Abstract
Rapid economic development in the past century has translated into severe pressures on species survival as a result of increasing land-use change, environmental pollution, and the spread of invasive alien species. However, though the impact of these pressures on biodiversity is substantial, it could be seriously underestimated if population declines of plants and animals lag behind contemporary environmental degradation. Here, we test for such a delay in impact by relating numbers of threatened species appearing on national red lists to historical and contemporary levels of socioeconomic pressures. Across 22 European countries, the proportions of vascular plants, bryophytes, mammals, reptiles, dragonflies, and grasshoppers facing medium-to-high extinction risks are more closely matched to indicators of socioeconomic pressures (i.e., human population density, per capita gross domestic product, and a measure of land use intensity) from the early or mid-, rather than the late, 20th century. We conclude that, irrespective of recent conservation actions, large-scale risks to biodiversity lag considerably behind contemporary levels of socioeconomic pressures. The negative impact of human activities on current biodiversity will not become fully realized until several decades into the future. Mitigating extinction risks might be an even greater challenge if temporal delays mean many threatened species might already be destined toward extinction.
Keywords: extinction debt, socioeconomic history, time lag
The progressive impact of environmental degradation on the loss of global biodiversity (1–4) is strongly linked to key socioeconomic indicators such as human population size (5), land use (6), and gross domestic product (GDP) (7, 8). However, species populations do not necessarily respond immediately to environmental degradation but might do so with a delay (9, 10). Such time-lags between environmental forcing, population decline, and, finally, extinction create a transient disequilibrium between environmental conditions and species’ abundance or range size, which has been conceptualized as “extinction debt” (9). Recent empirical research has shown that, at the scale of individual habitat patches, a delayed response of species to habitat loss and fragmentation is indeed often detectable, particularly among habitat specialists (9–11). The likelihood and magnitude of extinction debt is still contentious, however (12, 13), and seems to vary with the nature of environmental degradation and with the life history traits of the species concerned (14–18). In long-lived or less-mobile taxa (e.g., vascular plants, bryophytes, reptiles), a delayed response of populations to the deterioration and fragmentation of their habitats is especially likely and might extend over at least several decades.
If time-lags in population decline at the scale of individual habitats are a common phenomenon, the number of species facing extinction risks at larger spatial scales might easily be underestimated because many local populations of extant species might not survive in the long-term even if further environmental degradation is halted. Thus, red lists describing threatened species at either a national or global scale, which identify species extinction risks using criteria such as a reduction in population size, range size, and perceived rate of recent population or range decline (19), might be too optimistic. However, despite the recent increase in interest in the potential for temporal lags in population declines and extinction processes (8, 10, 12, 17), evidence for extinction debt has not yet been examined for a broad range of taxa at a spatial scale consistent with national or global decision-making. Given that many conservation policies in Europe (and elsewhere) are developed and implemented at regional to global scales (e.g., Ramsar Convention and Convention on Biological Diversity) and reported at the level of individual countries, assessing extinction debt at this scale would appear to be a crucial step.
Here, we provide such an assessment by analyzing national red lists (NRL) of threatened species of seven taxonomic groups (vascular plants, bryophytes, mammals, fish, reptiles, dragonflies, and grasshoppers) from 22 European countries (Tables S1 and S2) in relation to the magnitude of contemporary as well as historic drivers of environmental degradation. Red lists provide an objective framework for the classification of the broadest range of species according to their extinction risk based on the current trend in population size, geographic range, and area of occupancy (19). We focus on Europe because this is the only continent where NRLs have become recently available, and are regularly updated, for a range of taxonomic groups across more than 20 countries using standard approaches to the listing of threatened species. Our analysis is based on a rationale similar to the one used in Essl et al. (5) for evaluating delays in alien species invasions: if NRLs provide an accurate estimate of the number of species at risk under the current magnitude of human pressures on biodiversity, these numbers should best correlate with the spatial variation in contemporary levels of these drivers. If, by contrast, time-lags between different human pressures and subsequent species responses occur, the number of threatened species should better reflect historic rather than contemporary values of these drivers. We measure the values of these drivers by three indicators of human socioeconomic activities that are known proxies of environmental pressures on biodiversity (2, 7, 8, 20): human population density (PD), per capita GDP, and human appropriation of net primary production (HANPP) (21). We estimated HANPP as the ratio of the net primary productivity harvested by humans (NPPh) to the NPP of potential vegetation (NPP0). HANPP is therefore the hypothetical undisturbed vegetation cover of the harvested area and is a useful proxy for land use intensity.
We use generalized linear and mixed-effects models with a logistic link function (Materials and Methods) to relate these three indicators evaluated for both the early (1900–1910) and the mid-20th century (1950) as well as for the recent past (year 2000) to the proportions of national floras and faunas listed as threatened [International Union for Conservation of Nature (IUCN) categories endangered (EN), vulnerable (VU), and critically endangered (CR)] on NRLs and compare how the data support these three alternative models. We have chosen socioeconomic data from the early 20th century to represent pre-World War I conditions, whereas the situation in 1950 precedes the phase of rapid growth of the European economies after World War II.
Results
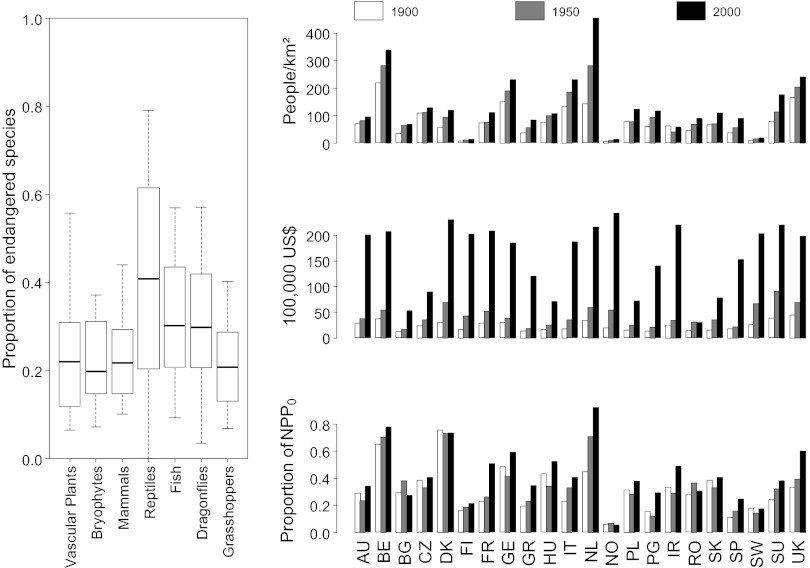
Averaged across the 22 countries, between ∼20% and 40% of the species examined are currently considered as facing medium-to-high extinction risks with reptiles, fish, and dragonflies figuring most prominently on NRLs (Fig. 1). The indicators of human socioeconomic activities have increased considerably during the 20th century, but at different rates in individual countries (Fig. 1). Consequently, the cross-national variation in the magnitude of these indicators is correlated across time, but with some scatter (Fig. S1).
Fig. 1.
Proportions of species facing medium-to-high extinction risks in NRLs and trends in socioeconomic indicators in Europe over the 20th century. (Left) Proportions of threatened species (IUCN categories EN, VU, CR) per taxonomic group across 22 European countries. (Right) Levels of three socioeconomic variables [human population density in people square kilometer; per capita GDP in 100,000 International Geary–Khamis dollars; and human appropriation of net primary productivity, which is defined here as the proportion of the total net primary productivity harvested by humans to the net primary productivity of the potential vegetation (NPP0)] for the years 1900, 1950, and 2000. See Table S1 for country codes.
Joint analysis across all seven taxonomic groups demonstrates that values of socioeconomic activity explain current red-listed species numbers better the longer they date back into the past (Tables 1 and 2). The difference is particularly pronounced between models using socioeconomic data from 1900 and those using data from the subsequent time points; this is true for multivariate models of the combined effect of all three indicators and for any single variable model comprising only one individual indicator.
Table 1.
Proportions of threatened species from seven taxonomic groups and 22 European countries as explained by multivariate models of historical or current socioeconomic indicators
| 1900 | 1950 | 2000 | |
| PC1 | −0.31 | −0.23 | −0.15 |
| PC2 | 0.13 | 0.04 | 0.06 |
| PC3 | −0.20 | −0.10 | −0.06 |
| R2MF | 0.22 | 0.09 | 0.04 |
| AIC | 4,830 | 5,637 | 5,943 |
| AW | >99.9 | <0.01 | <0.01 |
Fixed-effect estimates, McFadden’s R2, Akaike information criterion (AIC), and Akaike weights (AW) for generalized linear mixed-effects models relating the variation in the proportion of red-listed species to either historical (1900 and 1950) or contemporary (2000) values of impact indicators. The three impact indicators—human population density (PD), per capita GDP (GDP), and human appropriation of net primary productivity (HANPP)—were subjected to a principal component analysis before fitting the regression models to remove multicollinearity among independent variables (PC1–PC3). All fixed-effect estimates are different from 0 with a probability >0.99.
Table 2.
Proportions of threatened species from seven taxonomic groups and 22 European countries as explained by single variable models of historical or current socioeconomic indicators
| 1900 | 1950 | 2000 | 1900 | 1950 | 2000 | 1900 | 1950 | 2000 | |
| PD | 0.18 | 0.15 | 0.13 | ||||||
| GDP | 0.23 | 0.14 | 0.01 | ||||||
| HANPP | 0.35 | 0.23 | 0.16 | ||||||
| R2MF | 0.06 | 0.04 | 0.03 | 0.10 | 0.04 | 0.00 | 0.18 | 0.08 | 0.04 |
| AIC | 5,837 | 5,972 | 6,043 | 5,599 | 5,986 | 6,213 | 5,085 | 5,730 | 5,950 |
| AW | >99.9 | <0.01 | <0.01 | >99.9 | <0.01 | <0.01 | >99.9 | <0.01 | <0.01 |
Fixed-effect estimates, McFadden's R2, Akaike information criterion (AIC), and Akaike weights (AW) for generalized linear mixed-effects models relating the variation in the proportion of red-listed species to either historical (1900 and 1950) or contemporary (2000) values of impact indicators. All fixed-effect estimates are different from 0 with a probability >0.99.
The multivariate models of each individual taxonomic group demonstrate that the 1900 data best explain current proportions of threatened species of bryophytes, vascular plants, dragonflies, and grasshoppers (Fig. 2; Table 3). With respect to vertebrates, the results are less clear, with threatened fish being most closely correlated to contemporary indicator levels, whereas for mammals and reptiles, the models of 1900 and 1950 have similar support (Fig. 2).
Fig. 2.
Proportions of species facing medium-to-high extinction risks from seven taxonomic groups in 22 European countries (for data sources, see Table S2) as explained by current and historic socioeconomic models. (Left) Relative support (Akaike weights) for multiple logistic regressions models explaining the proportions of red-listed species by historical (1900, light gray; 1950, dark gray) or contemporary (2000, black) levels of socioeconomic impact indicators. The three indicators (human population density, per capita GDP, human appropriation of net primary productivity) were subjected to a principal component analysis before fitting the regression models to remove multicollinearity among explanatory variables. (Right) The deviance explained by simple logistic regression models relating the proportion of threatened species separately to either historical or contemporary levels of the three individual indicators: GDP, per capita GDP; LU, land use, i.e., human appropriation of net primary productivity; and PD, human population density.
Table 3.
Goodness of fit of models relating the proportion of threatened species from seven different taxonomic groups across 22 European countries to either historical or contemporary socioeconomic impact indicators
| AIC1900 | AIC1950 | AIC2000 | D21900 | D21950 | D22000 | |
| Vascular plants | 3,589 | 4,458 | 4,824 | 0.30 | 0.13 | 0.05 |
| Bryophytes | 828 | 861 | 873 | 0.14 | 0.10 | 0.09 |
| Mammals | 177 | 179 | 185 | 0.27 | 0.25 | 0.19 |
| Fish | 212 | 206 | 203 | 0.01 | 0.15 | 0.17 |
| Reptiles | 139 | 138 | 143 | 0.26 | 0.27 | 0.22 |
| Dragonflies | 171 | 177 | 193 | 0.26 | 0.22 | 0.10 |
| Grasshoppers | 103 | 111 | 111 | 0.51 | 0.39 | 0.40 |
Akaike Information Criterion (AIC), and Explained Deviance (D2) of multiple logistic regression models. The three indicators (human population density, per capita GDP, and human appropriation of net primary productivity) were subjected to a principal component analysis before fitting the regression models to remove multicollinearity among explanatory variables.
The environmental Kuznets curve hypothesis proposes that wealthier economies tend to reduce their impact on the environment by increasing expenditure toward environmental management and protection (20, 22). Because investment in such measures has played a much more important role toward the end of the 20th century than in the previous 100 y, its potential mitigating effects might bias correlations between indicators of socioeconomic activity and numbers of threatened species toward a historical explanation. To test for such a bias we repeated all analyses after adding country-specific values of recent environmental expenditures as an additional independent variable. This extension indeed improved model fit (compare Tables 1 and 2 and Table S3; Akaike weights >99.9 in favor of the models with the expenditures included); however, it did not change the relative ranks of the models from 1900, 1950, and 2000, except for fish, which appear most closely correlated to socioeconomic activity from 1950 rather than 2000, when expenditures are taken into account (Fig. S2).
Discussion
Even though human impacts on the environment greatly intensified following World War II (3, 6, 23, 24), our results suggest that the current threat status of many species reflects a legacy of several decades, and for four taxa even as much as a century. Such extended time-lags have indeed been observed in habitat-scale studies on vascular plants (12, 18) or cryptogams (25) for which historic indicators most markedly outperformed current indicators in explaining the proportion of threatened species in our country-scale study as well (Table 3). Contrary to common expectations (10, 26), however, the risks faced by taxonomic groups represented by short-lived species such as dragonflies and grasshoppers seem to reflect human impacts on the environment with a delay similar to that for plants. For a similar taxonomic group, butterflies, the available empirical evidence for delayed population response to habitat loss is mixed with some studies reporting rather fast (several years) (12, 27) and others relatively long (several decades or more) relaxation times (17). In addition, theoretical simulations have suggested that the delay in insect metapopulation extinctions can extend well beyond 100 y (28), and that their lag times are particularly long if available habitat networks are reduced to levels close to the extinction threshold (29), as might be the case in many landscapes of Europe where an intensively used agricultural matrix is interspersed by remnants of near-natural and nonintensively used habitats (30).
For the vertebrate taxa, time-lags between socioeconomic indicators and population decline appear to be less pronounced. In particular, fish were the only taxonomic group for which extinction risks appear more closely correlated to contemporary indicators. We do not know why fish behave differently, but it might be that anthropogenic impacts on freshwater ecosystems, such as water pollution, channelization, construction of dams, and water abstraction (31), have a more immediate effect because they not only reduce the quality and quantity of habitats, but directly and uniformly modify the medium in which species live. Additionally, historic legacies might be masked by the particularly intense but more recent modification of the aquatic environments (23). Mammals, however, might be particularly sensitive to habitat loss and fragmentation because they often need large contiguous habitats for survival (32) and can hence reach their extinction thresholds (29) during an earlier stage of habitat degradation and loss.
In conclusion, our results suggest that our perception of extinction risks at large geographical scales lags considerably behind increases in socioeconomic pressures on biodiversity across different taxonomic groups. With respect to the future, this long lag time implies that contemporary human pressures have already influenced the threat that biodiversity will face in the next decades, and this is underestimated by current NRLs. Indeed, the rapid recent increase in socioeconomic activity has triggered new, or at least greatly intensified, pressures on biodiversity arising from processes such as atmospheric nitrogen or acidic deposition (33), biological invasions (5, 34, 35), and climate change (2), which now reinforce and synergistically interact with habitat loss and fragmentation, the main drivers of population decline and extinction in the past. Although these novel pressures have rarely been studied in an extinction debt context (10), their effects on biodiversity might be characterized by similarly long lag times (5, 18). For example, both recent observations (36) and modeling studies (37) suggest that range adaptations of native species to changing climates considerably lag behind the velocity of climate change (38) and that remnant populations (39) currently occupy sites that are no longer climatically suitable for them in the long run (18).
Given this accumulating extinction debt from different sources, reducing further pressures on ecosystems might not be sufficient to reduce future biodiversity loss. In addition, long lag times might also blur cause–effect relationships, and if delayed responses by species to historic drivers (e.g., land use change) are confused with responses to more recent phenomena (e.g., climate change), mitigation measures might target the wrong drivers. Our results, combined with the evidence of both global (4) and European (40) failure to reach the 2010 biodiversity target, suggest current commitments to stop biodiversity loss in the region are even more inadequate than currently appreciated. From a global perspective, the extended time lags in extinction risk in Europe might also be a feature of other industrialized regions that have followed a similar development path—e.g., North America and Australia (6). Future measures to counter extinctions in these areas will probably require the mobilization of efforts well beyond current investment in conservation policies and importantly account for both new drivers of population declines as well as for those that have acted for over a century. Minimizing the magnitude of the “sixth extinction crisis” (41) might be an even greater challenge when temporal delays are taken into account.
Materials and Methods
Total and Red List Species Numbers.
We extracted total species numbers from national checklists, standard faunas, and floras, with some updates by national experts. Numbers of threatened species included on red lists were taken from the most recent NRLs, >90% of which have been published between 1995 and 2010 (Table S2). We have included only species that face medium-to-high extinction risks (IUCN categories EN, VU, and CR) (19), which are generally referred to as threatened species. We excluded species that had already gone extinct [extinct (EX) and extinct in the wild (EW)] to avoid bias in favor of an historical explanation. We used the recent NRLs in Europe that were developed following standardized criteria established for national to global red listing led by the IUCN since the 1990s (19). The five IUCN criteria for assessing extinction risks are based on current status and trends in population size, geographic range, and area of occupancy, and include quantitative analyses of extinction risks (e.g., population viability analysis).
Socioeconomic Indicators.
The scarcity of historical socioeconomic data limited our sample size to 22 countries (Table S1), but these are representative of the variation in European socioeconomic trajectories and include nations from both sides of the former Iron Curtain. Data on current and historical population densities and per capita GDP were taken from the Total Economy Database (www.ggdc.net/databases/ted.htm). We standardized current and historical per capita GDP to 1990 International (Geary–Khamis) dollars, a hypothetical currency unit with the same purchasing power that the US dollar had in the United States in 1990. In calculating net primary productivity harvested by humans NPPh, we used a broad definition of harvested NPP (21), which encompasses biomass extracted for further economic use and biomass destroyed during harvest.
Calculation of HANPP.
As a proxy for land use intensity we used an indicator that measures the human impact on trophic energy availability in ecosystems, thereby quantifying the human influence on certain important ecosystem processes (42, 43). We divided the total amount of harvested NPPh by the NPP of the potential vegetation, i.e., the vegetation assumed to exist in the absence of human land use (NPP0) to take biogeographic variation in natural productivity into account. The ratio NPPh/NPP0 is an indicator of land use intensity, and it is one variant of HANPP for which several empirical studies (44–46) support the hypothesis that it is a valid indicator of pressures on biodiversity (47). Different authors have used different definitions of HANPP. Vitousek et al. (42) proposed three definitions; the definition used here is similar, but somewhat less inclusive than Vitousek’s intermediate definition also adopted by Rojstaczer et al. (48) and Imhoff et al. (49). Vitousek’s intermediate definition includes the entire NPP of intensively managed ecosystems, whereas NPPh includes only plants actually harvested or destroyed during harvest.
We use an encompassing concept of biomass harvest that also includes biomass grazed by livestock and biomass destroyed during harvest, such as belowground biomass on cropland (21, 43). Data on the harvest of crops and timber are derived from statistical sources (50–54). Grazed biomass is estimated on the basis of feed balances using livestock and market feed data from the Food and Agriculture Organization and demand factors that considered species, region, and time (21, 55). Extraction of crop residues, harvest losses in forestry, and belowground biomass on cropland and harvested forest areas are estimated using region and time-dependent coefficients based on Krausmann et al. (56). NPP0 was calculated for 1910 and 2000 (Table S4) with the LPJmL model (57). Both NPPh and NPP0 are measured in terms of carbon content.
For a few countries, historic land-use data were only available for 1910. However, because land-use changes in Europe primarily occurred in the post-World War II period (6), we consider this approach to be justified.
Data Analysis.
National floras and faunas of European countries vary considerable in species richness due to differences in country area, climatic conditions, biogeographic setting, and other factors. We accounted for this uneven distribution of regional biodiversity by considering the numbers of species on NRLs as proportions of the countries’ total native floras and faunas (Table S2): our response variable was hence the average probability of a species from a country-specific species pool to be on a particular country’s red list in the early 21st century. For analyzing the data across all seven taxonomic groups, we related the proportions of threatened species with the three socioeconomic variables by means of generalized linear mixed-effects models (GLMMs) with a logit link function for binomial error distributions (function glmer of the R package lme4) (58). We used each of the seven taxonomic groups as a grouping variable and estimated random effects for the intercept. More complex model structures with random effects for each predictor variable did not converge with the available fitting algorithms, especially in the case of multivariate regression models (see below).
For contemporary and historical economic conditions, we fitted such models separately for each of the three socioeconomic variables and for all three of them together. In the latter case, we first subjected the three variables of contemporary and historic socioeconomic pressures to principal component analyses (PCA) separately for each of the time points and then used the scores of the 22 countries on the three axes of these PCAs as explanatory variables in the GLMMs to avoid problems possibly arising from multicollinearity. The PCAs were done by means of a singular value decomposition of the centered and scaled data matrices as implemented in the R function prcomp (59).
For each fitted GLMM, we calculated the corrected Akaike information criterion (AICc), which includes a second-order bias correction appropriate for small sample sizes (60). We then compared the AICc of each model pair [with the same independent variable(s) from 1900, 1950, or 2000] by means of Akaike weights. Akaike weights are derived from AICcs and quantify the relative support for individual candidate models as the probability that each of them delivers the best explanation for a given dataset (60). In addition, we assessed goodness-of-model fit by calculating McFadden’s R2 values (61), defining the respective null models as GLMMs with an intercept term as the only fixed effect (and the same random effects).
Regression models that include each of the socioeconomic variables separately or in combination were also fit for each taxon separately using logistic regression [generalized linear models (GLMs) with a logit link function] instead of GLMMs. Goodness-of-GLM fit was evaluated by calculating the explained deviance (62) (D2), and multivariable single-taxon models were compared by means of Akaike weights in the same way as described for GLMMs. Using the geographical coordinates of the country’s capitals, we checked the residuals of all GLMs for spatial autocorrelation by calculating Moran’s I for a neighborhood radius of 1,000 km (to guarantee that each country has at least one neighbor in the neighborhood matrix). The probability of getting an I value higher than the empirical one purely at random was assessed by means of 999 permutations of the vector of model residuals using the function moran.mc implemented in the R package spdep (63). To safeguard against possible bias from spatial autocorrelation, we refitted GLMs with a low such probability (<0.1) as autologistic models, i.e., by additionally introducing an autocovariate calculated by means of the function autocov_dist in the R library spdep (63). Residual autocorrelation was reduced in these autologistic models (with the exception of the grasshopper model for the year 2000, Table S5), and the probability of randomly generating an higher residual autocorrelation hence increased, but in no case was the ranking of goodness-of-fit reversed between historic and contemporary models compared with the respective ordinary GLMs. We hence consider our results robust and qualitatively unaffected by spatial autocorrelation.
To test for an eventual bias due to recent investments into environmental protection, we collected data on recent levels of public environmental expenditures of the 22 countries (in percent of GDP) extracted from the European Commission’s Eurostat Web site (http://epp.eurostat.ec.europa.eu/tgm/table.do?tab=table&init=1&language=en&pcode=ten00049&plugin=0, accessed May 10, 2011; Table S4). We calculated the mean of the available data from the years 1998–2009, and repeated all multivariable GLMMs and GLMs using these expenditures as an additional covariate. All statistical analyses were done in R 2.13.1 (60).
Supplementary Material
Acknowledgments
We thank the many colleagues who provided access to national red lists and total species numbers data. This research was funded by EcoChange Project Sixth Framework Programme 036866 (to S.D.); Academy of Sciences of the Czech Republic long-term research development project RVO 67985939 (to V.J., J.P., and P.P.); institutional resources of the Ministry of Education, Youth, and Sports of the Czech Republic (to V.J. and P.P.); Praemium Academiae award from the Academy of Sciences of the Czech Republic (to P.P.); and the Helmholtz Association Programme “Earth and Environment,” core subject area “Land Use Options—Strategies and Adaptation to Global Change” (I.K.). HANPP research was funded by Fonds zur Förderung der Wissenschaftlichen Forschung Project P20812-G11. This article contributes to the Global Land Project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216303110/-/DCSupplemental.
References
- 1.Hof C, Araújo MB, Jetz W, Rahbek C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature. 2011;480(7378):516–519. doi: 10.1038/nature10650. [DOI] [PubMed] [Google Scholar]
- 2.Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330(6010):1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- 3.Woodward G, et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science. 2012;336(6087):1438–1440. doi: 10.1126/science.1219534. [DOI] [PubMed] [Google Scholar]
- 4.Butchart SH, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328(5982):1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 5.Essl F, et al. Socioeconomic legacy yields an invasion debt. Proc Natl Acad Sci USA. 2011;108(1):203–207. doi: 10.1073/pnas.1011728108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein Goldewijk K, Beusen A, van Drecht G, de Vos M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob Ecol Biogeogr. 2011;20(1):73–86. [Google Scholar]
- 7.Burger JR, et al. The macroecology of sustainability. PLoS Biol. 2012;10(6):e1001345. doi: 10.1371/journal.pbio.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland TG, Peterson GD, Gonzalez A. A cross-national analysis of how economic inequality predicts biodiversity loss. Conserv Biol. 2009;23(5):1304–1313. doi: 10.1111/j.1523-1739.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 9.Tilman D, May RM, Lehman CL, Nowak MA. Habitat destruction and the extinction debt. Nature. 1994;371(6492):65–66. [Google Scholar]
- 10.Kuussaari M, et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol Evol. 2009;24(10):564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Ewers RM, Didham RK. Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc. 2006;81(1):117–142. doi: 10.1017/S1464793105006949. [DOI] [PubMed] [Google Scholar]
- 12.Krauss JR, et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett. 2010;13(5):597–605. doi: 10.1111/j.1461-0248.2010.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousins SAO. Extinction debt in fragmented landscapes: Paid or not? J Veg Sci. 2009;20(1):3–7. [Google Scholar]
- 14.Helm A, Hanski I, Pärtel M. Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett. 2006;9(1):72–77. doi: 10.1111/j.1461-0248.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 15.Wearn OR, Reuman DC, Ewers RM. Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science. 2012;337(6091):228–232. doi: 10.1126/science.1219013. [DOI] [PubMed] [Google Scholar]
- 16.Hahs AK, et al. A global synthesis of plant extinction rates in urban areas. Ecol Lett. 2009;12(11):1165–1173. doi: 10.1111/j.1461-0248.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 17.Sang A, et al. Indirect evidence for an extinction debt of grassland butterflies half century after habitat loss. Biol Conserv. 2010;143(6):1405–1413. [Google Scholar]
- 18.Bertrand R, et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479(7374):517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- 19. International Union for Conservation of Nature (IUCN) (2011) The IUCN Red List of Threatened Species. Available at www.iucnredlist.org. Accessed November 11, 2011.
- 20.Bradshaw CJA, Giam X, Sodhi NS. Evaluating the relative environmental impact of countries. PLoS ONE. 2010;5(5):e10440. doi: 10.1371/journal.pone.0010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haberl H, et al. Quantifying and mapping the human appropriation of net primary production in earth’s terrestrial ecosystems. Proc Natl Acad Sci USA. 2007;104(31):12942–12947. doi: 10.1073/pnas.0704243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern DI. The rise and fall of the environmental Kuznets curve. World Dev. 2004;32(8):1419–1439. [Google Scholar]
- 23.Lehner B, et al. High resolution mapping of the world’s reservoirs and dams for sustainable river flow management. Front Ecol Environ. 2011;9(9):494–502. [Google Scholar]
- 24.Storkey J, Meyer S, Still KS, Leuschner C. The impact of agricultural intensification and land-use change on the European arable flora. Proc Biol Sci. 2012;279(1732):1421–1429. doi: 10.1098/rspb.2011.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis CJ, Coppins BJ. Nineteenth century woodland structure controls stand-scale epiphyte diversity in present-day Scotland. Divers Distrib. 2007;13(1):84–91. [Google Scholar]
- 26.Morris WF, et al. Longevity can buffer plant and animal populations against changing climatic variability. Ecology. 2008;89(1):19–25. doi: 10.1890/07-0774.1. [DOI] [PubMed] [Google Scholar]
- 27.Thomas JA, et al. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science. 2004;303(5665):1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- 28.Bulman CR, et al. Minimum viable metapopulation size, extinction debt, and the conservation of a declining species. Ecol Appl. 2007;17(5):1460–1473. doi: 10.1890/06-1032.1. [DOI] [PubMed] [Google Scholar]
- 29.Hanski I, Ovaskainen O. Extinction debt at extinction threshold. Conserv Biol. 2002;16(3):666–673. [Google Scholar]
- 30.WallisDeVries MF, Poschlod P, Willems JH. Challenges for the conservation of calcareous grasslands in northwestern Europe: Integrating the requirements of flora and fauna. Biol Conserv. 2002;104(3):265–273. [Google Scholar]
- 31.Freyhof J, Brooks E. European Red List of Freshwater Fishes. Luxembourg: Publications Office of the European Union; 2011. [Google Scholar]
- 32.Temple HJ, Terry A. European mammals: Red list status, trends and conservation priorities. Folia Zool. 2009;58(3):248–269. [Google Scholar]
- 33.Bobbink R, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol Appl. 2010;20(1):30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- 34.Pyšek P, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA. 2010;107(27):12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulme PE, Pyšek P, Nentwig W, Vilà M. Will threat of biological invasions unite the European Union? Science. 2009;324(5923):40–41. doi: 10.1126/science.1171111. [DOI] [PubMed] [Google Scholar]
- 36.Devictor V, et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nature Climate Change. 2012;2(2):121–124. [Google Scholar]
- 37.Dullinger S, et al. Extinction debt of high-mountain plants under 21st-century climate warming. Nature Climate Change. 2012;2(8):619–622. [Google Scholar]
- 38.Loarie SR, et al. The velocity of climate change. Nature. 2009;462(7276):1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson O. Functional roles of remnant plant populations in communities and ecosystems. Glob Ecol Biogeogr. 2000;9(6):443–449. [Google Scholar]
- 40. European Commission (2011) Our Life Insurance, Our Natural Capital: An EU Biodiversity Strategy to 2020 (COM(2011) 244 final). Available at http://ec.europa.eu/environment/nature/biodiversity/comm2006/pdf/2020/1_EN_ACT_part1_v7%5b1%5d.pdf.
- 41.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 42.Vitousek PM, Ehrlich PR, Ehrlich AH, Matson PA. Human appropriation of the products of photosynthesis. Bioscience. 1986;36(6):363–373. [Google Scholar]
- 43.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 44.Haberl H, et al. Human appropriation of net primary production and species diversity in agricultural landscapes. Agric Ecosyst Environ. 2004;102(2):213–218. [Google Scholar]
- 45.Haberl H, et al. Human appropriation of net primary production as determinant of avifauna diversity in Austria. Agric Ecosyst Environ. 2005;110(3-4):119–131. [Google Scholar]
- 46.Haberl H, et al. Towards an integrated model of socioeconomic biodiversity drivers, pressures and impacts. A feasibility study based on three European long-term socio-ecological research platforms. Ecol Econ. 2009;68(6):1797–1812. [Google Scholar]
- 47.Wright DH. Human impacts on the energy flow through natural ecosystems, and implications for species endangerment. Ambio. 1990;19(4):189–194. [Google Scholar]
- 48.Rojstaczer S, Sterling SM, Moore NJ. Human appropriation of photosynthesis products. Science. 2001;294(5551):2549–2552. doi: 10.1126/science.1064375. [DOI] [PubMed] [Google Scholar]
- 49.Imhoff ML, et al. Global patterns in human consumption of net primary production. Nature. 2004;429(6994):870–873. doi: 10.1038/nature02619. [DOI] [PubMed] [Google Scholar]
- 50.Food and Agricultural Organization of the United Nations . FAO Statistical Databases: Agriculture, Fisheries, Forestry, Nutrition. Rome: Food and Agriculture Organization of the United Nations; 2007. [Google Scholar]
- 51.Institut International d'Agriculture . International Yearbook of Agricultural Statistics 1910. Rome: Institut International d'Agriculture; 1912. [Google Scholar]
- 52.Imprimerie de l'Institut International d'Agriculture . International Yearbook of Agricultural Statistics, 1909–1921. Rome: Imprimerie de l'Institut International d'Agriculture; 1922. [Google Scholar]
- 53.Zon R, Sparhawk WN. Forest Resources of the World. New York: McGraw-Hill; 1923. [Google Scholar]
- 54.Woytinsky W. The World in Figures. Book 3: Agriculture. Berlin: Rudolf Mosse; 1926. German. [Google Scholar]
- 55.Wirsenius S. 2000. Human use of land and organic materials. Modeling the turnover of biomass in the global food system. PhD thesis (Chalmers Univ of Technology, Göteborg, Sweden) [Google Scholar]
- 56.Krausmann F, Erb KH, Gingrich S, Lauk C, Haberl H. Global patterns of socioeconomic biomass flows in the year 2000: A comprehensive assessment of supply, consumption and constraints. Ecol Econ. 2008;65(3):471–487. [Google Scholar]
- 57.Bondeau A, et al. Modelling the role of agriculture for the 20th century global terrestrial carbon balance. Glob Change Biol. 2007;13(3):679–706. [Google Scholar]
- 58.Bates DM, Maechler B. 2011. lme4: Linear mixed effects models using S4 classes. R package version 0.999375-28m. Available at http://lme4.r-forge.r-project.org/. Accessed June 6, 2011.
- 59.R Development Core Team 2011. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org. Accessed June 2, 2011.
- 60.Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. [Google Scholar]
- 61.McFadden D. Quantitative Methods for Analyzing Travel Behaviour of Individuals: Some Recent Developments. London: Croom Helm; 1979. [Google Scholar]
- 62.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Modell. 2000;135(2-3):147–186. [Google Scholar]
- 63.Bivand L. 2011. spdep: Spatial dependence: Weighting schemes, statistics and models. R package version 0.4-24. Available at http://cran.r-project.org/web/packages/spdep/index.html. Accessed November 10, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.