Abstract
Gametocytes are essential for Plasmodium transmission, but little is known about the mechanisms that lead to their formation. Using piggyBac transposon-mediated insertional mutagenesis, we screened for parasites that no longer form mature gametocytes, which led to the isolation of 29 clones (insertional gametocyte-deficient mutants) that fail to form mature gametocytes. Additional analysis revealed 16 genes putatively responsible for the loss of gametocytogenesis, none of which has been previously implicated in gametocytogenesis. Transcriptional profiling and detection of an early stage gametocyte antigen determined that a subset of these mutants arrests development at stage I or in early stage II gametocytes, likely representing genes involved in gametocyte maturation. The remaining mutants seem to arrest before formation of stage I gametocytes and may represent genes involved in commitment to the gametocyte lineage.
Keywords: malaria, piggyBac insertional mutagenesis, gametocyte development, Plasmodium genetics
Malaria remains the most devastating parasitic disease, killing an estimated 1 million people every year. With ∼50% of the world’s population at risk, novel interventions are desperately needed (1). The disease is caused by the asexual multiplication of the protozoan parasite Plasmodium in the blood of its mammalian host, and transmission occurs by Anopheles mosquitoes. Although the vast majority of the parasites undergo asexual replication, in response to ill-defined environmental stimuli, a small subset commits instead to sexual differentiation and forms gametocytes (2). After feeding on an infected host, gametocytes are the only form of the parasite that is able to survive and develop in the mosquito vector. Thus, gametocytes are required for transmission of Plasmodium. However, the biology of gametocyte formation is poorly understood.
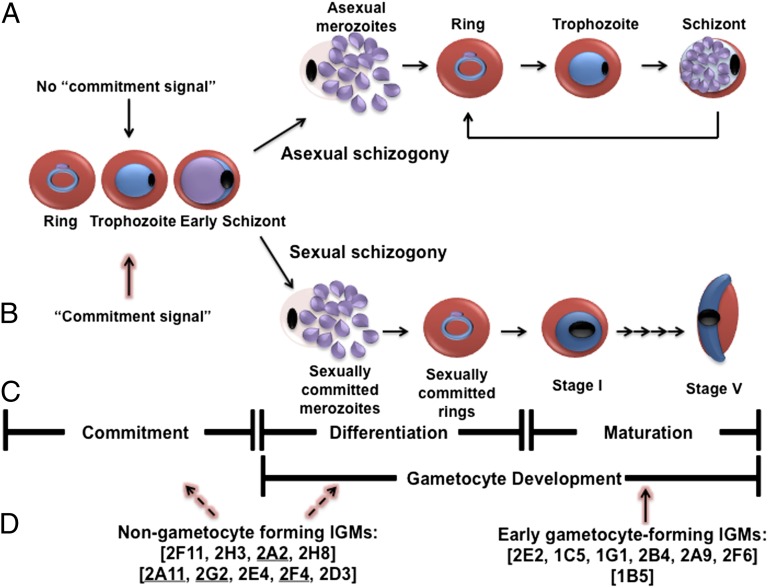
Gametocytogenesis (gametocyte formation) of the human malaria parasite P. falciparum can be broken down into three steps: commitment, prestage I gametocyte development, and poststage I gametocyte development. Early studies have shown that all merozoites from a single schizont follow either asexual or sexual fate (3, 4), and those merozoites that commit to sexual differentiation form exclusively male or exclusively female gametocytes (5, 6). These observations suggest the existence of a defined gene expression pathway leading to the commitment to gametocytogenesis that probably acts before DNA synthesis and nuclear division of the sexually committed schizont (7). Moreover, the extent of gametocyte production by a particular parasite strain can be influenced by the parasite’s environment (reviewed in ref. 7).
After the parasite commits to gametocytogenesis, it begins prestage I development; the committed parasite undergoes sexual schizogony, producing sexually committed merozoites that, on release from the host red blood cell, invade fresh erythrocytes, resulting in the formation of a sexually committed ring. Over the next ∼24–30 h, this committed ring stage parasite differentiates into a morphologically and molecularly identifiable stage I gametocyte (8–10). After the stage I gametocyte has formed, the poststage I development will ensue, where the stage I gametocyte will undergo a maturation process over an ∼5- to 7-d period, resulting in a mature stage V female or male gametocyte.
Very little is known about the molecular mechanisms governing the commitment of the dividing parasites to gametocytogenesis or about the genes required for gametocyte commitment and development. Microarray experiments with early or mature gametocytes revealed 246 gametocyte-specific genes (9). The majority (∼75%) encodes genes of unknown function. A small number of these gametocyte-specific genes—Pfs16, Pfg27, Pfmdv1/peg3, Pf11.1, Pfs230, and Pfg377—have been knocked out. However, in every case, mutant parasites still formed mature gametocytes, albeit in lower numbers (11–16). To date, no genes essential for gametocytogenesis have been identified.
We took a forward genetic approach to identify genes required for the formation of mature gametocytes. Using genome-wide piggyBac transposon-mediated insertional mutagenesis, we isolated 29 gametocyte-negative clones termed insertional gametocyte-deficient mutants (IGMs). Of 29 IGMs, 22 IGMs had a single transposon insertion at 16 unique loci, and none of them had previously been implicated in gametocytogenesis. Here, we report on the initial characterization of these IGMs.
Results
Isolation of Gametocyte-Deficient Mutant Parasites by piggyBac Transposon Mutagenesis.
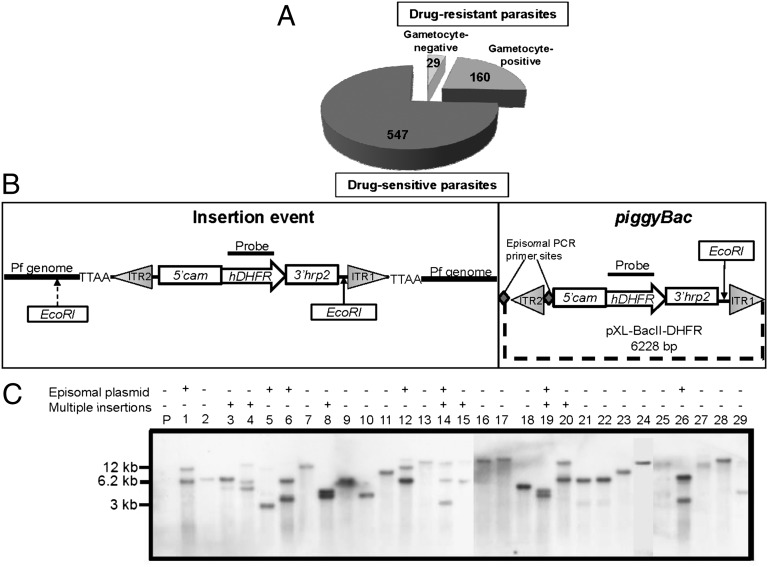
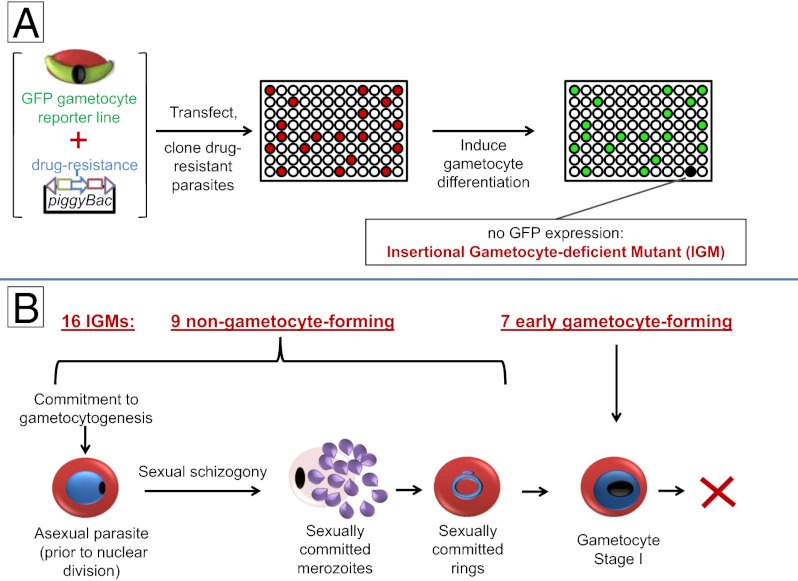
We used piggyBac transposon mutagenesis (17, 18) to identify genes required for P. falciparum gametocytogenesis. Parasites used for the screen (hereafter referred to as parental) contained a plasmid encoding a GFP gene driven by the Pfs28 promoter, which is active only in late stage male and female gametocytes (stages IV and V) (19). Any mutant derived from this line that is impaired in mature gametocyte formation will not produce fluorescent parasites on induction of gametocytogenesis. Parental parasites were transfected with a mixture of two plasmids: one plasmid carrying a drug-resistant marker flanked by the piggyBac inverted terminal repeats, and the other plasmid containing a helper transposase. Between one and four generations posttransfection, parasites were cloned in individual wells of 96-well plates and subjected to selection in the presence of drug followed by growth in the absence of drug to promote the loss of nonintegrated plasmids. Each clone was then screened for the ability to produce fluorescent gametocytes. Clones that did not form gametocytes were tested three additional times by absence of both fluorescent gametocytes and gametocytes in Giemsa-stained smears. From three independent transfection experiments, 189 drug-resistant clones were isolated, of which 29 (∼15%) clones failed to form gametocytes (Fig. 1A). These 29 clones (IGMs) were analyzed by Southern blot analysis and PCR to detect episomal plasmids (Fig. 1 B and C and Table S1). Of 29 IGMs, 22 IGMs had single insertions, and only these IGMs were further characterized.
Fig. 1.
piggyBac insertional mutagenesis results in 22 IGMs with single insertion events. (A) In three independent transfection experiments, 736 wells were seeded with parasites. Of these wells, 189 contained drug-resistant parasites. Of these wells, 29 (15%) failed to form gametocytes and were named IGMs. (B) Schematic diagram of the piggyBac plasmid and an insertion event. (Left) When digested with EcoRI and hybridized with an hdhfr probe, a Southern blot will yield a band with size that depends on the position of the closest EcoRI site in the P. falciparum genome. (Right) A nonintegrated episomal plasmid yields a 6.2-kb fragment (it has only one EcoRI site). ITR, transposon inverted terminal repeat. pXL-BacII-DHFR diagram was modified from ref. 59. PCR primer sites for the piggyBac plasmid are indicated by large gray diamonds. (C) Of 29 IGMs, 22 IGMs yielded a single novel band (different from the 6.2-kb band), indicating a single piggyBac insertion (lanes 2, 7, 9, 10, 11, 13, 16, 17, 18, 21, 22, 23, 24, 25, 27, 28, and 29). Some IGMs with integrated transposons also yielded an ∼6.2-kb band, suggesting that a nonintegrated episomal plasmid is still present in these parasites. PCR analysis was used to determine whether an episome is present in the mutant clone (Table S1) or a separate piggyBac integration event occurred in another region of the genome. IGMs with multiple insertion events (lanes 3, 4, 8, 14, 15, 19, and 20) were not further characterized. No signal was detected with DNA from parental parasites as expected.
Identification of the Genes Disrupted by piggyBac Insertion.
The sites of transposon insertion in the IGMs were identified by inverse PCR (Table S2). Sequence analysis revealed that insertion events in 22 IGMs with single piggyBac elements occurred at 16 unique loci. In several cases, insertions at the same site were found in distinct clones. With the exception of IGM 1G1 and IGM 1A2, the IGMs with insertions at the same site were recovered from separate transfection experiments, showing that they were independent events (Table 1 and Tables S1 and S2). The insertions of all IGMs were widely distributed among 9 of 14 chromosomes (Table 1), and all occurred into a canonical TTAA target sequence (Table S2); 5 of 16 insertion events occurred within the protein-coding sequence, whereas the remaining 11 insertion events occurred in intergenic regions (Table 1). The effect of piggyBac insertions on transcript abundance was assessed by semiquantitative RT-PCR (Fig. S1). For IGMs that had insertions outside the coding region, mRNA abundance of both flanking genes was assayed. In all cases, mRNA abundance of only one of two genes was affected (Table 1 and Fig. S1). The gene with the lower abundance is presumed to be the affected gene.
Table 1.
piggyBac screen identifies 16 insertion events that putatively disrupt gametocytogenesis
| Insertion number | IGM | Accession | Annotation | Chromosome | Insertion position | mRNA abundance | ORF size (bp) |
| 1 | 2F11 | PF14_0532 | LCCL domain-containing (PfCCP2) | 14 | ORF | Decreased | 5,115 |
| 2 | 2H3 | PFE1370w | Putative Hsp70-interacting protein (PfHip) | 5 | 5′ of ORF | Decreased | 1,742 |
| 3 | 2A2 | PFC0200w | 60S ribosomal protein (Rpl36a) | 3 | 5′ of ORF | Absent | 549 |
| 4 | 2A11 | PFD0800c | Repressor of RNA polymerase III (MAF1) | 4 | 5′ of ORF | Decreased | 1,170 |
| 5 | 2H8 | PF14_0097 | CDP-DAG synthase | 14 | 5′ of ORF | Absent | 2,004 |
| 6 | 2A9, 2H2 | PFL1770c | p25a/TPPP | 12 | 5′ of ORF | Absent | 580 |
| 7 | 2B4 | PF11_0167 | ZDHHC-domain containing Palmitolyation factor | 11 | 5′ of ORF | Absent | 2,040 |
| 8 | 2D3 | PFL2550w | Type IV HSP-interacting (PfGECO) | 12 | 5′ of ORF | Decreased | 1,579 |
| 9 | 2E4, 2F12 | PF13_0097 | AP2 transcription factor | 13 | ORF | Decreased | 8,019 |
| 10 | 2G2, 2G11 | PFI1215w | SF3A3 | 9 | 5′ of ORF | Absent | 1,770 |
| 11 | 2F4 | PFE1615c | Small PEXEL-containing hypothetical protein | 5 | ORF | Absent | 699 |
| 12 | 2E2 | MAL7P1.171 | Large PEXEL-containing hypothetical protein | 7 | 5′ of ORF | Decreased | 6,439 |
| 13 | 2F6 | PFI0980w | Long-chain fatty acid elongase enzyme (ELO3) | 9 | ORF | Absent | 1,929 |
| 14 | 1B5 | PF07_0055 | AAT-1/C. reinhardtii flagellar-associated protein 91 component of eukaryotic flagellar regulatory complex | 7 | 5′ of ORF | Absent | 2,049 |
| 15 | 1C5 | PF14_0595 | Calponin homology domain protein | 14 | ORF | Absent | 4,962 |
Clones with single piggyBac insertions were named IGM. Inverse PCR was used to identify piggyBac insertion sites (Table S1). A total of 16 insertion sites was identified among 22 IGMs with single piggyBac insertions, because some of the independently isolated IGMs had insertions at identical sites (Tables S1 and S2). In cases where the same disruption site was identified in multiple IGMs, the IGM used for additional characterization is indicated in italics. Sequence analysis revealed that insertions occurred in nine different chromosomes, indicating genome-wide distribution of piggyBac insertion events. mRNA abundance was estimated by semiquantitative RT-PCR (Fig. S1). AP2, Apicomplexan Apetala 2; HSP, heat shock protein; LCCL, Limulus clotting factor C; PEXEL, plasmodium export element; ZDHHC, zinc-finger aspartate-histidine-histidine-cysteine.
We also verified the expression pattern of each of 16 genes in the parental line (Fig. S2). In all cases, the mRNA expression profiles closely matched those profiles previously reported (20). The majority of the genes identified were unannotated in the 3D7 genome. We used extensive BLAST searches (21) and pairwise comparisons by hidden Markov models on HHPred (22) to determine remote homologs and functional domains to assign putative identities to all 16 genes (Table 1).
Episomal Complementation Restores Gametocytogenesis to 5 of 22 IGMs.
Because P. falciparum can spontaneously lose the ability to produce gametocytes in vitro (23, 24) and cloning of P. falciparum 3D7 was previously shown to produce gametocyte-negative clones (25), it was important to confirm that the piggyBac insertions were causally related to the loss of the ability to form mature gametocytes. Accordingly, we set out to episomally express the putatively disrupted gene in the context of its own promoter and terminator regions (26) (Fig. 2A). Despite numerous attempts and alternative strategies, the large size and AT-rich nature of the putatively affected genes precluded us from producing rescue constructs for all 16 genes.
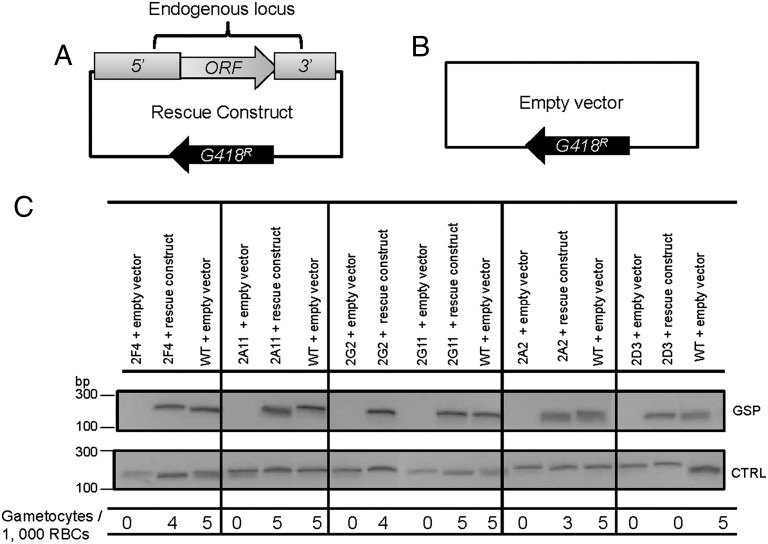
Fig. 2.
Episomal complementation restores gametocytogenesis to five of the IGMs. (A) The rescue construct contains the endogenous putatively disrupted gene along with ∼1.5–2 kb upstream of the ATG translational initiator (5′) and ∼0.3–0.8 kb downstream (3′) of the stop codon. The construct confers resistance to G418/Geneticin. (B) As a control, the IGMs and the parental line were also transfected with an empty vector, which only contains the Geneticin/G418 selectable marker. (C) RT-PCR showing that the complementation constructs restored mRNA expression to levels similar to parental parasites. CTRL, control seryl-tRNA synthetase; GSP, gene-specific primer. The bottom line shows that microscopic analysis revealed that complemented parasites produced gametocytes in numbers comparable with the parental line, whereas IGMs transfected with the empty construct did not produce gametocytes. Gametocyte numbers were assayed three independent times, and each time, 10,000 RBCs were counted. Except for IGM 2D3, differences in gametocyte numbers between the rescued IGMs and the parental line were not statistically significant (Poisson). Whereas IGM 2D3 transfected with the rescue vector produced target mRNA of abundance comparable to the parental line, it did not produce gametocytes, suggesting that a second site mutation accounts for impaired gametocytogenesis.
Complementation constructs for five of the smaller genes were produced, and complementation experiments were conducted with six IGM clones corresponding to the presumed five disrupted genes (IGMs 2G2 and 2G11 have a piggyBac insertion at the same site). Each rescue construct restored transcription of the affected gene to levels comparable with the levels of the parental line (Fig. 2). However, gametocytogenesis was restored in only five of six IGMs (Fig. 2). Gametocytogenesis of IGM 2D3 was not restored, although mRNA abundance of the disrupted gene, pfl2550w, was restored by the rescue construct, suggesting that this line carries a second site mutation that disrupts gametocytogenesis. Importantly, two independent clones, IGM 2G2 and IGM 2G11, with identical insertions affecting pfi1215w (Table 1 and Tables S1 and S2) were complemented using the same rescue construct (Fig. 2C), confirming that, for both clones, disruption of this gene was responsible for the loss of gametocytogenesis.
Investigation of the Ability of Mutant Parasites to Initiate Gametocyte Maturation.
Because the parental reporter strain used for the screen carried a GFP gene driven by a promoter (Pfs28) that is active only in late stage gametocytes, it is conceivable that some of the IGMs could initiate gametocytogenesis (form stages I–III) and subsequently arrest. We investigated this possibility for all of the IGMs using immunofluorescence assays with an anti-Pfmdv1/peg3 antibody (Fig. 3), which specifically identifies gametocytes of all stages (13). These assays identified seven IGMs—2E2, 1C5, 1G1, 2B4, 2A9, 2F6, and 1B5—that formed stage I gametocytes (Fig. 3A), of which one (IGM 1B5) also forms early stage II gametocytes (Fig. 3B). As expected, the antibody consistently identified gametocytes of all stages from the parental parasite line (Fig. 3C). The remaining nine IGMs did not produce Pfmdv1/peg3-positive stage I gametocytes. Because none of seven IGMs differentiated into later stage gametocytes, the mutations likely disrupt very early (poststage I) events of gametocyte maturation. Most likely, the remaining nine IGMs are defective at earlier stages of commitment or differentiation into stage I gametocytes.
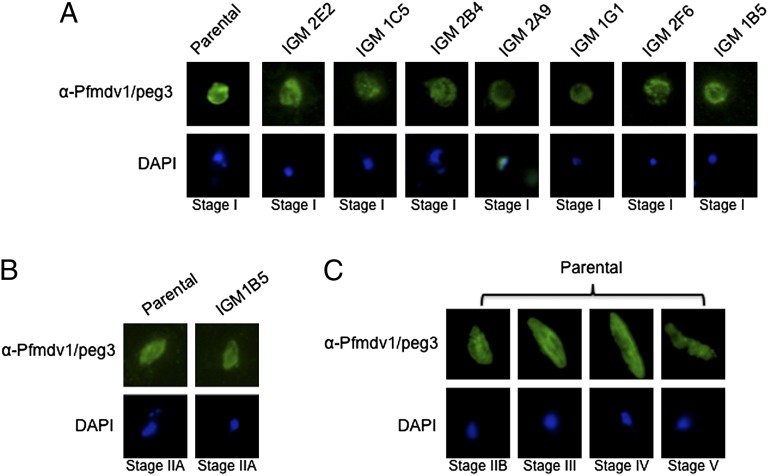
Fig. 3.
A subset of the IGMs forms stage I gametocytes. (A) Pfmdv1/peg3 is a gametocyte-specific gene expressed in all gametocyte stages (13). Immunofluorescence using antisera against Pfmdv1/peg3 identified the protein in stage I gametocytes from IGMs 2E2, 1C5, 2B4, 1G1, 2F6, and 1B5. (B) Pfmdv1/peg3 staining also reveals that IGM 1B5 is able to produce what appears to be stage IIA gametocytes but not stage IIB or later gametocytes (8). None of the IGMs produced gametocytes that mature beyond stage I or IIA. (C) Under the same experimental conditions, the antibody consistently identified the protein in stages I–V gametocytes from the parental line.
Hierarchical Ordering of the IGMs.
The developmental period from the commitment of a parasite to a sexual fate to the first appearance of an early stage II gametocyte covers a time span of 48 h or more. This differentiation process likely involves several distinct and sequential regulatory events that have yet to be described. Although expression of the Pfmdv1/peg3 marker divides the IGM mutants into two broad groups, one arresting development before and the other arresting development after formation of stage I gametocytes (Fig. 3), the IGM mutants likely represent a much wider spectrum of developmental arrest points across this 48-h period. We reasoned that early developmental mutants may fail to initiate the transcription of genes hierarchically linked to them and normally expressed at later stages of development. Semiquantitative RT-PCR was performed on each of the IGMs using primers for the remaining 16 putatively disrupted IGM transcripts as well as known early and late gametocyte-specific transcripts. We classified the results in a binary fashion (expressed or not expressed according to the presence or absence of a PCR product in at least one biological replicate) (Fig. S3) and hierarchically clustered the expression profiles of each mutant and the parental line based on their similarity to each other. The results are presented in Fig. 4. The IGMs appear (from left to right in Fig. 4) in order of increasing transcript representation. We hypothesize that those IGMs that are defective in the expression of the largest number of genes arrest at an earlier step of the commitment/differentiation pathway. In agreement with this hypothesis, all IGMs that progress to stage I gametocytes cluster into a group that is closest to the expression profile of the WT parental line, and among these IGMs, IGM 1B5 (a mutant that produces early stage II gametocytes) is the most similar to the parental line. Of note, IGM 2D3 produces Pfmdv1/peg3 transcripts, but the protein is undetectable in these parasites, raising the possibility that posttranscriptional regulatory mechanisms operate in the regulation of gametocyte differentiation.
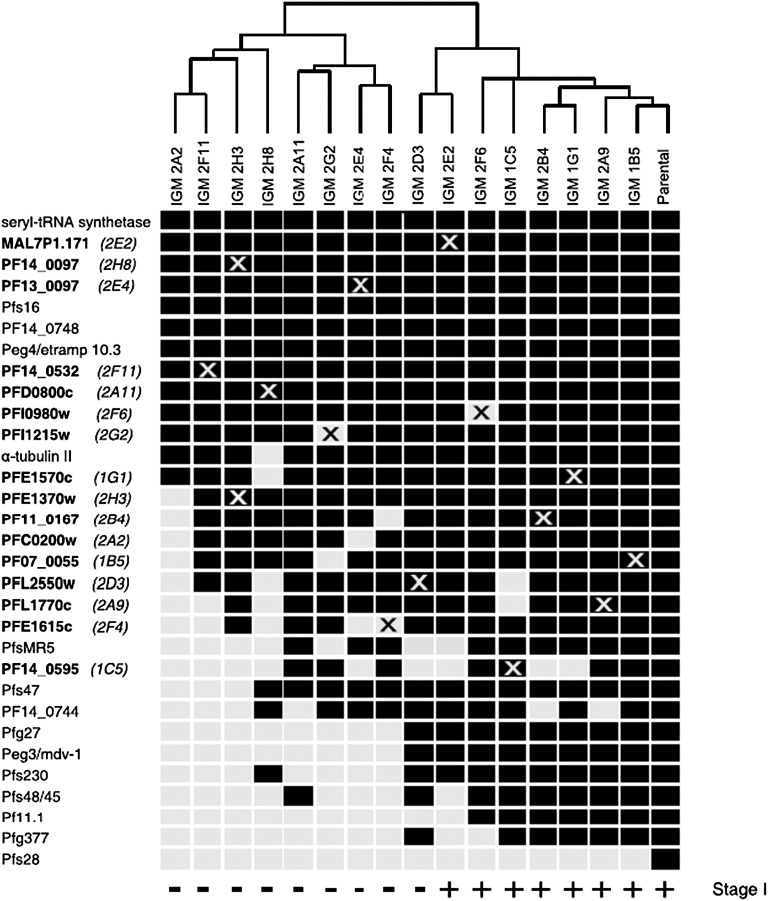
Fig. 4.
Hierarchical ordering of the IGMs by RT-PCR expression profiling. The expression profiles indicate transcripts that were expressed (black boxes) or not expressed (light gray boxes) as measured by semiquantitative RT-PCR (Fig. S3). The transcripts listed correspond to 16 putatively disrupted genes as well as known early (Pfs16, PF14_0748, Peg4/etramp10.3, Pfg27, PF14_0744, and Pfmdv1/peg3) and late (Pf11.1, Pfg377, Pfs48/45, PfMR5, Pfs47, and Pfs28) gametocyte-specific genes. An X indicates the transcript from the putatively disrupted IGM gene. Transcript abundance from some putatively disrupted genes was only decreased and not completely absent in some IGMs (gray Xs on black boxes). The IGMs are hierarchically clustered based on the similarity of their expression profiles to each other and the parental line. All IGMs that are able to differentiate into stage I gametocytes and express the Pfmdv1/peg3 marker (Fig. 3, indicated along the bottom) cluster to the right next to the WT parental line. Gametocyte-specific transcript accession numbers are in Table S3.
Discussion
Despite the essential role gametocytes play in malaria transmission, the molecular mechanisms that govern Plasmodium gametocytogenesis remain largely undefined. Although a small number of gametocyte-specific genes were previously disrupted, all of the resulting KO lines were still able to produce mature gametocytes (11–16). To address this subject from a different angle, we took a forward genetics approach using transposon-mediated mutagenesis. Three independent transfection experiments yielded 22 single IGMs that were unable to produce mature gametocytes; these 22 IGMs accounted for insertions at 16 unique sites. With one exception, all of the IGMs with identical insertion positions were isolated from independent transfection experiments (Table 1 and Table S1), indicating the occurrence of piggyBac insertion hotspots. Although piggyBac is known to have fewer hotspot regions than other transposons, such as the P element, such hotspots were also observed in insertional mutagenesis experiments with other organisms (27). However, the present results contrast with the results in the work by Balu et al. (18), which did not find multiple piggyBac insertion events at the same site. The reasons for this difference are not clear; 5 of the transposon insertions were in coding regions, whereas 11 of the transposon insertions were in intergenic regions presumed to encode transcriptional regulatory elements (Table 1). A previous screen using piggyBac also found a bias for insertion into the intergenic regions of the genome (18). The intergenic regions may be more accessible to the transposase, because the chromatin is typically not as tightly packed in these regions (17, 27).
Because P. falciparum can spontaneously lose the ability to produce gametocytes in vitro (23, 24), it was important to show that the disrupted gene was causally related to the loss of gametocytogenesis using genetic complementation. Technical considerations limited our ability to produce rescue constructs for all disrupted genes given the large size of the coding plus regulatory regions (∼5.5–18 kb) and the extremely rich AT content of the P. falciparum genome. We were able to assemble five rescue constructs for some of the smaller genes, namely IGMs 2A2, 2A11, 2G2, 2G11, 2F4, and 2D3. Importantly, we were able to complement five IGMs (IGMs 2A2, 2A11, 2G2, 2G11, and 2F4), which corresponded to 4 of 16 putatively disrupted genes. Interestingly, none of these IGMs form stage I gametocytes. The mutant genes may play a role in the early events, either commitment to gametocytogenesis or differentiation of the committed parasites into stage I gametocytes. IGM 2A2 carries an insertion near pfc0200w, a gene predicted to code for ribosomal protein L36 (Rpl36a or L42). Recent studies in other systems have shown that differential expression of ribosomal proteins is important for both development and stress response (28–30). Given that gametocyte numbers increase in response to environmental stimuli, particularly stress (31), one may speculate that Rpl36a plays a role in commitment or sexual schizogeny (Fig. 5 A and B). IGM 2A11 carries a piggyBac insertion upstream of pfd0800c, a gene related to Maf1, which is a repressor of RNA polymerase III. In yeast, Maf1 normally localizes to the cytoplasm, but at times of nutrient deprivation and in response to stress, it is translocated into the nucleus, where it down-regulates transcription of tRNAs and 5S RNA through repression of RNA polymerase III (32). Changes in tRNA abundance may lead to translational regulation (33) of mRNAs that encode proteins involved in the early stages of sexual development. IGM 2G2 has an insertion upstream of pfi1215w, which codes for the splice factor 3a subunit 3 (SF3A3), an essential component of the 17S U2 small nuclear ribonucleoprotein alternative splicing complex (34). Several P. falciparum genes with alternative splice forms have been detected in gametocytes (35), raising the possibility that SF3A3 is essential for proper splicing of RNAs involved in gametocyte differentiation. Finally, 2F4 has an insertion in the ORF of pfe1615c, which is a protein predicted to be exported to the erythrocyte cytoplasm (36), indicating a possible role in signal transduction for commitment of cells to the sexual pathway.
Fig. 5.
Model for gametocyte commitment, differentiation, and maturation. P. falciparum has two developmental fates: (A) cyclic asexual propagation or (B) terminal sexual differentiation. (A) In humans, the majority of the blood stage parasites undergo asexual propagation (default pathway). Here, the parasite undergoes asexual schizogny, producing asexual merozoites that, when released, invade new erythrocytes and continue the cycle every 48 h. The asexual propagation of the parasite accounts for disease symptoms such as cyclic fever. (B) The parasite undergoes sexual differentiation (gametocytogenesis) if, before DNA replication, a commitment signal is received (the position of the up arrow is arbitrary, because the timing of commitment is unknown). This commitment signal is ill defined, but in vitro, it is typically associated with environmental conditions that negatively impact asexual reproduction, such as high parasitemia or changes in hematocrit (2, 31). After committed, the parasite initiates gametocyte development through sexual schizogony that results in formation of sexually committed merozoites. When released, the committed merozoites invade fresh erythrocytes to form sexually committed rings. There are currently no known markers for this initial process. During the next ∼24 h, the parasite differentiates into a morphologically and molecularly distinguishable stage I gametocyte. Thereafter, the gametocyte undergoes a stepwise maturation process to yield a mature stage V male or female gametocyte. Only stage V gametocytes circulate in the bloodstream, and this stage is the only stage that is infectious to the Anopheles mosquito vector. (C) Gametocytogenesis can be divided into three steps: commitment, prestage I gametocyte development, and poststage I gametocyte development. Commitment is the process that commits the parasite to sexual differentiation. Prestage I gametocyte development begins with sexual schizogony and concludes when the committed ring stage parasite transforms into an identifiable stage I gametocyte (∼24–30 h postinvasion). Poststage I gametocyte development is the process that transforms a stage I gametocyte into a mature stage V gametocyte. (D) One group of IGMs does not form stage I gametocytes. These mutants likely arrest during commitment prestage I development. Because there are no known markers that discriminate between the two processes, at present, there are no means to determine at which point these IGMs arrest (as indicated by the dashed arrows). A second group of IGMs forms stage I gametocytes but then arrests (as indicated by the solid arrow). The genes disrupted in these IGMs likely play a role in gametocyte maturation. The underlined IGMs are the complemented lines.
Although stable lines carrying the rescue plasmid were established for IGM 2D3 (in three independent experiments) that reverted mRNA abundance to WT level, no gametocytes were observed (Fig. 2C). In agreement with these results, the putatively disrupted gene in IGM 2D3, pfl2550w, was knocked out independently and found to produce mature gametocytes (37). Thus, we believe that IGM 2D3 carries a second site mutation that accounts for the loss of gametocytogenesis. In addition, pf14_0532 (IGM 2F11) was previously knocked out and shown to produce mature, functional gametocytes (38), raising the possibility that this clone has a second mutation as well. We note that, because the rescue constructs contained the entire endogenous locus (including the putative promoter and terminator regions), we cannot completely rule out the possibility that the regulatory regions influenced expression of adjacent genes (39), especially when piggyBac inserted between divergent transcription units. For genes that have not been complemented, the assignment of the disrupted gene to gametocytogenesis will need to be verified by episomal complementation, protein destabilization, or targeted gene KO (40).
A gametocyte-specific marker (Pfmdv1/peg3) allowed us to classify the IGMs as belonging to one of two distinct groups: IGMs that form early gametocytes and IGMs that form no gametocytes. Although IGMs 2E2, 1C5, 1G1, 2B4, 2A9, and 2F6 only make stage I gametocytes (Fig. 3A), we noticed what appeared to be very early stage II gametocytes in clone 1B5 (Fig. 3B). By Giemsa stain, these stage II gametocytes appear to be malformed and truncated, and staining with Pfmdv1/peg3 identified them as stage IIA gametocytes (Fig. 3B) (8). Whereas the parental line consistently produced stages IIB–V gametocytes (Fig. 3C), repeated experiments revealed that IGM 1B5 never progressed beyond stage IIA gametocytes. The putatively disrupted gene in IGM 1B5, pf07_0055, bears homology to the human amylase alpha-associated protein expressed in testis 1 (AAT-1) (Table 1). AAT-1 has been shown to interact with A-kinase anchor protein, which tethers the regulatory subunits of protein kinase A to the active subunits in the mitochondria of both somatic and sperm cells (41) and has been postulated to play a regulatory role in spermatogenesis (42). Furthermore, AAT-1 shares considerable homology with Chlamydomonas reinhardtii flagellar-associated protein 91, which seems to play a role in the regulation of flagellar activity near the base of the axoneme spokes (43). Given the sequence similarity and mutant phenotype, it is tempting to speculate that PF07_0055 may play an important role in the structural and morphological changes that occur, especially in transitioning between the round stage I and the D-shaped stage II gametocytes. Additional studies on PF07_0055 may provide new insights on the molecular mechanisms that underlie changes in gametocyte morphology during differentiation.
Transcript analysis provided an independent measure of hierarchical relationships among the IGMs (Fig. 4). Interestingly, all of the IGMs that were able to initiate gametocytogenesis (Fig. 3) formed a cluster in the independent transcript analysis that was closest to the parental profile (Fig. 4), providing support for the validity of the latter approach. Bioinformatic searches (20, 21, 36) provided some clues to potential functional groups (Fig. 5). Genes of the earliest arresting IGMs (2A2, 2F11, 2H3, and 2H8) (Fig. 5D) code for proteins involved in gene regulation and signal transduction. IGM 2H8 has an insertion upstream of pf14_0097 that codes for cytidine diphosphate-diacylglycerol (CDP-DAG) synthase, a gene that plays an important role in signal transduction (44). It has been hypothesized that the parasite must use some sort of signaling system to sense changes in the outside environment that eventually results in commitment to sexual differentiation (45). P. falciparum CDP-DAG contains an N-terminal extension that localizes the protein to the parasitophorous vacuole (46) and thus, could play a role in the signal transduction system involved in commitment to gametocytogenesis.
The next group of IGMs (2A11, 2G2, 2E4, 2F4, and 2D3) (Fig. 5D) is impaired in the expression of fewer genes than the preceding group (Fig. 4); thus, we speculate that they may arrest later in the commitment/differentiation pathway. This group includes genes that are also predicted to code for proteins involved in gene regulation. The piggyBac insertion of IGM 2E4 interrupts the ORF of a gene that contains an AP2 domain. This domain is found in the only known family of P. falciparum transcription factors (47). Gene KOs of other AP2-containing genes have been shown to disrupt other developmental processes (48). Thus, PF13_0097 may be an essential transcription factor for gametocyte-specific genes.
A later-acting group of IGMs arrests in poststage I gametocyte development (2E2, 2F6, 2A9, 2B4, 1G1, and 1B5) (Fig. 5D). A subset of these IGMs seems to be involved in cell structure and could play a role in the dramatic morphological changes associated with gametocyte development. Another gametocyte-specific protein, Pfg27, has also been shown to play a role later in gametocyte structural integrity (16), but it is not involved in the early structuring of the gametocyte. IGMs 2A9, 2H2, and 1C5 have insertions in genes putatively involved in cytoskeletal remodeling. IGM 1C5 encodes a protein containing a calponin homology domain that can bind to actin and play a role in signal transduction (49). IGMs 2A9 and 2H2 have an insertion upstream of a gene homologous to mammalian tubulin polymerization-promoting protein (TPPP), which was originally identified in the brain as a phosphoprotein and has been shown to promote tubulin polymerization (50). Thus, both of these proteins may be critical for the dramatic cytoskeletal rearrangements that occur during gametocyte development. Recently, two publications have shown that the inner membrane complex (formerly the subpellicular complex) plays an important role in the structural changes of the gametocyte during its development (51, 52). Analysis using Tubulin Tracker revealed that the microtubule network assembles at the flat section of the stage II gametocyte and that it continues to expand around the periphery of the gametocyte in stage III and totally encase the gametocyte by stage IV; it is the microtubule network that drives the structural changes associated with gametocyte maturation rather than gliding motility (51). It is unknown how the microtubules are organized, and it is possible that this TPPP homolog plays an important role in this process. Along these same lines, IGM 2B4 has an insertion upstream of a gene that putatively encodes for a zinc-finger aspartate-histidine-histidine-cysteine domain that acts as a palmitoyl transferase. Palmitoylation of soluble proteins results in the association of proteins with membranes and can increase membrane stability (53). In addition, in Toxoplasma gondii, integral members of the inner membrane complex must be palmitoylated for proper localization (54). Possibly, specific members of the inner membrane complex that play a role in gametocyte development (51, 52) depend on palmitoylation for their proper localization.
It is of interest to note that gametocytogenesis in P. falciparum differs from other model Plasmodium spp. in several features (55). For P. falciparum, all merozoites from a single schizont are committed to either an asexual or a sexual fate (3–6), whereas for P. berghei and presumably, the other model rodent Plasmodium spp., merozoites from a single schizont can commit to either fate (55). In addition, the P. falciparum gametocytes undergo dramatic structural changes during their long maturation period (∼5–7 d), resulting in crescent-shaped or falciform mature gametocytes (56). However, all other human Plasmodium parasites and the model rodent parasites form round gametocytes within 36 h postinvasion (55). Although most genes from our screen have orthologs in the other available sequenced Plasmodium genomes, two of the genes seem to be P. falciparum-specific: pfe1615c and mal7p1.171. PFE1615c (IGM 2F4) does not form stage I gametocytes but seems to arrest sometime during prestage I development (Fig. 5). MAL7P1.171 (IGM 2E2) forms stage I gametocytes and then arrests (Fig. 5). Both proteins are predicted to be exported and recently, a proteomics study showed that the majority of the proteins expressed in early gametocytes is exported (57). These proteins may play a role in the events of commitment or differentiation (PFE1615c) or the structural changes of early gametocyte maturation (MAL7P1.171).
Transposon mutagenesis is ideally suited for the investigation of Plasmodium gametocytogenesis, because disruption of genes specifically required for gametocytogenesis does not affect maintenance of the parasite in culture. Our screen led to the identification of a new set of genes essential for gametocytogenesis, as previous knockout experiments did not result in complete interruption of gametocyte formation. Of note, none of the genes identified here were previously known to be involved in gametocytogenesis. The relatively high rate of mutant recovery (29/189 clones) was initially surprising. However, considering that Plasmodium is haploid, the high frequency of mutant recovery was probably influenced by the fact that insertions disrupting essential genes or genes strongly affecting parasite growth are never recovered. However, the screen was certainly not saturating, and it is likely that many more genes remain to be identified. Because gametocytogenesis is essential for parasite survival in nature, this line of investigation promises to lead to the identification of novel targets to interfere with disease transmission.
Materials and Methods
General Parasite Culturing and Gametocyte Production.
P. falciparum strain 3D7 parasites were previously transformed with reporter construct pCBM.BSD.52/28.GFP (Pfs28 base pairs −1263 to 1; P. falciparum–Pfs28:GFP) (19). These parasites are referred to as parental throughout this article. Asexual and gametocyte P. falciparum parasites were maintained in culture according to standard methods (2, 58). For gametocyte induction, cultures were seeded at 0.5% parasitemia at 5% hematocrit. Media was changed daily until the parasitemia reached ∼5–6%, at which point media was supplemented with 50 nM GlcNAc until the asexual parasites disappeared, and mature gametocytes were obtained as described (9).
Plasmid Transfection and Screening for P. falciparum IGMs.
P. falciparum–Pfs28:GFP was transformed with piggyBac mutagenic plasmid (pXLBacIIDHFR) containing the dihydrofolate reductase resistance marker and the helper plasmid (pHTH) containing the piggyBac transposase using the RBC preloading method as previously described (59). Parasites were sorbitol synchronized as previously described (60). Mature schizont stage parasites were purified on a MACS magnet (Miltenyl Biotec) (61). The preloaded RBCs were mixed with the schizonts in complete medium and cultured as before.
After one to four generations of culturing (Table S1), the parasites were cloned in, and one parasite per well was placed into 96-well plates. At this point, drug pressure with WR99210 was applied to select for parasites with the piggyBac transposon. WR99210 drug pressure was taken off for 21 d to promote the loss of episomal plasmids and reapplied for 21 d to select for parasites with a piggyBac insertion. Gametocytogenesis was then induced in drug-resistant parasites. Drug-resistant parasites from three independent transfections were screened for the presence or absence of gametocytes by both fluorescence microscopy and Giemsa-stained smears. The parasites that did not produce gametocytes were termed IGMs. The IGMs were isolated and frozen, and loss of the ability to produce gametocytes was confirmed four independent times by both fluorescence and Giemsa staining.
Genomic DNA Extraction.
P. falciparum genomic DNA was isolated from blood stage parasites using a DNeasy Blood & Tissue Kit according to the manufacturer’s instructions (Qiagen).
Southern Blot Analysis.
Total genomic DNA extracted from 29 isolated IGMs was digested with EcoRI overnight and separated on a 0.8% agarose gel, and the blot was hybridized with a 32P-labeled hdhfr probe and exposed to a Kodak photographic film at −80 °C to visualize the hybridized fragments.
Sequence Analysis and Identification of Insertion Sites.
The sequences obtained by sequencing inverse PCR products were analyzed using Genetyx software, and the insertion sites in the genome were identified by performing a blast search using the PlasmoDB database (36).
Comparison of mRNA Abundance with the Parental Parasite Line.
RNA was extracted from each of the IGMs as described in SI Materials and Methods. Semiquantitative RT-PCR was carried out using Standard Taq and conditions according to the manufacturer (New England Biolabs).
Episomal Complementation of piggyBac Disruption Mutants.
Rescue constructs were assembled by amplification of the coding region of the disrupted gene along with ∼2,000 bp of upstream (5′) and 300–800 bp of downstream (3′) sequence from WT genomic DNA; they were cloned first into pJET (Fermentas) and sequenced using the pJET forward and reverse primers (Fermentas). After the correct sequence was verified (Macrogen), the insert was excised with either KpnI or ApaI (FastDigest; Fermentas) and cloned into pINTp3 (62), or the insert was excised with SpeI and NotI (FastDigest; Fermentas) and cloned into pCBM.NEO (63), both of which confer resistance to Geneticin/G418. Parasites were transfected with 100 μg rescue construct or 100 μg empty vector with the drug resistance marker but no rescue insert (pINT or pCBM.NEO) as described above; 48 h posttransfection, 800–1,000 μg/mL G418 (Sigma) were added to culture, and medium was changed daily until parasites disappeared from culture. After disappearance, medium supplemented with G418 was changed every other day until parasites reappeared. After stable lines were established, stocks were frozen, and Giemsa-stained smears were examined every other day for presence of gametocytes. When gametocytes were present, gametocyte numbers were compared with parental parasites that had been transfected with an empty vector. Gametocyte numbers were assayed three independent times. Each time, 10,000 RBCs were counted, and gametocyte numbers per 1,000 RBCs were calculated. Complementation was repeated at least two to three times with independent transfection experiments for each IGM.
mRNA Expression in Complemented Lines.
To measure mRNA expression from the genes encoded in the complementation plasmids, semiquantitative RT-PCR was carried out as before using the RNA from the stable complemented lines and controls with the same primer sets that were used to verify KO/knockdown of each gene after the original screen (Table S3).
Purification of Early Gametocytes and Pfmdv1/peg3 Immunostaining.
Induction of gametocytogenesis and purification of early gametocytes were done as described (64). Immunofluorescence with rabbit α-Pfmdv1/peg3 [1:1,000, 2% (wt/vol) BSA/PBS] was also done as described (13).
RT-PCR and Hierarchical Ordering of the IGMs.
IGMs were cultured and sorbitol synchronized. Synchronized parasites were grown until the culture reached ∼10–15% parasitemia. RNA and then cDNA were separately prepared from rings, trophozoites, or schizonts. Equal amounts of rings, trophozoites, or schizonts cDNA were combined to serve as templates for semiquantitative RT-PCR. The primer sets for the putatively disrupted genes for known gametocyte-specific transcripts are listed in Table S3. RT-PCR products were fractionated by agarose gel electrophoresis, and the results were scored in a binary fashion: expressed if a product was detected in at least one of the replicates and not expressed if no product was detected. The resulting binary expression profile of each IGM was used to construct a dissimilarity matrix by Euclidean distance and subsequently hierarchically clustered by Ward's method.
Supplementary Material
Acknowledgments
We thank Dr. John Adams for helpful discussions about the use of piggyBac for the mutagenesis screen and provision of the pXL-BacII-DHFR and pHTH plasmids. We thank Dr. Tetsuya Furuya for providing the Pfmdv1/peg3 antisera. We also thank Dr. David Fidock for providing the pINTp3 plasmid and MR4 for providing the pCBM.NEO plasmid. We thank the Johns Hopkins Malaria Research Institute Parasitology Core for assistance with human blood preparation. Financial support from the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation is gratefully acknowledged. H.I. was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad, the Kato Memorial Bioscience Foundation, and a Kitasato University Research Grant for Young Researchers. K.S.S. was supported by a Johns Hopkins Malaria Research Institute Pre-Doctoral Fellowship. S.M.K. was supported by a Johns Hopkins Malaria Research Institute Post-Doctoral Fellowship. This research was supported by National Institute of Allergy and Infectious Diseases Grants R01-AI069314 (to K.C.W.) and R21-AI67640 (to M.J.-L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 7121 (volume 110, number 18).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217712110/-/DCSupplemental.
References
- 1.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57(Suppl 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Inselburg J. Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. J Parasitol. 1983;69(3):584–591. [PubMed] [Google Scholar]
- 4.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 5.Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121(Pt 5):465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- 6.Smith TG, Lourenço P, Carter R, Walliker D, Ranford-Cartwright LC. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology. 2000;121(Pt 2):127–133. doi: 10.1017/s0031182099006265. [DOI] [PubMed] [Google Scholar]
- 7.Dixon MWA, Thompson J, Gardiner DL, Trenholme KR. Sex in Plasmodium: A sign of commitment. Trends Parasitol. 2008;24(4):168–175. doi: 10.1016/j.pt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: The puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JA, et al. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143(1):67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Silvestrini F, Tibúrcio M, Bertuccini L, Alano P. Differential adhesive properties of sequestered asexual and sexual stages of Plasmodium falciparum on human endothelial cells are tissue independent. PLoS One. 2012;7(2):e31567. doi: 10.1371/journal.pone.0031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 12.Kongkasuriyachai D, Fujioka H, Kumar N. Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (Pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption. Mol Biochem Parasitol. 2004;133(2):275–285. doi: 10.1016/j.molbiopara.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Furuya T, et al. Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc Natl Acad Sci USA. 2005;102(46):16813–16818. doi: 10.1073/pnas.0501858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eksi S, et al. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol. 2006;61(4):991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 15.de Koning-Ward TF, et al. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2008;67(2):278–290. doi: 10.1111/j.1365-2958.2007.06039.x. [DOI] [PubMed] [Google Scholar]
- 16.Olivieri A, et al. The Plasmodium falciparum protein Pfg27 is dispensable for gametocyte and gamete production, but contributes to cell integrity during gametocytogenesis. Mol Microbiol. 2009;73(2):180–193. doi: 10.1111/j.1365-2958.2009.06762.x. [DOI] [PubMed] [Google Scholar]
- 17.Balu B, Adams JH. Functional genomics of Plasmodium falciparum through transposon-mediated mutagenesis. Cell Microbiol. 2006;8(10):1529–1536. doi: 10.1111/j.1462-5822.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 18.Balu B, et al. piggyBac is an effective tool for functional analysis of the Plasmodium falciparum genome. BMC Microbiol. 2009;9:83. doi: 10.1186/1471-2180-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eksi S, Suri A, Williamson KC. Sex- and stage-specific reporter gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 2008;160(2):148–151. doi: 10.1016/j.molbiopara.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Roch KG, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M, et al. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008;36(Web Server issue):W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(Web Server issue):W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day KP, et al. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc Natl Acad Sci USA. 1993;90(17):8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gissot M, et al. Transcriptome of 3D7 and its gametocyte-less derivative F12 Plasmodium falciparum clones during erythrocytic development using a gene-specific microarray assigned to gene regulation, cell cycle and transcription factors. Gene. 2004;341:267–277. doi: 10.1016/j.gene.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Alano P, et al. Plasmodium falciparum: Parasites defective in early stages of gametocytogenesis. Exp Parasitol. 1995;81(2):227–235. doi: 10.1006/expr.1995.1112. [DOI] [PubMed] [Google Scholar]
- 26.Miao J, et al. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J Cell Sci. 2010;123(Pt 7):1039–1049. doi: 10.1242/jcs.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibault ST, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36(3):283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 28.Kearse MG, Chen AS, Ware VC. Expression of ribosomal protein L22e family members in Drosophila melanogaster: rpL22-like is differentially expressed and alternatively spliced. Nucleic Acids Res. 2011;39(7):2701–2716. doi: 10.1093/nar/gkq1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondrashov N, et al. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145(3):383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vesper O, et al. Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell. 2011;147(1):147–157. doi: 10.1016/j.cell.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172(2):57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cieśla M, Boguta M. Regulation of RNA polymerase III transcription by Maf1 protein. Acta Biochim Pol. 2008;55(2):215–225. [PubMed] [Google Scholar]
- 33.Johnson SS, Zhang C, Fromm J, Willis IM, Johnson DL. Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol Cell. 2007;26(3):367–379. doi: 10.1016/j.molcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9(4):374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Iriko H, et al. A small-scale systematic analysis of alternative splicing in Plasmodium falciparum. Parasitol Int. 2009;58(2):196–199. doi: 10.1016/j.parint.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Aurrecoechea C, et al. PlasmoDB: A functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37(Database issue):D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morahan BJ, et al. Functional analysis of the exported type IV HSP40 protein PfGECO in Plasmodium falciparum gametocytes. Eukaryot Cell. 2011;10(11):1492–1503. doi: 10.1128/EC.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradel G, et al. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med. 2004;199(11):1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Shitrit T, et al. Systematic identification of gene annotation errors in the widely used yeast mutation collections. Nat Methods. 2012;9(4):373–378. doi: 10.1038/nmeth.1890. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg DE, Janse CJ, Cowman AF, Waters AP. Has the time come for us to complement our malaria parasites? Trends Parasitol. 2011;27(1):1–2. doi: 10.1016/j.pt.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda E, Ishizaki R, Taira T, Iguchi-Ariga SMM, Ariga H. Structure and characterization of AAT-1 isoforms. Biol Pharm Bull. 2005;28(5):898–901. doi: 10.1248/bpb.28.898. [DOI] [PubMed] [Google Scholar]
- 42.Yukitake H, Furusawa M, Taira T, Iguchi-Ariga SMM, Ariga H. AAT-1, a novel testis-specific AMY-1-binding protein, forms a quaternary complex with AMY-1, A-kinase anchor protein 84, and a regulatory subunit of cAMP-dependent protein kinase and is phosphorylated by its kinase. J Biol Chem. 2002;277(47):45480–45492. doi: 10.1074/jbc.M206201200. [DOI] [PubMed] [Google Scholar]
- 43.Dymek EE, Smith EF. A conserved CaM- and radial spoke associated complex mediates regulation of flagellar dynein activity. J Cell Biol. 2007;179(3):515–526. doi: 10.1083/jcb.200703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin D, et al. Characterization of Plasmodium falciparum CDP-diacylglycerol synthase, a proteolytically cleaved enzyme. Mol Biochem Parasitol. 2000;110(1):93–105. doi: 10.1016/s0166-6851(00)00260-7. [DOI] [PubMed] [Google Scholar]
- 45.Dyer M, Day KP. Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol Today. 2000;16(3):102–107. doi: 10.1016/s0169-4758(99)01608-7. [DOI] [PubMed] [Google Scholar]
- 46.Shastri S, et al. Plasmodium CDP-DAG synthase: An atypical gene with an essential N-terminal extension. Int J Parasitol. 2010;40(11):1257–1268. doi: 10.1016/j.ijpara.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Painter HJ, Campbell TL, Llinás M. The Apicomplexan AP2 family: Integral factors regulating Plasmodium development. Mol Biochem Parasitol. 2011;176(1):1–7. doi: 10.1016/j.molbiopara.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Mol Microbiol. 2010;75(4):854–863. doi: 10.1111/j.1365-2958.2009.07005.x. [DOI] [PubMed] [Google Scholar]
- 49.Waller KL, et al. Interaction of the exported malaria protein Pf332 with the red blood cell membrane skeleton. Biochim Biophys Acta. 2010;1798(5):861–871. doi: 10.1016/j.bbamem.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovádi J. The tubulin polymerization promoting protein, TPPP/p25. IUBMB Life. 2008;60(9):637–642. doi: 10.1002/iub.112. [DOI] [PubMed] [Google Scholar]
- 51.Dearnley MK, et al. Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. J Cell Sci. 2012;125(Pt 8):2053–2063. doi: 10.1242/jcs.099002. [DOI] [PubMed] [Google Scholar]
- 52.Kono M, et al. Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol Biol Evol. 2012;29(9):2113–2132. doi: 10.1093/molbev/mss081. [DOI] [PubMed] [Google Scholar]
- 53.Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191(7):1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fung C, Beck JR, Robertson SD, Gubbels M-J, Bradley PJ. Toxoplasma ISP4 is a central IMC sub-compartment protein whose localization depends on palmitoylation but not myristoylation. Mol Biochem Parasitol. 2012;184(2):99–108. doi: 10.1016/j.molbiopara.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mons B, Janse CJ, Boorsma EG, Van der Kaay HJ. Synchronized erythrocytic schizogony and gametocytogenesis of Plasmodium berghei in vivo and in vitro. Parasitology. 1985;91(Pt 3):423–430. doi: 10.1017/s0031182000062673. [DOI] [PubMed] [Google Scholar]
- 56.Aingaran M, et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell Microbiol. 2012;14(7):983–993. doi: 10.1111/j.1462-5822.2012.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silvestrini F, et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9(7):1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen JB, Trager W. Plasmodium falciparum in culture: Use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977;63(5):883–886. [PubMed] [Google Scholar]
- 59.Balu B, Shoue DA, Fraser MJ, Jr, Adams JH. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc Natl Acad Sci USA. 2005;102(45):16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418–420. [PubMed] [Google Scholar]
- 61.Ribaut C, et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar J. 2008;7:45. doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nkrumah LJ, et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3(8):615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamoun CB, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96(15):8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saliba KS, Jacobs-Lorena M. Production of Plasmodium falciparum gametocytes in vitro. In: Ménard R, editor. Malaria Methods in Molecular Biology. Vol 923. Heidelberg: Springer; 2013. pp. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]