Abstract
Rheumatoid arthritis (RA) is an inflammatory disease in which interleukin 17 (IL-17)-producing T helper 17 (TH17) cells have been critically involved. We show that in patients with RA, the expression of a multifunctional regulator β-arrestin1 was significantly up-regulated in peripheral and synovial CD4+ T cells, which correlated well with active phases of RA. In collagen-induced arthritis, deficiency of β-arrestin1 ameliorated disease with decreased TH17 cell differentiation, proinflammatory cytokine production, synovitis, and cartilage and bone destruction. Further mechanistic study reveals that β-arrestin1 promoted signal transducer and activator of transcription 3 (STAT3) activation required for TH17 cell differentiation through scaffolding the interaction of Janus kinase 1 and STAT3. These findings indicate a critical role for β-arrestin1 in the pathogenesis of collagen-induced arthritis and TH17 cell differentiation and suggest β-arrestin1 as a potential diagnostic biomarker and therapeutic target for RA.
Inflammation has been known to be an essential immune response that can be initiated upon infection or injury to maintain tissue homeostasis (1). Rheumatoid arthritis (RA) is a chronic, painful, and disabling disease associated with a typical unrestrained inflammation in diarthrodial joints (2). To elucidate the molecular mechanism of RA development, several animal models of arthritis, in particular collagen-induced arthritis (CIA), have been widely used to explore the key inflammatory inducers and mediators by genetic manipulation of specific genes (3, 4). There is strong evidence that CD4+ T-cell–mediated adaptive immunity is involved in the pathogenesis of RA (5). Interleukin 17 (IL-17)–producing T helper 17 (TH17) cells represent a distinct subset of CD4+ T cells that are essential in clearing foreign pathogens, but dysregulation of TH17 cells would induce tissue inflammation in a variety of inflammatory conditions including RA (3, 6). The current understandings of TH17 cells involvement in RA have been mostly drawn from murine models, whereas less is known about those in human pathologic conditions.
Signal transducer and activator of transcription 3 (STAT3) is one of the nuclear transcription factors from a highly conserved family, which has been involved in various biological processes, including immune response (7). Upon cytokines, such as IL-6, binding to their receptors, STAT3 is associated with and then activated by Janus kinases (JAKs) through phosphorylation at tyrosine residue 705 (Tyr705), and activated STAT3 regulates its target genes transcription and CD4+ T-cell lineage commitment (8). Because IL-6, in combination with TGF-β, drives TH17 cell differentiation, depletion of STAT3 greatly impairs this process (9–11). Several STAT3-interacting proteins, such as PIAS3, GRIM-19, and Rac1, have been reported to regulate STAT3 activation (12–14). However, it remains unknown whether any of those regulators functions in TH17 cells or is under control by any physiological or pathological signal.
β-Arrestins are multifunctional proteins that play critical roles in G protein-coupled receptor (GPCR) signaling (15). Emerging observations reveal that they could also act as essential adaptors to modulate many other signaling pathways (16). A previous report shows that β-arrestin1 serves as an adaptor to bring the oncoprotein E3 ubiquitin ligase MDM2 to the activated insulin-like growth factor-1 (IGF1) receptor, thereby promoting receptor ubiquitination and subsequent proteasomal degradation (17). In response to GPCR stimulation, β-arrestin1 regulates histone H4 acetylation and contributes to CD4+ T-cell survival (18). Interestingly, a microRNA, miR-326, encoded by the first intron of β-arrestin1 gene, regulates TH17 cell differentiation, and its expression level is associated with the pathogenesis of multiple sclerosis (MS) (19). Here, we identify β-arrestin1 as a critical regulator in the pathogenesis of CIA and TH17 cell differentiation. β-arrestin1 exerts its function through scaffolding the interaction of Janus kinase 1 (JAK1) and STAT3, thereby promoting STAT3 activation. Thus, our findings suggest β-arrestin1 as a potential diagnostic biomarker and therapeutic target for RA.
Results
Expression of β-Arrestin1 Is Up-Regulated in Patients with Active RA and Mice with CIA.
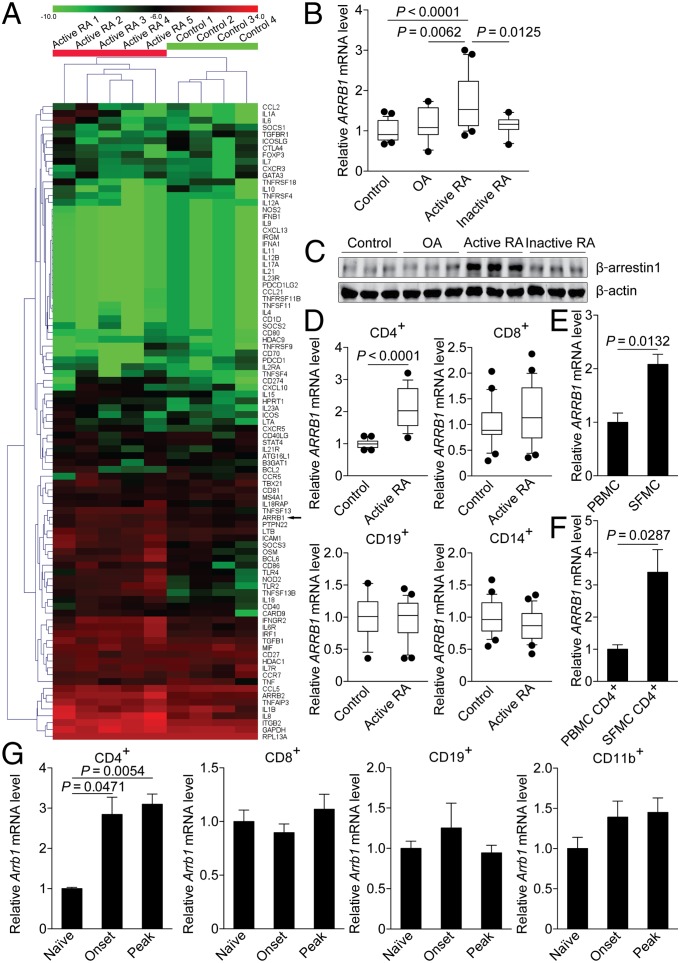
In an attempt to identify genes that are differentially expressed in peripheral blood mononuclear cells (PBMC) from patients with active RA and age-matched normal controls, we performed a custom PCR array containing a panel of immune response genes. Among the 93 genes evaluated, the expression of a subgroup of genes was up-regulated in patients with active RA, including ARRB1 (Fig. 1A). We further confirmed the increased expression of ARRB1 in PBMC from patients with active RA, compared with normal controls, patients with osteoarthritis (OA), or inactive RA (Fig. 1B). The expression of β-arrestin1 was also up-regulated in patients with active RA at the protein level (Fig. 1C). Next, we examined the expression of ARRB1 in purified CD4+ T cells, CD8+ T cells, CD19+ B cells, or CD14+ monocytes from patients with active RA and normal controls, respectively. Noticeably, the increased expression of ARRB1 occurred predominantly in CD4+ T cells, but not in CD8+ T cells, CD19+ B cells, or CD14+ monocytes (Fig. 1D). The expression of ARRB1 was comparable in CD4+ T cells from normal controls, patients with OA, and inactive RA (Fig. S1). Moreover, the expression of ARRB1, in either synovial fluid mononuclear cells (SFMC) or purified CD4+ T cells from SFMC, was even higher than that in PBMC or purified CD4+ T cells from PBMC (Fig. 1 E and F).
Fig. 1.
Expression of β-arrestin1 is up-regulated in CD4+ T cells from patients with active RA and mice with CIA. (A) Heat map of a custom PCR array comparing gene expression in PBMC from normal controls (n = 4) or patients with active RA (n = 5). (B) Expression of ARRB1 in PBMC from normal controls (n = 26), patients with OA (n = 16), active RA (n = 20), or inactive RA (n = 10). Boxes = 25–75 percentiles. Lines in center of box, median. Whiskers = 10–90 percentiles. Dot, outliers. Results are normalized to expression of the housekeeping gene RPL13A. (C) Immunoblot of β-arrestin1 in PBMC from normal controls (n = 3), patients with OA (n = 3), active RA (n = 3), or inactive RA (n = 3). (D) Quantitative RT-PCR of ARRB1 mRNA in CD4+ T cells (Upper Left), CD8+ T cells (Upper Right), CD19+ B cells (Lower Left), or CD14+ monocytes (Lower Right) from normal controls (n = 17) or patients with active RA (n = 18). Boxes = 25–75 percentiles. Lines in center of box, median. Whiskers = 10–90 percentiles. Dot, outliers. (E and F) Quantitative RT-PCR of ARRB1 mRNA in PBMC or SFMC (E) and CD4+ T cells from PBMC or SFMC (F) from patients with active RA (n = 3). (G) Expression of Arrb1 in CD4+ T cells (Left), CD8+ T cells (Center Left), CD19+ B cells (Center Right), or CD11b+ myeloid cells (Right) from naïve mice or mice with CIA (onset or peak) (n = 4 per group). Results are normalized to expression of the housekeeping gene Hprt.
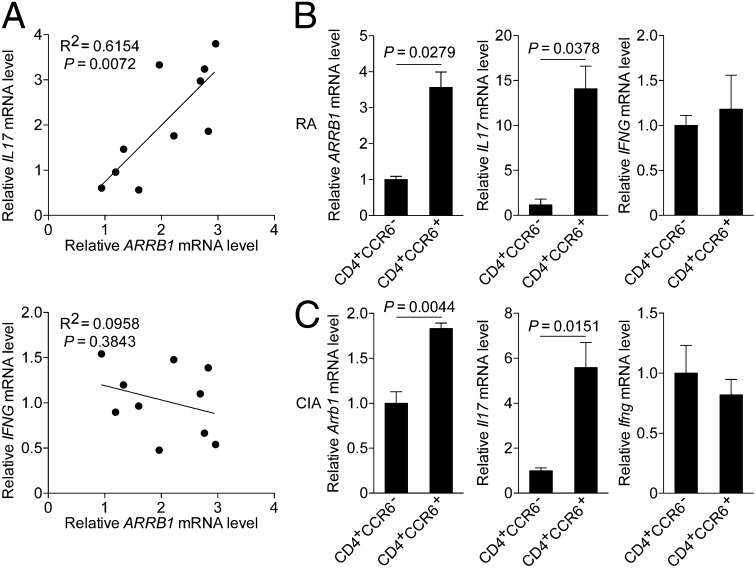
Interestingly, linear correlation analysis between transcripts of β-arrestin1 and the cytokines showed that the expression of IL17 correlated well with that of ARRB1 (Fig. 2A and Fig. S2). Furthermore, ARRB1 was highly expressed in CD4+CCR6+ T cells (Fig. 2B), a subpopulation enriched for TH17 cells (20–22). Although CCR6 was also expressed on TH1 cells (20, 21), the expression of IFNG was similar in CD4+CCR6− and CD4+CCR6+ T cells (Fig. 2B). Collectively, these data show that the expression of β-arrestin1 is up-regulated in CD4+ T cells, particularly in CD4+CCR6+ T cells, suggesting it as a potential diagnostic biomarker for RA.
Fig. 2.
Expression of β-arrestin1 correlates with that of IL-17 in CD4+ T cells. (A) Linear correlation analysis between transcripts of β-arrestin1 and IL-17 (Upper) and β-arrestin1 and IFN-γ (Lower) in CD4+ T cells from patients with active RA (n = 10). (B) Expression of ARRB1, IL17, or IFNG in CD4+CCR6− or CD4+CCR6+ T cells from patients with active RA (n = 5). (C) Expression of Arrb1, Il17, or Ifng in CD4+CCR6− or CD4+CCR6+ T cells from mice with CIA (n = 4).
To further explore the expression profile of β-arrestin1 in the development of RA, we induced CIA on C57BL/6 mice as reported (4). Consistent with the data obtained from clinical samples, the expression of Arrb1 was up-regulated in sorted CD4+ T cells (Fig. 1G and Fig. S3A), especially in CD4+CCR6+ T cells from mice with CIA at the onset and peak of disease (Fig. 2C). The expression pattern of β-arrestin1 was also confirmed at the protein level (Fig. S3B). All above results indicate a potential role of β-arrestin1 in the pathogenesis of CIA.
Deficiency of β-Arrestin1 Ameliorates CIA.
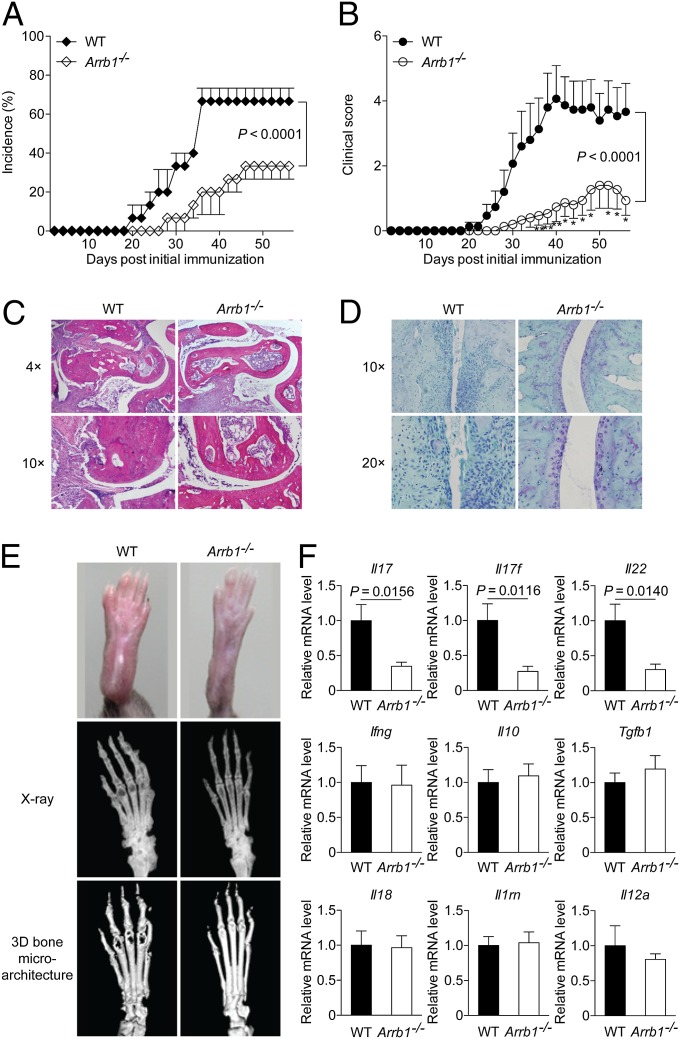
We next examined the clinical and histopathological features of β-arrestin1–deficient (Arrb1−/−) mice in CIA. Although most of the wild-type (WT) littermates developed arthritis, Arrb1−/− mice exhibited significantly reduced incidence of disease (Fig. 3A). The severity of arthritis was also markedly attenuated in Arrb1−/− mice throughout the chronic phase of disease (Fig. 3B). Compared with WT littermates, Arrb1−/− mice displayed much milder joint swelling (Fig. 3E). Hispathological analysis of ankle joints showed a remarkable decrease in synovial inflammation, pannus formation, and cartilage and bone destruction in Arrb1−/− mice (Fig. 3C). The loss of matrix proteoglycan content in the articular cartilage, another hallmark of chronic destructive arthritis, was also decreased in Arrb1−/− mice (Fig. 3D). Further assessment by microscopic computed tomography (micro-CT) showed that Arrb1−/− mice exhibited less severe bone destruction (Fig. 3E). Additionally, the expression of proinflammatory cytokines including Il17, Il17f, and Il22 in the joints was down-regulated in Arrb1−/− mice (Fig. 3F). These observations demonstrate that deficiency of β-arrestin1 ameliorates CIA, further suggesting a proinflammatory role of β-arrestin1.
Fig. 3.
Deficiency of β-arrestin1 ameliorates CIA. (A and B) Incidence (A) and severity (B) of CIA in Arrb1−/− mice and WT littermates (n = 15 per group). *P < 0.05 and **P < 0.01, versus WT littermates. (C and D) Hematoxylin and eosin (H&E) staining (C) and safranin O staining (D) of paraffin sections of ankle joints at day 56 after initial immunization (n = 8 per group). (E) Representative photograph and radiography of the hind paws (n = 8 per group). (F) Expression of various cytokines in the joints of collagen II (CII)-immunized Arrb1−/− mice and WT littermates (n = 8 per group).
Knockout or Knockdown of β-Arrestin1 Reduces the Production of TH17-Associated Cytokines.
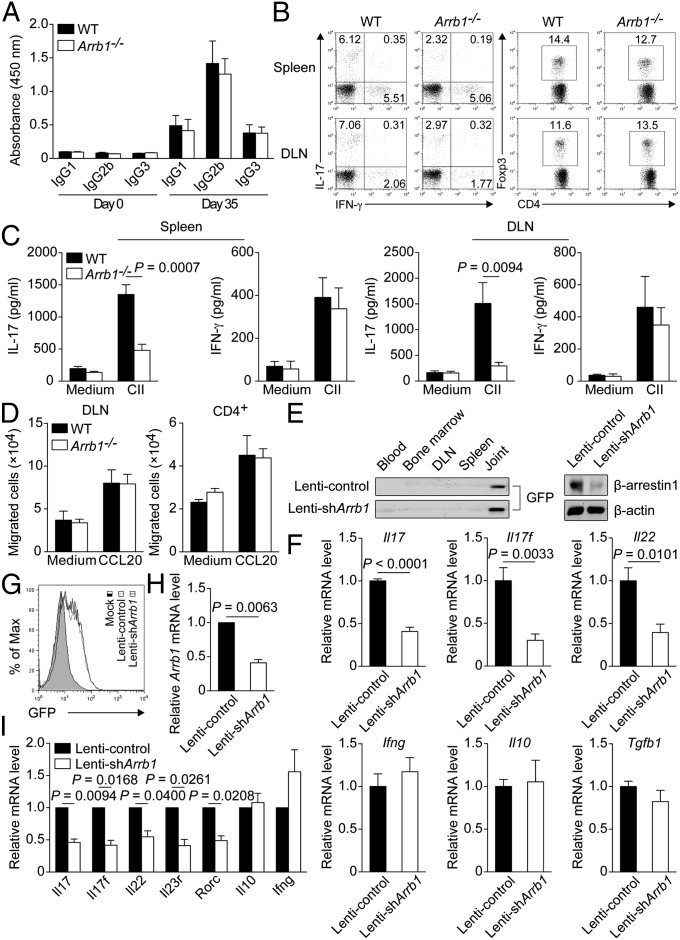
Because both T-cell–mediated and humoral immune responses have been described to be essential in the pathogenesis of CIA, we first examined the serum levels of collagen II (CII)-specific antibodies, which have been suggested to correlate well with the severity of CIA (23, 24). Circulating levels of CII-specific IgG1, IgG2b, and IgG3 were comparably elevated in Arrb1−/− mice and WT littermates at day 35 after initial immunization (Fig. 4A). We next performed multilineage analysis to evaluate the infiltration of immune cells in the spleen and draining lymph node (DLN). Although the frequency and absolute number of TH1 cells or regulatory T (Treg) cells were similar in both groups of mice, those of TH17 cells were markedly reduced in Arrb1−/− mice (Fig. 4B and Fig. S4A). Concurrently, the absolute number of B220+ cells, CD11b+ cells, or CD11c+ cells was not different between Arrb1−/− mice and WT littermates (Fig. S4B). Furthermore, the production of IL-17, but not of IFN-γ, in the supernatants of cell cultures after in vitro CII challenge, was profoundly decreased in Arrb1−/− mice (Fig. 4C). In addition, chemotaxis assay showed no significant difference in CCL20-mediated migration of DLN cells or CD4+ T cells between Arrb1−/− mice and WT littermates (Fig. 4D and Fig. S5).
Fig. 4.
Knockout or knockdown of β-arrestin1 reduces the production of TH17-associated cytokines. (A) ELISA of CII-specific IgG1, IgG2b, or IgG3 in the serum of Arrb1−/− mice and WT littermates at day 0 and day 35 after initial immunization (n = 7 per group). (B) Flow cytometry of intracellular IL-17, IFN-γ, or Foxp3 in gated CD4+ T cells from the spleen and draining lymph node (DLN) of CII-immunized Arrb1−/− mice and WT littermates (n = 8 per group). (C) Concentration of IL-17 or IFN-γ in the supernatants of cell cultures challenged with CII (100 μg/mL) for 4 d (n = 6 per group). (D) Chemotaxis assay of DLN cells or CD4+ T cells from CII-immunized Arrb1−/− mice and WT littermates (n = 5 per group). (E and F) Immunoblot of GFP or β-arrestin1 (E) and expression of various cytokines (F) in the joints of CII-immunized mice intraarticularly injected with lentivirus expressing GFP and shRNA for Arrb1 (Lenti-shArrb1) or GFP and control shRNA (Lenti-control) (n = 5 per group). (G–I) Flow cytometry of GFP (G) and expression of various genes (H and I) in CD4+CCR6+ T cells from mice with CIA transduced with Lenti-shArrb1 or Lenti-control.
To further confirm the potential role of β-arrestin1 in vivo, we used lentiviral vectors expressing shRNA to silence β-arrestin1 gene for long-lasting suppressive capacity. Local administration of lentivirus expressing GFP and shRNA for Arrb1 (Lenti-shArrb1) into inflamed joints of mice with CIA could effectively reduce the protein level of β-arrestin1, compared with lentivirus expressing GFP and control shRNA (Lenti-control) (Fig. 4E). We then intraarticularly injected Lenti-shArrb1 or Lenti-control into the joints of CII-immunized mice at the onset of disease. Consistent with the data obtained from Arrb1−/− mice and WT littermates, lentivirus-mediated silencing of β-arrestin1 in vivo in the joints led to significant decreased expression of proinflammatory cytokines, including Il17, Il17f, and Il22 (Fig. 4F). Based on the increased expression of Arrb1 in CD4+CCR6+ T cells from mice with CIA, knockdown of β-arrestin1 ex vivo in these cells remarkably reduced the expression of several TH17-associated genes, such as Il17 and Il22 (Fig. 4 G–I), which have been critically involved in CIA (3, 25). Together, these results indicate that knockout or knockdown of β-arrestin1 reduces the production of TH17-associated cytokines.
Depletion of β-Arrestin1 Impairs TH17 Cell Differentiation in Vitro.
To determine whether β-arrestin1 directly regulated TH17 cell differentiation, we performed an in vitro T-cell differentiation assay. We first examined the expression of Arrb1 in CD4+ T-cell subsets and found that Arrb1 was most highly expressed in TH17 cells (Fig. 5A). Bioinformatic analysis revealed a putative binding site for RORγt, the TH17 lineage-specific transcription factor, in the 2-kb region of mouse Arrb1 promoter. In cells expressing a reporter containing the Arrb1 promoter, RORγt substantially enhanced luciferase activity in a dose-dependent manner (Fig. S6), indicating that RORγt may regulate β-arrestin1 expression in TH17 cells. In addition, the up-regulation of Arrb1 expression in TH17 cell differentiation was inhibited by Digoxin (Fig. 5B), a RORγt-specific inhibitor in TH17 cells (26, 27). Further investigation is needed for clarification of β-arrestin1 expression regulated by RORγt.
Fig. 5.
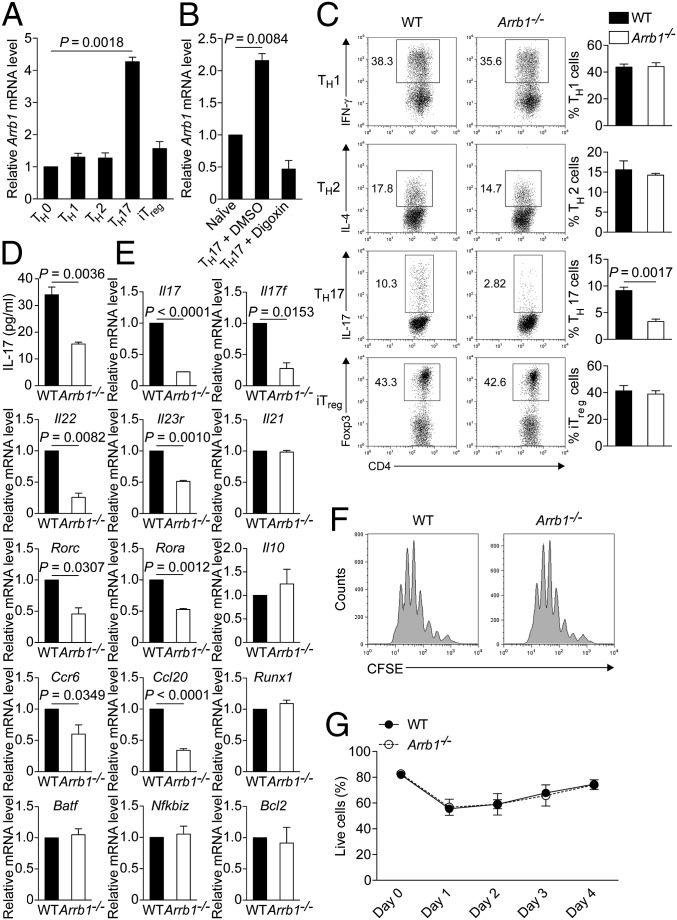
Depletion of β-arrestin1 impairs TH17 cell differentiation in vitro. (A) Expression of Arrb1 in TH0, TH1, TH2, TH17, or inducible Treg (iTreg)-polarized cells from C57BL/6 mice. (B) Quantitative RT-PCR of Arrb1 mRNA in naïve CD4+ T cells or TH17-polarized cells in the presence of DMSO or Digoxin (10 μM). (C) Flow cytometry of naïve CD4+ T cells from Arrb1−/− mice and WT littermates cultured for 4 d under TH1, TH2, TH17, or iTreg-polarizing conditions. Percentage of each subset is shown. (D) Production of IL-17 in the supernatants of TH17-polarized cells. (E) Expression of TH17-associated genes in naïve CD4+ T cells from Arrb1−/− mice and WT littermates cultured for 2 d under TH17-polarizing conditions. (F) Flow cytometry of naïve CD4+ T cells labeled with carboxyfluorescein succinimidyl ester (CFSE) from Arrb1−/− mice and WT littermates cultured for 4 d under TH17-polarizing conditions. (G) Naïve CD4+ T cells were cultured under TH17-polarizing conditions, and live cells were identified by propidium iodide exclusion.
We then assessed TH17 cell differentiation in Arrb1−/− mice and WT littermates. Obviously, the frequency of IL-17–positive cells, Il17 mRNA expression, and IL-17 production were markedly decreased in Arrb1−/− mice (Fig. 5 C–E). However, the frequency of IFN-γ–positive cells, IL-4–positive cells, or Foxp3-positive cells was comparable in Arrb1−/− mice and WT littermates (Fig. 5C), suggesting no significant impact of β-arrestin1 on the differentiation of TH1, TH2, or inducible Treg (iTreg). Besides Il17, the expression of other TH17-associated genes including Rorc, Rora, Il17f, Il22, Il23r, Ccr6, and Ccl20, was also remarkably down-regulated in Arrb1−/− T cells (Fig. 5E and Fig. S7). Our previous report showed that β-arrestin1 regulates CD4+ T-cell survival via Bcl2 (18). Under TH17-polarizing conditions, we observed no apparent difference in proliferation or viability between WT and Arrb1−/− T cells (Fig. 5 F and G and Fig. S8). The expression of Bcl2 was also unchanged in the same conditions (Fig. 5E). These data clearly demonstrate the pivotal role of β-arrestin1 in TH17 cell differentiation.
Interaction of JAK1/β-Arrestin1/STAT3 Regulates STAT3 Activation.
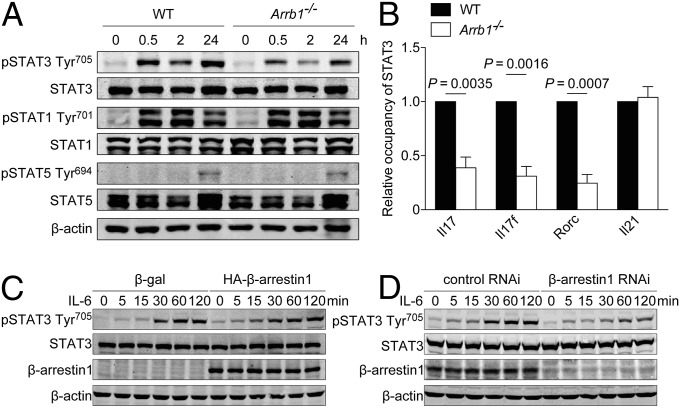
Given the essential role of STAT3 in TH17 cell differentiation (9–11, 28), we next investigated whether loss of β-arrestin1 affected STAT3 Tyr705 phosphorylation, which is required for its activation. Under TH17-polarizing conditions, STAT3 Tyr705 phosphorylation in Arrb1−/− T cells was significantly attenuated (Fig. 6A), resulting in decreased occupancy of STAT3 on the promoter of Il17, Il17f, and Rorc (Fig. 6B). However, STAT1 Tyr701 phosphorylation or STAT5 Tyr694 phosphorylation was not altered under these conditions (Fig. 6A). The effect of β-arrestin1 on STAT3 phosphorylation was further confirmed by overexpression or knockdown of β-arrestin1 (Fig. 6 C and D). In addition, the expression of other transcription factors involved in TH17 cell differentiation, such as Runx1, Batf, and Nfkbiz, was comparable in WT and Arrb1−/− T cells (Fig. 5E).
Fig. 6.
Regulation of STAT3 activation by β-arrestin1. (A) Immunoblot of phosphorylation and expression of STAT3, STAT1, or STAT5 in naïve CD4+ T cells under TH17-polarizing conditions for the indicated times. (B) ChIP assay of the binding of STAT3 to the Il17, Il17f, Rorc, or Il21 promoter in Arrb1−/− mice and WT littermates. (C and D) Immunoblot of phosphorylation and expression of STAT3 in HEK293 cells expressing β-galactosidase (β-gal) or HA–β-arrestin1 (C) and control RNAi or β-arrestin1 RNAi (D).
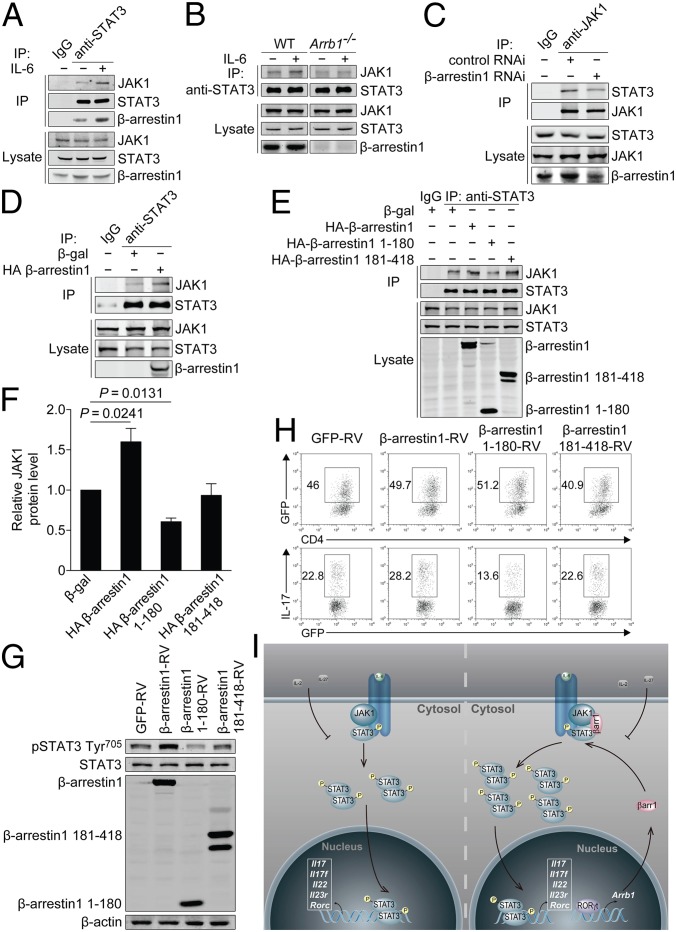
Because β-arrestins have been reported to function as adaptors in various signaling pathways (29, 30), we examined whether a similar scaffolding mechanism was involved in STAT3 activation. In CD4+ T cells from C57BL/6 mice, endogenous β-arrestin1, JAK1, and STAT3 associated with each other (Fig. 7A). Interestingly, administration of IL-6 trigged a remarkable increase in JAK1/STAT3 interaction as well as β-arrestin1/STAT3 interaction (Fig. 7A), and this interaction was further confirmed in TH17-polazired cells (Fig. S9). These results implicate that IL-6 stimulation promotes the JAK1/β-arrestin1/STAT3 interaction. However, the interaction of JAK1 and STAT3 was markedly reduced in β-arrestin1–knockout or –knockdown cells (Fig. 7 B and C) and, conversely, promoted by β-arrestin1 overexpression (Fig. 7D), which suggest that β-arrestin1 is indispensable for promoting the IL-6–induced interaction of JAK1 and STAT3. More importantly, neither β-arrestin1 1–180 (a C-terminal deletion mutant consisting of β-arrestin1 amino acids 1–180) nor β-arrestin1 181–418 (a N-terminal deletion mutant consisting of β-arrestin1 amino acids 181–418) possessed the potential to promote the JAK1/STAT3 interaction (Fig. 7 E and F). Therefore, STAT3 activation and TH17 cell differentiation were unchanged in the presence of both β-arrestin1 mutants (Fig. 7 G and H). Taken together, these findings indicate that β-arrestin1 positively regulates STAT3 activation through scaffolding the JAK1/STAT3 interaction.
Fig. 7.
Interaction of JAK1/β-arrestin1/STAT3 regulates STAT3 activation and TH17 cell differentiation. (A) Endogenous interaction of JAK1/β-arrestin1/STAT3 in naïve CD4+ T cells from C57BL/6 mice. (B) Endogenous interaction of JAK1 and STAT3 in naïve CD4+ T cells from Arrb1−/− mice and WT littermates. (C and D) Endogenous interaction of JAK1 and STAT3 in HEK293 cells expressing control RNAi or β-arrestin1 RNAi (C) or β-gal or HA–β-arrestin1 (D). (E) Endogenous interaction of JAK1 and STAT3 in HEK293 cells expressing β-gal, HA–β-arrestin1, HA–β-arrestin1 1–180, or HA–β-arrestin1 181–418. (F) Densitometric analysis of E. (G) Immunoblot of phosphorylation and expression of STAT3 in naïve CD4+ T cells transduced with β-arrestin1–RV, β-arrestin1 1–180-RV, β-arrestin1 181–418-RV, or GFP-RV under TH17-polarizing conditions. (H) Flow cytometry of intracellular IL-17 in naïve CD4+ T cells transduced with β-arrestin1–RV, β-arrestin1 1–180-RV, β-arrestin1 181–418-RV, or GFP-RV under TH17-polarizing conditions. (I) Schematic model of JAK1/β-arrestin1/STAT3 interaction in TH17 cells. βarr1, β-arrestin1.
Discussion
The essential role of STAT3 in TH17 cell differentiation has been clearly described (9–11, 28). However, the understanding of STAT3 activation remains limited. Our observations have demonstrated that in response to IL-6, β-arrestin1 may function as a scaffold protein to promote the JAK1/STAT3 interaction (Fig. 7I). Subsequently, the phosphorylation of STAT3 by JAK1 is increased, and the expression of STAT3 target genes, particularly a group of TH17-associated genes including Il17, Il17f, Il22, and Rorc, is up-regulated. Although the phosphorylation of STAT3 by JAK1 has been well documented (7, 8), our data indicate that promotion of the JAK1/STAT3 interaction by β-arrestin1 is also critical for STAT3 activation, which provide insights into regulation of STAT3 activation. Collectively, our findings suggest β-arrestin1 as an important regulator of STAT3 activation in TH17 cell differentiation. Interestingly, our preliminary results reveal that RORγt, the TH17 lineage-specific transcription factor, transactivates Arrb1 promoter. This molecular mechanism might account for the selective higher expression of β-arrestin1 in TH17 cells. Therefore, β-arrestin1 possesses the potential to specifically regulate TH17 cell differentiation, but not the differentiation of TH1, TH2, or Treg cells. Because STAT3 regulates the expression of RORγt through binding to its promoter (28), based on our data presented, we propose a feed-forward loop of β-arrestin1–STAT3–RORγt to regulate TH17 cell differentiation. Furthermore, whether any extracellular signal could terminate this loop is of interest. It is noteworthy that IL-2, a growth factor for most T cells, has been reported to inhibit TH17 cell differentiation via STAT5 activation (9). A recent study has confirmed this finding and further proposed the reciprocal actions of STAT3 and STAT5 on the genetic locus encoding IL-17 (31). Likewise, IL-27 has been shown to have antiinflammatory activity through its restraint of TH17 cell differentiation, in a STAT1-dependent manner (32, 33). Thus, IL-2/STAT5 or IL-27/STAT1 axis might restrain this β-arrestin1–STAT3–RORγt loop under certain physiological or pathological conditions.
It has been well documented that β-arrestin1 functions as multiprotein scaffolds to coordinate complex signal transduction networks in diverse cellular processes, including differentiation, cytokine production, proliferation, cell viability, and migration (15, 34). It could be of interest to examine whether β-arrestin1 regulates the above events in TH17 cells. Our observations show that (i) depletion of β-arrestin1 impairs in vitro TH17 cell differentiation; (ii) knockout or knockdown of β-arrestin1 reduces the production of TH17-associated cytokines in vivo; (iii) proliferation of CFSE-labeled cells is not altered by loss of β-arrestin1; (iv) viability of TH17 cells is unchanged in the absence of β-arrestin1; and (v) deletion of β-arrestin1 does not affect CCL20-mediated migration. Together, these observations suggest that β-arrestin1 could selectively be involved in TH17 cell differentiation and production of TH17-associated cytokines.
Besides TH17 cells, fibroblast-like synoviocytes have also been reported to be involved in the pathogenesis of CIA (2). Recent reports show that β-arrestin1 is up-regulated in fibroblast-like synoviocytes, the primary effector of cartilage destruction and inflammatory pathogenesis in CIA (35, 36). Because β-arrestin1 is reported to play an important role in fibroblast invasion and pulmonary fibrosis (34), further investigation is needed to clarify the role of β-arrestin1 in fibroblast-like synoviocytes.
Our investigation on clinical samples demonstrates that the expression of β-arrestin1 is significantly up-regulated in peripheral or synovial CD4+ T cells from patients with active RA, and it correlates well with the active phases of RA. These data are consistent with our previous finding that β-arrestin1 expression is remarkably up-regulated in peripheral CD4+ T cells from patients with relapsing-remitting MS (18). Our preliminary results indicate that β-arrestin1 expression seems to be up-regulated also in CD4+ T cells from patients with inflammatory bowel disease (IBD). Taken together, all of the evidence accumulated so far indicate the functional involvement of β-arrestin1 in many inflammatory and autoimmune diseases. Although it is not exactly known at this point how β-arrestin1 is extensively involved, it could be further explored as a potential diagnostic biomarker or even a prognostic indicator for those diseases. Furthermore, silencing of β-arrestin1 either in joints or in CD4+CCR6+ T cells from mice with CIA results in decreased production of proinflammatory cytokines. These results suggest that β-arrestin1 might serve as a potential therapeutic target for RA. It is noticeable that the expression of β-arrestin1 in SFMC is markedly higher than that in PBMC. This increased expression of β-arrestin1 in specific lesions may also occur in other inflammatory and autoimmune diseases, such as MS and IBD. Given that MS has been characterized as a central nervous system (CNS) disorder and IBD has been classified as an intestinal disease, tissue-specific targeting of β-arrestin1 in individual disease is necessary, although β-arrestin1 expression in those lesions remains to be further explored.
Materials and Methods
Study Subjects.
Volunteer subjects included patients from Renji Hospital of Shanghai Jiaotong University, who fulfilled the American College of Rheumatology (ACR) classification criteria for RA, and age-matched healthy volunteers from personnel of Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences). Blood and synovial fluid samples were obtained from subjects after informed-consent procedures, and the sampling was completed in accordance with the guidelines of the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine.
Mice.
C57BL/6 mice were obtained from Shanghai Laboratory Animal Center (Chinese Academy of Sciences). Arrb1−/− mice on a C57BL/6 background were provided by Robert J. Lefkowitz (Duke University Medical Center, Durham, NC). All mice were maintained in pathogen-free conditions. Animal care and use were in accordance with the guidelines of the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Statistics.
Data are presented as mean ± SEM. A two-tailed Student t test was used to compare two groups, or where appropriate, the two-way analysis of variance followed by Bonferroni post hoc test for multiple comparisons and a Mann–Whitney test for nonparametric data (CIA scoring). For all tests, a P value of 0.05 or less was considered statistically significant.
Details of T-cell differentiation, induction of CIA, histopathology, ELISA, flow cytometry, immunoprecipitation and immunoblot, and ChIP are provided in SI Materials and Methods and Tables S1 and S2.
Supplementary Material
Acknowledgments
We thank Robert J. Lefkowitz (Duke University Medical Center) for Arrb1−/− mice; Biao Zheng (GlaxoSmithKline Research and Development Center) for technical assistance on CIA induction; Dan R. Littman (New York University) for plasmid FLAG-RORγt; Dangsheng Li (Shanghai Institutes for Biological Sciences), Jiuhong Kang (Tongji University), and Jian Zhao for helpful discussions; and Wenjing Li, Shunmei Xin, Hui Xu, and Xianglu Zeng for technical assistance. We also thank all laboratory members for sharing reagents and advice. This research was supported by Chinese Academy of Sciences (XDA01010302), Ministry of Science and Technology (2011CB946102), and Ministry of Health (2012BAI10B03).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221608110/-/DCSupplemental.
References
- 1.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140(6):771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 4.Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57BL/6 (H-2b) mice: New insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30(6):1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Toh ML, Miossec P. The role of T cells in rheumatoid arthritis: New subsets and new targets. Curr Opin Rheumatol. 2007;19(3):284–288. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 6.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 7.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 8.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 11.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 12.Chung CD, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278(5344):1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 13.Simon AR, et al. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290(5489):144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- 14.Lufei C, et al. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J. 2003;22(6):1325–1335. doi: 10.1093/emboj/cdg135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol Cell. 2006;24(5):643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Girnita L, et al. beta-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280(26):24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8(8):817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 19.Du CS, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 20.van Hamburg JP, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63(1):73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 22.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki N, et al. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988;140(5):1477–1484. [PubMed] [Google Scholar]
- 24.Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154(3):688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geboes L, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60(2):390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 26.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472(7344):486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita-Sato S, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286(36):31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32(5):605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Luan B, et al. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457(7233):1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 31.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 33.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 34.Lovgren AK, et al. β-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3(74):74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li P, et al. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: Isoform specific regulation of inflammation. Mol Immunol. 2011;49(1-2):64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang QT, Zhang LL, Wu HX, Wei W. The expression change of β-arrestins in fibroblast-like synoviocytes from rats with collagen-induced arthritis and the effect of total glucosides of paeony. J Ethnopharmacol. 2011;133(2):511–516. doi: 10.1016/j.jep.2010.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.