity of these cells in vivo (31, 32). To address whether postnatal SVZ NSCs are Wnt/β-catenin responsive, we induced labeling of Axin2CreERT2/+; R26RmTmG/+ mice in adolescence (P14–16) or adulthood (≥8 wk). Initial labeling analysis 2 d posttamoxifen showed rare GFP+ GFAP+ (red) cells around the lateral ventricle (Fig. 4A). Some ependymal cells were also labeled (Fig. 4A, Inset). GFP+ RMS neuroblasts (Fig. 4B) or olfactory bulb neurons (Fig. 4C) were not detected, suggesting that Wnt/β-catenin pathway is not sufficiently activated in neuroblasts and differentiated neurons to evoke Axin2CreERT2 expression and GFP labeling. The absence of labeling in postmitotic cells did not appear to be due to inefficient recombination of the reporter allele, as we were able to detect GFP+ cells in the vasculature in both locations (Fig. 4C).
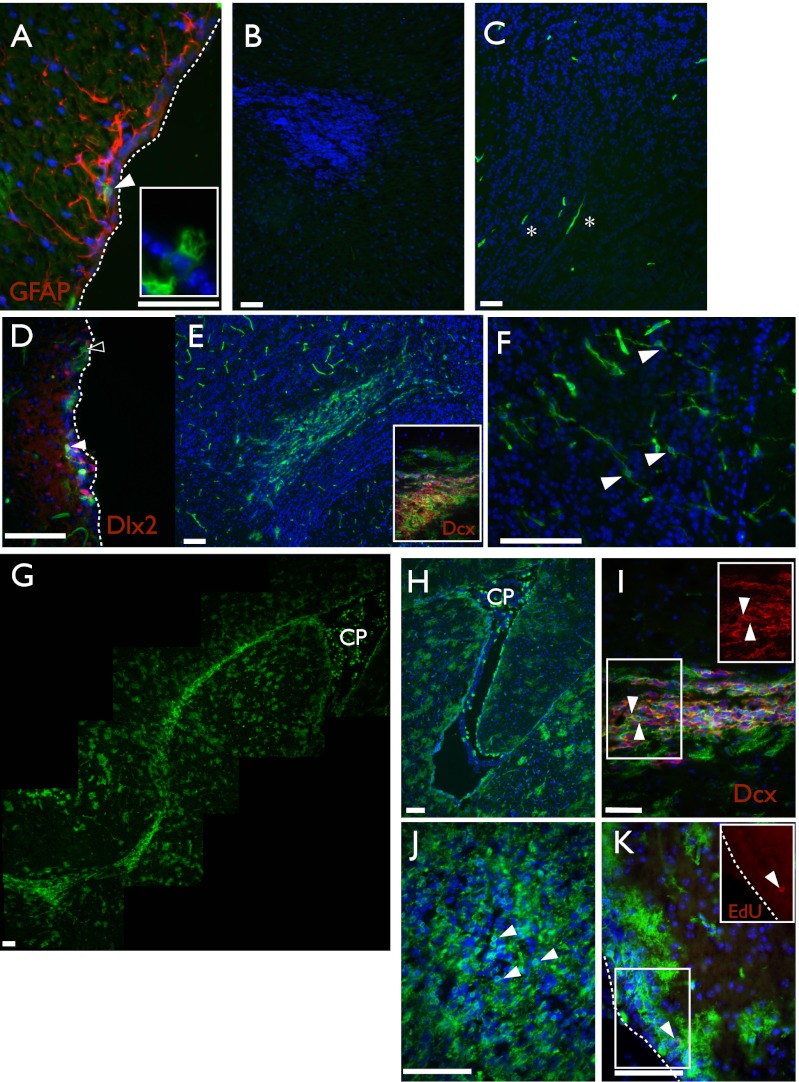
Fig. 4.
Adult SVZ neural stem cells are Wnt/β-catenin responsive. Representative images of brain sections from Axin2CreERT2/+; R26RmTmG/+ traced postnatally for various lengths of time. (A, D, H, and K) lateral ventricle; (B, E, and I) rostral migratory stream; (C, F, and J) olfactory bulb; and (G) photomontage of a sagittal section. (A) P14–P16 trace, rare subependymal GFP+ GFAP+ cells are present, as are some GFP+ ependymal cells (Inset). (B and C) No initial labeling is detected in the rostral migratory stream or olfactory bulb, apart from vasculature (asterisks). (D) P14–P24, 10 d after tamoxifen, increasing numbers of GFP+ cells can be found around the ventricle. Some GFP+ cells are Dlx2+ (closed arrowheads) and some are Dlx2− (open arrowhead). (E) P14–P24, many GFP+ cells are detected in the rostral migratory stream, and many coexpress doublecortin (Inset). (F) P14–P24, GFP+ cells are found in the olfactory bulb. (G) P14–P81, many GFP+ cells can be found remaining at the ventricle and populating the entire rostral migratory stream. (H) P14–P379, 1 y after initial labeling, GFP+ cells are retained at the ventricle, (I) are present in the rostral migratory stream, and (J) fill the olfactory bulb. (K) P14–P65, rare GFP+ EdU+ subependymal cells can be found 30 d after EdU administration (Inset shows EdU). CP, choroid plexus. Asterisks in C mark vasculature; closed arrowheads point to cells of interest and double positive cells; open arrowhead in D marks a Dlx− GFP+ cell; dashed lines in A, D, and K mark the edge of the ventricle. In all cases DAPI is shown in blue, GFP is shown in green, and all other markers in red. (Scale bar, 50 μm.)
Ten days after tamoxifen injection, the number of labeled cells at the ventricle was increased and some cells coexpressed distal-like homeobox (Dlx2), a marker of transient amplifying neuroblasts (Fig. 4D, closed arrowhead). Interestingly, some GFP+ cells that remained in contact with the ventricle were Dlx2−, suggesting that they represent a less differentiated NSC population (Fig. 4D, open arrowhead). Furthermore, in contrast to the analysis performed after 2 d, after 10 d of tracing we detected doublecortin positive GFP+ migrating neuroblasts in the RMS (Fig. 4E). GFP+ olfactory neurons were also detected, whereas previously there had been none (compare Fig. 4 B and C with E and F). To ascertain whether this initially labeled subependymal cell was a transitory progenitor or a long-term stem cell, we increased the trace length to 8 wk. Previous studies using retroviral labeling have demonstrated that the transit time from an initial stem cell division to a differentiated neuron is conservatively 10–14 d (33). Therefore, a 2-mo trace, which is in vast excess of this time, should distinguish between short-lived progenitors and longer-lived NSC populations. Upon examining these long traces, we detected the continued presence of GFP+ cells at the ventricle, and the RMS remained populated with abundant GFP+ migrating neuroblasts (Fig. 4G). Astrocyte-like cells that display a typical cobblestone distribution are also abundant in the surrounding tissues (Fig. 4G). Longer traces of up to 1 y confirmed the longevity of the labeled subependymal population and demonstrated its potent neurogenic capacity, as revealed by the continuous presence of GFP+ neuroblasts in the RMS, and many GFP+ olfactory bulb neurons (Fig. 4 H–J). In addition, Wnt/β-catenin–responsive cells in the SVZ were label retaining, a suggested characteristic of many long-term stem cell populations (34–36). GFP+ 5-ethynyl-2′-deoxyuridine (EdU+) cells were present 4 wk after the administration of tamoxifen and the DNA label (Fig. 4K). Taken together, these results demonstrate the presence of a long-lived Wnt-responsive adult NSC in the SVZ of the lateral ventricle, which gives rise to differentiated neuronal progeny.
In contrast to embryonically initiated labeling, Axin2CreERT2+ NSCs labeled postnatally lined all walls of the lateral ventricle and gave rise to all major subtypes of neurons in the olfactory bulb, suggesting that most, if not all, NSCs in the postnatal SVZ are Wnt/β-catenin responsive (Fig. S2).
Axin2CreERT2 Labels Hippocampal Stem Cells and Multiple Adult Radial Glial Cell Populations.
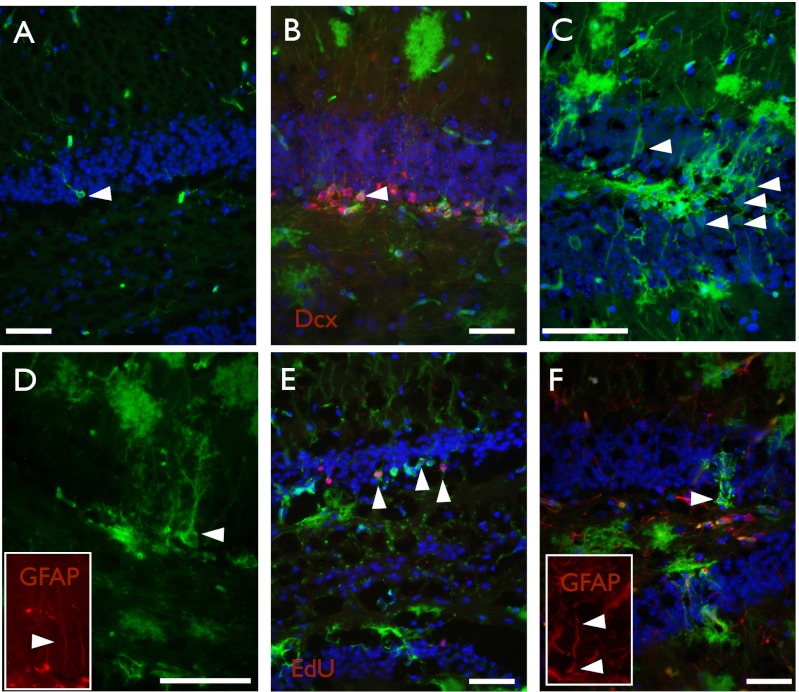
In addition to the SVZ, stem cells also persist postnatally in the DG of the hippocampus. SVZ and DG stem cell populations share numerous characteristics; most notably, both are astrocytic cells that retain a radial morphology. In addition, they express similar markers, including GFAP (5–7). Given our finding that adult NSCs in the SVZ are Wnt/β-catenin responsive and that Wnt signaling has been previously demonstrated to control proliferation and neuronal commitment of adult hippocampal progenitors (12), we wondered whether DG stem cells might also be controlled by Wnt/β-catenin signaling. When we administered tamoxifen to postnatal Axin2CreERT2/+; R26RmTmG/+ mice, we detected Axin2CreERT2+ cells in the DG of the hippocampus. Short trace experiments (2 d) revealed only rare subgranular zone cells to be Wnt/β-catenin responsive (Fig. 5A). A 2-mo-long trace revealed that these GFP+ cells had given rise to Dcx+ progenitors as well as differentiated neurons (Fig. 5 B and C). Additionally, some of GFP+ cells present at this 2-mo time point coexpressed GFAP (Fig. 5D), which labels DG stem cells and mature astrocytes. We were also able to detect GFP+ EdU label retaining cells in the SGZ (Fig. 5E). Furthermore, GFAP+ GFP+ cells remained present 1 y after tamoxifen-mediated recombination (Fig. 5F). Together with the observation that during the course of this time many GFP+ differentiated neurons were born, these studies argue that at least a subset of hippocampal stem cells in the postnatal mouse brain are Wnt/β-catenin responsive.
Fig. 5.
Axin2CreERT2 labels stem cells of the dentate gyrus. Representative images of brain sections from Axin2CreERT2/+; R26RmTmG/+ traced postnatally for various lengths of time. (A) P14–16, 2 d after tamoxifen, rare GFP+ cells are found in the subgranular zone, but no neurons are labeled. (B) P14–P81, GFP+ Dcx+ cells are present, as well as differentiated neurons (D) and subgranular cells that are GFP+ GFAP+ (E) (Inset shows GFAP staining). (F) P14–P65, GFP+ EdU+ cells can be found in the subgranular zone 30 d after EdU administration. (G) P14–P379, 1 y after tamoxifen, GFP+ GFAP+ cells are still present (Inset shows GFAP staining). DAPI is shown in blue, GFP is shown in green, and all other markers are shown in red. Arrowheads point to cells of interest and double positive cells. (Scale bar, 50 μm.)
Intriguingly, we noticed that other radial glial populations in the central nervous system—the Bergmann glia of the cerebellum and the Müller glia of the neural retina—were also Axin2CreERT2 positive at multiple developmental time points (Fig. S3), suggesting a conserved role for Wnt/β-catenin signaling in specific radial glia populations from many regions of the developing CNS.
Discussion
Our results show that in the developing embryo early ectodermal, neuroepithelial, and radial glial cells are Wnt/β-catenin responsive, demonstrating that at all major developmental stages, a subset of stem and progenitor-like cells of the central nervous system are responding to Wnt/β-catenin signaling. Although most embryonic precursors are not maintained into postnatal life, we find that Wnt-responsive embryonic neuroepithelial cells, as well as RGCs, are able to give rise to adult NSCs. As early as E8.5 (the earliest time we traced to postnatal times), Axin2CreERT2 labels cells that eventually convert into adult NSCs in the ventricular wall, and these cells persist throughout adult life and continue to produce new olfactory bulb neurons. To our knowledge, this is the earliest reported inducible cre-mediated labeling which can still produce functional adult SVZ stem cells. In the subgranular zone of the DG, labeling from E8.5 to adulthood is very sparse, making definitive interpretation difficult (Fig. S1B), but robust labeling of adult NSCs in the DG can be seen when tamoxifen is administered from E12.5 onwards (Fig. 2 H–K). This is consistent with the unique establishment of a Wnt-dependent secondary germinal zone that occurs late in development and ultimately becomes the SGZ of the adult hippocampus (37).
Previously, studies with glioblastoma oncogene (GliCreERT2), which reports Hedgehog (Hh) signaling, have also addressed the potential of embryonic RGCs to contribute to NSCs in the adult brain. Interestingly, embryonically labeled GliCreERT2 cells could only be detected in the adult SVZ when tamoxifen was administered from E15.5 onwards. For the SGZ of the DG, embryonically labeled GliCreERT2 cells could not contribute earlier than E17.5 (27). Thus, Axin2CreERT2 labels stem and progenitor pools at an earlier stage in development. As such, Axin2CreERT2 might be a more powerful tool for assessing the developmental potential of cells during earlier neural development than previously described Cre lines.
Interestingly, we determined that adult SVZ NSCs derived from an embryonically labeled Axin2CreERT2+ precursor are regionally and functionally restricted; RGCs labeled in utero only populate the medial and dorsal wall of the lateral ventricle and give rise to populations of olfactory bulb progeny that are similarly derived from medial but not lateral ventricular domains in the adult. The domains are derived from the dorsal telencephalon, a region where we find Axin2CreERT2-mediated recombination during embryogenesis. These results are in agreement with previously published reports suggesting that canonical Wnt signaling is restricted to the dorsal telencephalon (23, 24). On the other hand, Gulacsi and Anderson (25) have shown that other transgenic Wnt reporters have wider expression domains than Axin2, and have suggested a different role for Wnt signaling during midneurogenesis based on regional β-catenin elimination.
That these particular domains are Wnt responsive is intriguing, as they are found in a complimentary pattern to those marked by Hh responsiveness using GliCreERT2. Embryonic labeling in GliCreERT2 mice, marks a ventrally restricted population of SVZ NSCs (38). Together, these results suggest that both spatially and temporally restricted Wnt and Hh morphogen gradients function to pattern the embryonic and adult brain. The fact that these two signaling activities appear to be largely nonoverlapping suggests a distinct anatomical and/or temporal origin for different populations of adult NSCs.
Of note, our studies in the adult mouse brain argue that long-lived NSCs in the SVZ and DG retain Wnt/β-catenin responsiveness over development. In addition, the fact that NSCs along all walls of the ventricle are Axin2CreERT2+ in the adult suggests that the NSCs derived from a Gli+ Axin2− precursor also become dependent on Wnt signaling to maintain their self-renewal in the adult.
Materials and Methods
Animals.
Axin2CreERT2 and R262RmT/mG mice have been described previously (18, 19). All animal protocols were approved by the Animal Care and Use Committee of Stanford University School of Medicine.
Tamoxifen and EdU Injections.
Unless otherwise noted, tamoxifen (Sigma) was administered intraperitoneally at 4 mg/25 g body weight for adult mice or 0.5 mg/25 g dam body weight for embryonic studies. EdU was administered intraperitoneally at 1 mg/25 g body weight. Label-retaining studies were modified from those previously described (39). Briefly, beginning at P28, littermates were given 1 mg/mL EdU for 7 consecutive days. One day after the final dose, mice were euthanized and examined for initial label incorporation. Thirty days later, the remaining mice were examined for the presence of EdU+ cells in neurogenic regions. For all labeling studies at least three separate experiments were conducted for each time point.
Tissue Processing and Immunohistochemistry.
Brains were fixed overnight in 4% (wt/vol) paraformaldehyde at 4 °C, cryoprotected 24 h in 30% (wt/vol) sucrose at 4 °C, then equilibrated in 2:1 optimal cutting temperature (TissueTek):30% sucrose for 1 h at 4 °C. For X-gal staining, fixation was limited to 1 h at 4 °C in 4% paraformaldehyde. Antibodies used: chicken anti-GFP (Abcam; 1:1,000–1:2,000), mouse anti-GFAP (Millipore; 1:1,000), rabbit antityrosine hydroxylase (1:1,000; Pel-Freez), rabbit anticalbindin (1:300; Swant), goat anticalretinin (1:100; Millipore), guinea pig anti-Dcx (1:1,000; Millipore), and rabbit anti-Dlx2 (1:200; Millipore).
Supplementary Material
Acknowledgments
We thank members of our laboratories for valuable input and discussions. A.N.B. was supported by a National Science Foundation graduate research fellowship and a fellowship from the California Institute for Regenerative Medicine. R.v.A. was funded by a European Molecular Biology Organization long-term fellowship (ALTF 122-2007) and a Koningin Wilhelmina Fonds (KWF) fellowship from the Dutch Cancer Society. Additional funding came from the California Institute for Regenerative Medicine (Grant TR1-01249). R.N. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305411110/-/DCSupplemental.
References
- 1.Noctor SC, et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22(8):3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41(6):881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 3.Mori T, Buffo A, Götz M. The novel roles of glial cells revisited: The contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 4.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 6.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lois C, García-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 9.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo P-M. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6(5):507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 11.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 12.Lie D-C, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Michaelidis TM, Lie DC. Wnt signaling and neural stem cells: Caught in the Wnt web. Cell Tissue Res. 2008;331(1):193–210. doi: 10.1007/s00441-007-0476-5. [DOI] [PubMed] [Google Scholar]
- 16.Zechner D, et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258(2):406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 17.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 18.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11(3):387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 20.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32(4):591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 21.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125(12):2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 22.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129(9):2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 23.Backman M, et al. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279(1):155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn. 2005;233(2):528–539. doi: 10.1002/dvdy.20365. [DOI] [PubMed] [Google Scholar]
- 25.Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008;11(12):1383–1391. doi: 10.1038/nn.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barolo S. Transgenic Wnt/TCF pathway reporters: All you need is Lef? Oncogene. 2006;25(57):7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- 27.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 28.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101(50):17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27(31):8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama A, et al. GFP transgenic mice reveal active canonical Wnt signal in neonatal brain and in adult liver and spleen. Genesis. 2007;45(2):90–100. doi: 10.1002/dvg.20268. [DOI] [PubMed] [Google Scholar]
- 32.Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29(3):528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- 33.Craig CG, D’sa R, Morshead CM, Roach A, van der Kooy D. Migrational analysis of the constitutively proliferating subependyma population in adult mouse forebrain. Neuroscience. 1999;93(3):1197–1206. doi: 10.1016/s0306-4522(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 34.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 35.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25(3):333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 36.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127(3):457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 38.Ihrie RA, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71(2):250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renault VM, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.