Abstract
Biogenesis of iron–sulfur cluster proteins is a highly regulated process that requires complex protein machineries. In the cytosolic iron–sulfur protein assembly machinery, two human key proteins—NADPH-dependent diflavin oxidoreductase 1 (Ndor1) and anamorsin—form a stable complex in vivo that was proposed to provide electrons for assembling cytosolic iron–sulfur cluster proteins. The Ndor1–anamorsin interaction was also suggested to be implicated in the regulation of cell survival/death mechanisms. In the present work we unravel the molecular basis of recognition between Ndor1 and anamorsin and of the electron transfer process. This is based on the structural characterization of the two partner proteins, the investigation of the electron transfer process, and the identification of those protein regions involved in complex formation and those involved in electron transfer. We found that an unstructured region of anamorsin is essential for the formation of a specific and stable protein complex with Ndor1, whereas the C-terminal region of anamorsin, containing the [2Fe-2S] redox center, transiently interacts through complementary charged residues with the FMN-binding site region of Ndor1 to perform electron transfer. Our results propose a molecular model of the electron transfer process that is crucial for understanding the functional role of this interaction in human cells.
Keywords: Fe/S protein maturation, CIAPIN1 domain, diflavin reductase
Iron–sulfur clusters (ISCs) are ancient inorganic cofactors that are crucial for many protein functions in eukaryotic and bacterial cells (1–3). The clusters are composed of inorganic sulfide and ferric/ferrous iron atoms, the latter being preferentially coordinated by cysteinyl residues (4–6). Because inorganic sulfide and ferrous/ferric iron atoms are toxic in vivo, biosynthesis of ISC proteins within cells is a highly regulated process that requires complex protein machineries for the mobilization of Fe and S atoms from appropriate sources, for their assembly into ISC forms and their final delivery to the recipient proteins (7–9). Three distinct protein machineries are operative and essential in the (nonplant) eukaryotic cells for the biogenesis of ISC proteins: (i) the ISC assembly machinery in the mitochondrial matrix, (ii) the ISC export machinery located in the mitochondrial intermembrane space, and (iii) the cytosolic iron–sulfur protein assembly (CIA) machinery.
The CIA machinery comprises several proteins (10, 11), among which one named Dre2 has been recently identified in yeast (12). The C-terminal domain of Dre2 (residues 228–348) is able to bind two ISCs, a [2Fe-2S] and a [4Fe-4S] (12, 13). The [2Fe-2S] cluster of Dre2 receives electrons from a cytosolic diflavin reductase, termed Tah18 (13), which contains a FAD and a FMN prosthetic group, respectively, bound in two distinct structural domains, to accept electrons from NADPH (14). Dre2 and Tah18 are protein partners forming a stable complex in vivo (13, 15). The C-terminal region of Dre2 (residues 173–348) is fundamental to form, both in vivo and in vitro, the stable complex with Tah18 (16). The Tah18-Dre2 interaction is essential for yeast viability and it has been implicated in cellular death regulation mechanisms (16). This complex was proposed to provide electrons necessary for the CIA machinery (13) and for assembling the diferric tyrosyl radical cofactor of ribonucleotide reductase, Rnr2 (17). However, the molecular basis of these processes has not been elucidated. The electron transfer process observed in yeast is possibly conserved in humans as the functional orthologs, NADPH-dependent diflavin oxidoreductase 1 (Ndor1) and anamorsin, form a stable complex in vivo that can functionally replace the Tah18-Dre2 complex in yeast cells (13, 15). Similar to Dre2 but with significant differences in the number of residues, anamorsin contains two domains (an N-terminal domain of 172 residues and a C-terminal domain of 90 residues, named cytokine-induced apoptosis inhibitor 1 (CIAPIN1) hereafter, which contains two highly conserved cysteine rich motifs, CX8CX2CXC and CX2CX7CX2C) connected by a linker of 51 residues (18). Recently, we reported the solution structure of the well-folded N-terminal domain of anamorsin [Protein Data Bank (PDB) ID: 2LD4] and showed that the CIAPIN1 domain of anamorsin, at variance with Dre2, does not bind a [4Fe-4S] cluster but binds a [2Fe-2S] cluster through the CX8CX2CXC motif of CIAPIN1 (18). Recent EPR data also support the presence of only the [2Fe-2S] cluster(s) in Dre2 (19).
Like yeast Tah18, human Ndor1 belongs to the family of diflavin reductases and consists of two domains: the first binds FMN (FMN-binding domain, hereafter), and the second binds FAD and NADPH (FAD-binding domain, hereafter). On the basis of the well-known electron transfer mechanism occurring in diflavin reductase enzymes (14) (i.e., electrons are transferred from NADPH to FAD and then to FMN, which serves as a donor for one-electron terminal acceptors), the FMN-binding domain is the part of Ndor1 directly interacting with the terminal electron acceptor anamorsin. On this basis, the FMN-binding domain of Ndor1 has been used within this study.
In the present work we unravel the molecular basis of the recognition and of the electron transfer process between Ndor1 and anamorsin. This is based on the structural characterization of the FMN-binding domain of Ndor1 and of the C-terminal region (linker and CIAPIN1 domain) of anamorsin containing the [2Fe-2S] cluster in the oxidized state ([2Fe-2S]-anamorsin, hereafter) and on the identification of the protein regions between [2Fe-2S]-anamorsin and the FMN-binding domain of Ndor1 responsible for the formation of their stable complex and of those regions interacting in the electron transfer process. The molecular model of the electron transfer process proposed here provides significant information on the functional processes in which the anamorsin–Ndor1 interaction has been implicated, i.e., the assembly of ISCs (13) and diferric (17) proteins and the regulation of cell survival/death mechanisms (15, 16).
Results
Structural Characterization of the FMN-Binding Domain of Ndor1.
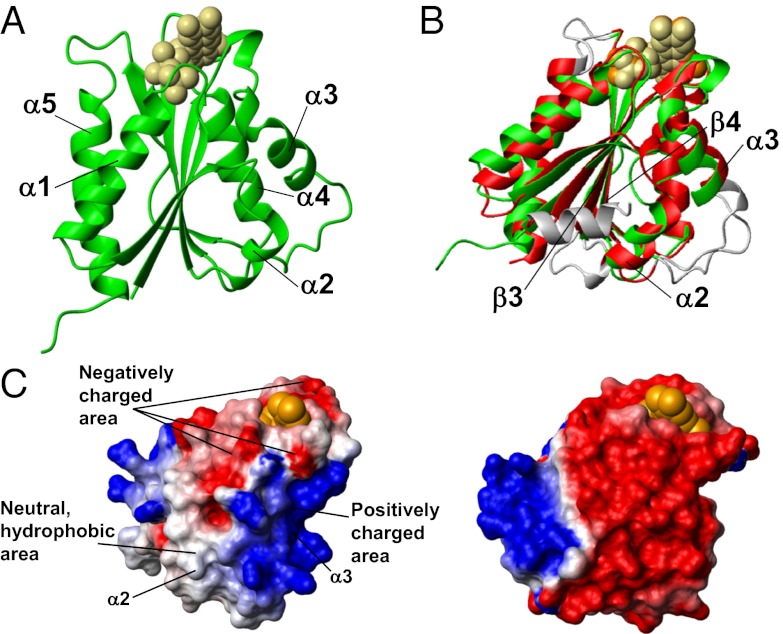
The crystal structure of the FMN-binding domain of Ndor1 (FMN-Ndor1 hereafter) shows the classical fold of FMN-binding domains of diflavin reductases (20, 21) and consists of a wound α-β-α fold with five parallel β-strands (the fifth strand divided in two short β-strands with a gap of four residues) in the core of the molecule flanked by two helices on one side (α1 and α5) and three (α2, α3, and α4) on the other (Fig. 1A and Table S1). This structural organization is fully maintained in solution (SI Materials and Methods). A search for structurally related proteins using the Dali server identifies the FMN-binding domain of the diflavin reductase NADPH-cytochrome P450 reductase (FMN-CytP450r hereafter, PDB ID 1B1C, Z-score of 23.9, rmsd 1.5 Å) as the closest structural homolog in the human proteome. With the exception of the absence, in FMN-Ndor1, of a N-terminal helix, which is spatially close to helix α2 in FMN-CytP450r, the α-helices and β-strands involve a similar number of residues (Fig. 1B). On the contrary, the loops show more variability, in the main affecting two spatially close loops connecting helix α2 and strand β3 and helix α3 and strand β4, respectively (Fig. 1B). These loops in FMN-Ndor1 are, respectively, shorter and longer than those in FMN-CytP450r, thereby making helix α2 and helix α3 shorter by two residues in FMN-Ndor1 (Fig. 1B). Although structurally very similar, FMN-Ndor1 and FMN-CytP450r are quite different in terms of charged residues and charge surface distribution. The majority of these differences are located in helices α2 and α3, which are close to each other on the same face of the β-sheet. They are both negatively charged in FMN-CytP450r whereas they are, respectively, neutral and positively charged in FMN-Ndor1 (Fig. 1C). A negative electrostatic potential around the FMN-binding site is still maintained in FMN-Ndor1, even if to a lesser extent than that in FMN-CytP450r (Fig. 1C).
Fig. 1.
Structure of the FMN-binding domain of Ndor1. (A) Ribbon representation of the crystal structure of the FMN-binding domain of Ndor1. FMN is shown as beige spheres. (B) Superimposition of the crystal structures of the FMN-binding domains of Ndor1 (green, FMN in beige spheres) and of the human NADPH-cytochrome P450 reductase (PDB ID: 1B1C) (red, FMN in orange spheres). The regions with structural variations between the two proteins are shown in gray. (C) Molecular surface of the FMN-binding domain of Ndor1 (Left) and of the human NADPH-cytochrome P450 reductase (Right) colored according to their electrostatic potential. The views are equivalent in terms of the orientation of the protein backbone. The colors of the molecular surface indicate positive (blue), neutral (white), and negative (red) electrostatic potentials. The FMN molecule is shown as orange spheres.
Structural Characterization of the C-Terminal Region of [2Fe-2S]-Anamorsin.
The C-terminal region of [2Fe-2S]-anamorsin, which includes the linker (residues 173–223) and the CIAPIN1 domain (residues 224–312) coordinating an oxidized, paramagnetic [2Fe-2S]2+ cluster (18) was structurally characterized through solution NMR. The linker does not show any specific secondary structural element with the exception of the short hydrophobic stretch 192–195 showing some α-helical secondary structure propensity (Fig. S1). The simultaneous occurrence of paramagnetic effects on resonance linewidths, of poor chemical shifts dispersion of the NMR signals, and of extensive resonance overlap made the structural characterization of the CIAPIN1 domain in full-length anamorsin essentially impossible. Therefore, we cloned and produced it as an independent domain in a construct reported as isoform 2 in the UniProt database (residues 205–312, CIAPIN1-single hereafter). The electrospray ionization (ESI)-MS, UV/visible, and EPR spectra of CIAPIN1-single showed the same spectroscopic features for the [2Fe-2S] cluster as those in [2Fe-2S]-anamorsin (Fig. S2 and SI Materials and Methods), indicating that CIAPIN1-single maintains the same coordination and electronic properties of the [2Fe-2S] cluster bound in the full-length protein. The circular dichroism spectrum of [2Fe-2S]-CIAPIN1-single (Fig. S3 and SI Materials and Methods) shows that the protein adopts conformations typically found in unfolded proteins with a little α-helical content. In the 1H-15N heteronuclear single quantum coherence (HSQC) spectrum of the [2Fe-2S]-CIAPIN1-single (Fig. S3) cross-peaks are highly crowded in the spectral region between 8 and 8.5 ppm and well superimposable to the corresponding cross-peaks of the 1H-15N HSQC spectrum of [2Fe-2S]-anamorsin. These data indicate that the CIAPIN1 domain also in the full-length anamorsin is largely unstructured with no large tertiary structure organization and that there are no specific interactions of the CIAPIN1 domain with the linker and the N-terminal domain. Close to zero or negative 15N{1H}-NOE values, typical of unstructured proteins, as well as R2/R1 ratios lower than expected for a globular protein of the CIAPIN1-single size, are found along the amino acid sequence of [2Fe-2S]-CIAPIN1-single, with the exception of some residues in the CX2CX7CX2C motif that have positive 15N{1H}-NOE values, indicating their tendency to adopt a partially defined conformation, but still not characterized by a stable 3D structure (Fig. S3). It can be concluded from the available chemical shift values that residues 254–264, a stretch that connects the two cysteine motifs, residues 229–235, which precede the metal-binding motif, and some residues located in the CX2CX7CX2C motif, have a propensity to be in an α-helical conformation (Fig. S3), whereas all of the other residues have essentially a random coil conformation, in full agreement with the low α-helical content observed in the circular dichroism spectrum.
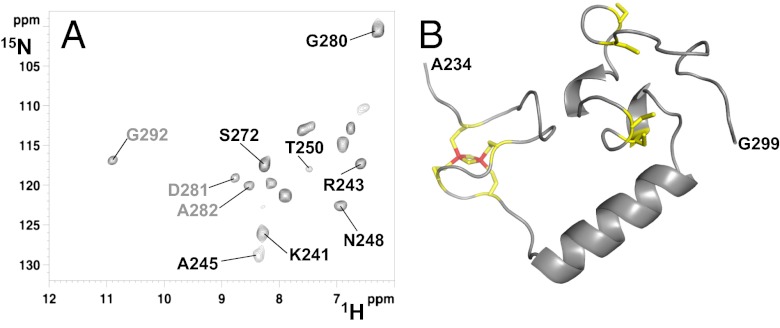
Backbone NHs affected by fast, paramagnetic relaxation properties were identified through a 1H-15N inversion recovery filtered HSQC experiment observed in antiphase (IR-HSQC-AP) specifically designed to identify scalar couplings between fast relaxing 1H resonances. Thirteen backbone NH resonances, 10 of which were lost in standard diamagnetic 1H-15N HSQC experiments, were detected with this experiment (Fig. 2A). 1H- and 13C-detected triple resonance experiments tailored to assign fast relaxing 1H, 15N, and 13C resonances were also performed (22–25). An assignment can be proposed for 7 of the 10 NH resonances observed only in the 1H-15N IR-HSQC-AP experiment (Fig. 2A) and for 7 of the 11 Cα and CO resonances observed only in the 13C-detected paramagnetic experiments. These fast relaxing, assigned residues are located in the regions encompassing both CX8CX2CXC and CX2CX7CX2C motifs. Their 1H and 13C longitudinal relaxation rates were measured, providing precious information on the relative position of these residues with respect to the [2Fe-2S] cluster. A structural model of [2Fe-2S]-CIAPIN1-single was calculated using 20 distance restraints that define the [2Fe-2S] cluster binding to the protein, 17 paramagnetic-based distance restraints, obtained from longitudinal 1HN and 13Cα relaxation rates, and 51 dihedral angle restraints (see SI Materials and Methods for details). This structural model was then subjected to an unrestrained molecular dynamics (MD) simulation of 100 ns to explore possible, further conformations. The MD simulation showed that the [2Fe-2S]-cluster–bound CX8CX2CXC region and the CX2CX7CX2C region sample a restricted range of conformations that are close in space and with an α-helix stably formed between them (residues 254–268, Fig. 2B), in full agreement with the backbone diamagnetic NMR chemical shifts (Fig. S3) and the paramagnetic longitudinal relaxation rates.
Fig. 2.
Structural characterization of [2Fe-2S]-CIAPIN1-single. (A) 1H-15N IR-HSQC-AP spectrum, optimized to detect fast relaxing 1H resonances, showing 13 backbone NH resonances of the [2Fe-2S]-CIAPIN1-single, 10 of which (in black) were completely lost in the standard diamagnetic 1H-15N HSQC experiment (the three residues also detected in the diamagnetic 1H-15N HSQC are shown in gray. (B) Representative model of the CIAPIN1 domain of anamorsin derived from a molecular dynamics simulation of 100 ns. The [2Fe-2S] cluster bound to the CX8CX2CXC motif is shown; the four cysteines coordinating the [2Fe-2S] cluster and the four cysteines of the CX2CX7CX2C motif are shown.
Molecular Recognition and Electron Transfer Between the FMN-Binding Domain of Ndor1 and [2Fe-2S]-Anamorsin.
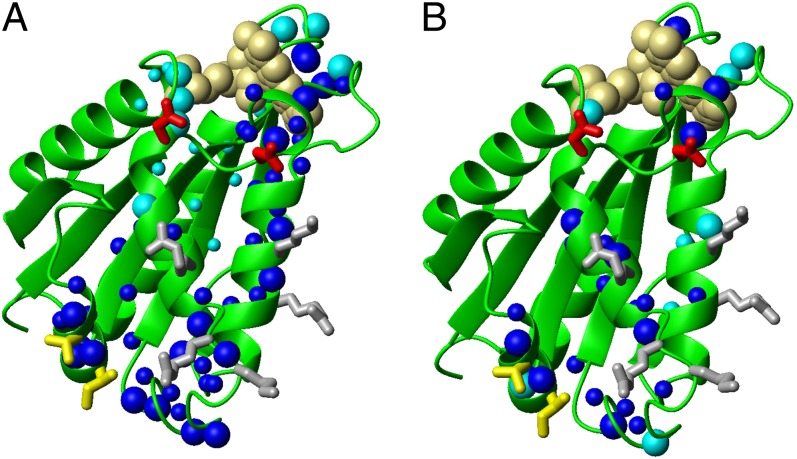
When 15N-labeled oxidized FMN-Ndor1 is titrated with unlabeled oxidized [2Fe-2S]-anamorsin, the 1H-15N HSQC spectra are drastically affected, indicating that the two proteins interact. The majority of the NH cross-peaks broaden beyond detection upon protein addition, with the exception of the last 15 residues of the C-terminal unstructured tail of FMN-Ndor1 that remain unaffected in the 1H-15N HSQC maps (Fig. S4), indicating that they are not involved in the interaction and still reorient faster than the overall protein tumbling rate. The majority of the NH signals can be recovered in a transverse relaxation optimized spectroscopy (TROSY)-type 1H-15N HSQC experiment, which can detect signals with broader linewidths (Fig. 3A). These NMR data indicate the formation of a tight, large molecular mass complex, which is in slow exchange, on the NMR timescale, with the isolated proteins. The chemical shift variations observed in the 1H-15N HSQC spectra of the complex vs. FMN-Ndor1, mapped on the structure of the FMN-binding domain, indicated a well defined protein–protein recognition region (Fig. 4A). Specifically, the major spectral changes are observed on the spatially close helices α2, α3, and α4 and on the two loops following helices α2 and α3, described above as structurally different from FMN-CytP450r (Fig. 4A). Smaller spectral changes are also observed for several residues in the four loops surrounding the FMN moiety (Fig. 4A). The residues directly involved in protein–protein recognition, based on their large chemical shift changes as well as their high solvent accessibility (relative solvent accessibility above 50%), identify three main areas characterized by negatively charged, positively charged, and hydrophobic residues, respectively (Figs. 1C and 4A), with the negatively charged area surrounding only the FMN-binding site.
Fig. 3.
Protein–protein interaction between the FMN-binding domain of Ndor1 and [2Fe-2S]-anamorsin as characterized by NMR. (A) TROSY 1H-15N HSQC at 308 K, acquired at 900 MHz, of the 15N-labeled oxidized FMN domain (construct 1–174 aa) before (red) and after (black) the addition of 1 eq of unlabeled oxidized [2Fe-2S]-anamorsin. (B) Overlay of 1H-15N HSQC spectra of 15N-labeled oxidized FMN-binding domain at 298 K, acquired at 800 MHz, before (black) and after (red) addition of 1 eq of [2Fe-2S]-CIAPIN1-single. (Inset) Chemical shift variations of selected FMN-binding domain residues upon addition of increasing amounts of [2Fe-2S]-CIAPIN1-single (0%, 50%, and 100%).
Fig. 4.
Mapping the binding interface of the FMN-binding domain of Ndor1 interacting with [2Fe-2S]-anamorsin. (A and B) Ribbon representations of the FMN-binding domain showing as spheres backbone NHs of the residues experiencing chemical shift variations upon interaction with (A) [2Fe-2S]-anamorsin and (B) [2Fe-2S]-CIAPIN1-single. Large blue spheres, residues showing large chemical shift changes and characterized by relative solvent accessibility above 50%; small blue spheres, residues showing large chemical shift changes but featuring relative solvent accessibility lower than 50%; large cyan spheres, residues showing small chemical shift changes with concomitant broadening effects and with relative solvent accessibility above 50%; small cyan spheres, residues showing small chemical shift changes with concomitant broadening effects and with relative solvent accessibility lower than 50%. Positively charged (gray), negatively charged (red), and hydrophobic (yellow) side chains which are highly solvent exposed in the interacting region are indicated.
When analyzing the interaction from the anamorsin side, it appears that chemical shift changes in the 1H-15N HSQC maps of oxidized, 15N-labeled [2Fe-2S]-anamorsin involve only signals present in the crowded 1H(NH) region between 8 and 8.5 ppm, whereas all of the resonances of the folded N-terminal domain remain essentially unaffected (Fig. S5). This behavior indicates that the unstructured C-terminal region of anamorsin interacts with oxidized FMN-Ndor1, whereas the N-terminal, well-structured domain of anamorsin is not involved in protein–protein recognition. Accordingly, no chemical shift perturbations were observed when oxidized, 15N-labeled FMN-Ndor1 was titrated with the N-terminal domain of [2Fe-2S]-anamorsin produced as an independent domain (Fig. S4). This result is consistent with the absence of any interaction between the N-terminal domain of Dre2 and Tah18, as monitored both in vitro and in vivo (16). When the anamorsin/FMN-Ndor1 interaction was monitored on a 13C,15N-labeled oxidized [2Fe-2S]-anamorsin sample by 13C-direct detection CON, CACO, and CBCACO NMR experiments (Fig. S5), which experience increased spectral resolution for unstructured proteins (26), the spectral changes are located in the region preceding the CX8CX2CXC motif (residues 185–223), encompassing the last 38 residues of the linker. This region can be partitioned in two main areas characterized by specific amino acid content: a highly hydrophobic area (residues 188–202) and a negatively charged area (residues 204–223) (Fig. S6). These two areas match, respectively, the hydrophobic and positively charged areas of FMN-Ndor1 involved in protein recognition, which are not directly in contact with the FMN cofactor (Fig. 1C). Such interactions are thus those responsible for the formation of a specific and stable protein complex between Ndor1 and anamorsin.
Oxidized [2Fe-2S]-CIAPIN1-single interacts also with oxidized FMN-Ndor1, but on a fast exchange regime on the NMR timescale (Fig. 3B). The chemical shift changes on FMN-Ndor1 are smaller than those detected when the complex is formed with [2Fe-2S]-anamorsin and involve a lower number of residues (Figs. 3 and 4). Still, on the FMN-Ndor1 structure the interacting residues are localized in an area that is part of the interacting surface of the complex between [2Fe-2S]-anamorsin and FMN-Ndor1 (Fig. 4B). These results indicate that a specific protein–protein interaction still occurs but the two proteins do not form a stable complex (Fig. 3). Therefore, the hydrophobic interacting residues of the linker (188–202, Fig. S6), which are absent in the [2Fe-2S]-CIAPIN1-single construct, are essential to determine an effective recognition of anamorsin.
To detect electron transfer between the FMN-binding domain of Ndor1 and [2Fe-2S]-anamorsin, we used UV-visible spectroscopy. FMN-Ndor1 can be reduced anaerobically to form the neutral blue semiquinone state (FMNH•) and the fully reduced state (FMNH2) of FMN by addition of 0.50 and 1.0 eq of dithionite, respectively (Fig. S7 and SI Materials and Methods for details). FMN-Ndor1 in either of the reduced states was titrated with [2Fe-2S]-anamorsin or [2Fe-2S]-CIAPIN1-single in their oxidized states and the reactions were followed by UV-visible spectroscopy. When FMNH• was mixed with [2Fe-2S]-anamorsin or with [2Fe-2S]-CIAPIN1-single, no changes were observed in the UV-visible spectra, indicating that no electron transfer occurs between the FMNH• moiety and the [2Fe-2S] redox center. On the contrary, when FMNH2 was mixed with [2Fe-2S]-anamorsin or with [2Fe-2S]-CIAPIN1-single, electrons were stoichiometrically transferred from FMN to [2Fe-2S], producing the reduced state of the [2Fe-2S] cluster and the semiquinone FMNH• species. Indeed, the absorbance at 432 nm, i.e., the isosbestic point of the FMNH2 and FMNH• species, decreases over time, indicating the reduction of the [2Fe-2S] cluster because the latter absorbs at this wavelength only in its oxidized state (Fig. S7); and the formation of the FMNH• state is monitored by the increase of the absorbance at 650 nm that is due only to the presence of the FMNH• species (Fig. S7). The rate of the [2Fe-2S] cluster reduction is of the same order of magnitude in [2Fe-2S]-anamorsin and in [2Fe-2S]-CIAPIN1-single (Fig. S7). Because the midpoint reduction potentials of the oxidized/semiquinone and semiquinone/dihydroquinone couples present in the FMN-binding domain of Ndor1 are −146 mV and −305 mV (27), respectively, the reduction potentials of the [2Fe-2S]+2/[2Fe-2S]+1 cluster center in anamorsin have to be in between these two values.
To specifically monitor the interaction between the two redox centers ([2Fe-2S] cluster and FMN) involved in the electron transfer process, paramagnetic 1H-15N IR-HSQC-AP maps of oxidized [2Fe-2S]-anamorsin were collected before and after the addition of 1 eq of unlabeled, oxidized FMN-Ndor1. With the exception of one signal, all of the others show Δavg(HN) values [i.e.,((ΔH)2 + (ΔN/5)2)/2)1/2, where ΔH and ΔN are chemical shift differences for 1H and 15N, respectively] with less than 0.03 ppm variations (Fig. S5), despite the fact that the two proteins form the tight, large molecular mass complex. The occurrence of small chemical shift variations suggests the presence of interactions with no specific orientation at the redox centers. Rather, there is a dynamic ensemble of orientations governed predominantly by long-range electrostatic forces as already observed in other electron transfer protein complexes (28, 29). This is in agreement with the presence of a positively charged region nearby the [2Fe-2S] cluster (Fig. S6) and a negatively charged region surrounding the FMN-binding site (Fig. 1C). Electron transfer over a sufficiently short distance is still possible in multiple orientations and the requirement to form a specific interaction is less stringent (28). This is especially true in the present case in which the specificity in protein–protein recognition is already guaranteed by intermolecular contacts involving hydrophobic and complementary charged residues far from the [2Fe-2S] cluster and FMN regions.
As the interaction between anamorsin and FMN-Ndor1 still occurs also between apo-anamorsin and oxidized FMN-Ndor1 and between oxidized [2Fe-2S]-anamorsin and fully reduced FMNH2-Ndor1, as monitored through NMR experiments (Fig. S8), it results that the redox state of the FMN moiety as well as the presence or not of the [2Fe-2S] cluster does not affect the formation of the protein complex. This behavior suggests that the protein–protein interaction interface is not affected by the redox centers and is fully consistent with a protein–protein recognition process determined by residues far from the redox centers. The same behavior was reported for the in vitro interaction of the yeast homologs, where apo-Dre2 showed stable interaction with Tah18 (16).
Discussion
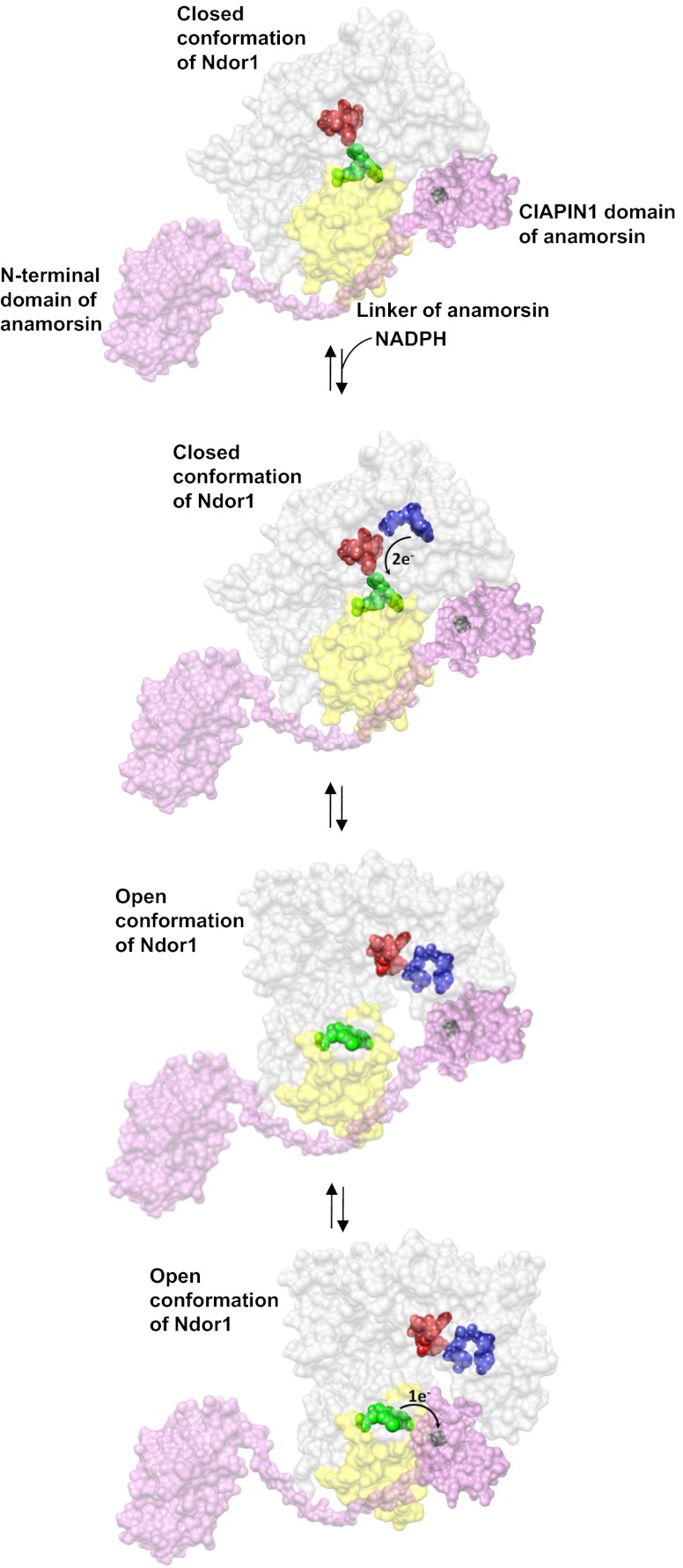
In this work, we defined the molecular basis for the recognition between the diflavin reductase Ndor1 and the iron–sulfur protein anamorsin and for the electron transfer process between them. We showed that the two proteins form a stable complex where one electron is transferred from the hydroquinone state of the FMN moiety of Ndor1 to the oxidized [2Fe-2S] cluster of anamorsin. The stable complex is achieved thanks to specific protein–protein recognition between a completely unstructured region of anamorsin (residues 185–223), which is part of the linker separating the N-terminal domain from the C-terminal CIAPIN1 domain, and a α-helical–containing face of the FMN-binding domain of Ndor1. This molecular recognition is specifically governed by hydrophobic and complementary charged interacting residues. In particular, the hydrophobic interactions are essential to ensure the formation of a stable complex, the electrostatic interactions are determining only a transient complex between anamorsin and the FMN-binding domain of Ndor1, and the N-terminal domain of anamorsin is not involved in the recognition process. The formation of the stable complex is independent of the presence of the [2Fe-2S] center as well as of the redox state of the FMN moiety, indicating that the two protein partners do interact permanently and no dissociation occurs along the electron transfer process. The two redox centers, on the other hand, transiently interact via electrostatic interactions between a negatively charged region surrounding the FMN moiety and a positively charged region surrounding the [2Fe-2S] cluster. All these data lead to a molecular model for the protein–protein recognition and for the electron transfer process according to which (a) the two proteins form a stable complex through specific interactions involving regions that are not in direct contact with the redox cofactors; (b) the areas surrounding the FMN and [2Fe-2S] redox moieties transiently and weakly interact with each other, exchanging one electron; and (c) the unstructured region comprising residues 185–223 is essential for the complex formation and can contribute in positioning the CIAPIN1 domain containing the [2Fe-2S] cluster in those orientations that can efficiently receive the electron(s) from the hydroquinone state of the FMN redox center. This molecular model nicely fits with the overall electron transfer chain occurring in diflavin reductases, which deliver electrons from NADPH to FMN through the mediation of FAD. Indeed, when the region of the FMN-binding domain of Ndor1, which stably interacts with anamorsin, is mapped on a structural model of entire Ndor1 (obtained from the crystal structure of the homologous human cytochrome P450 reductase in the closed conformation where the FMN and FAD moieties are in close contact to each other, thus ensuring an efficient interflavin electron transfer), this region is still largely solvent exposed and therefore still able to interact with anamorsin (Fig. 5). Consistent with this, coimmunoprecipitation data showed that anamorsin and Ndor1 form a stable complex in vivo (13). The transient interaction observed for the protein regions containing the two redox cofactors FMN and [2Fe-2S] also fits well in the overall electron transfer process of diflavin reductases. For diflavin reductase enzymes, it is well established that a conformational change from the closed conformation to a more open one is required to promote the final electron transfer from FMNH2 to its substrate (30). Otherwise, the reduced FMN would have too restricted solvent accessibility in the closed conformation to efficiently deliver electrons to the substrates (30–32). It has been also found that the open conformational state is significantly populated in the NADPH-bound, reduced state of diflavin reductases (31, 33, 34). At variance with the stable interaction between the FMN-binding domain of Ndor1 and the unstructured region of anamorsin, the transient interaction observed in the regions containing the FMN and [2Fe-2S] cofactors can be easily regulated upon the occurrence of the conformational closed-to-open equilibrium of the FAD/FMN domains of Ndor1. Indeed, the FMN-[2Fe-2S] interaction could be favored when the interacting area of Ndor1 surrounding the FMN moiety is solvent exposed in the open conformation and unfavored in the closed conformation, which does not expose this interacting area (Fig. 5). An electron transfer process coupled with tightly regulated conformational rearrangements can therefore be proposed to occur in the anamorsin–Ndor1 complex (Fig. 5): (i) Anamorsin is stably bound to both closed and open conformations of Ndor1 due to a specific recognition between an unstructured region of anamorsin and a region of the FMN-binding domain that is solvent exposed in both open and closed conformations; (ii) upon NADPH binding by Ndor1, electrons can efficiently be transferred within the closed conformation of Ndor1 to produce the hydroquinone state of FMN; (iii) NADPH binding and interflavin electron transfer within Ndor1 significantly populate the open conformation, which allows the formation of the transient interaction between the FMN and the [2Fe-2S] cluster regions; and (iv) the latter interaction allows efficient transfer of one electron from the hydroquinone state of FMN to the [2Fe-2S] cluster.
Fig. 5.
Model of the electron transfer process between Ndor1 and anamorsin. Anamorsin (in pink) can be tightly bound to both closed and open conformational states of Ndor1 (in gray) due to the specific recognition between an unstructured region of anamorsin and a region of the FMN-binding domain that is solvent exposed in both open and closed conformations (in yellow). The N-terminal domain of anamorsin is not involved in the protein–protein recognition process. Upon NADPH (in blue) binding, electrons can efficiently be transferred in the closed conformation of Ndor1 to FAD (in red) and then to FMN (in green) to produce the hydroquinone state of FMN. Interflavin electron transfer in Ndor1 significantly populates the open conformation, which exposes to the solvent the FMN moiety and allows the formation of a transient interaction with the [2Fe-2S] cluster region of anamorsin located in the CIAPIN1 domain. The latter interaction allows an efficient transfer of one electron from the hydroquinone state of FMN to the [2Fe-2S] cluster (in black).
Our study lays out the molecular basis for the comprehension of the two functional processes in which the anamorsin–Ndor1 complex is implicated, i.e., assembly of Fe/S proteins and regulation of cell survival/death mechanisms. From our data we can propose that the electron transfer process responsible for the assembly of ISC (13) and diferric (17) proteins occurs within a stable complex without the dissociation of the two protein partners but just through the modulation of the interactions of the domains involved in the electron transfer process. Although the molecular targets of the electron transfer flow generated by this protein–protein complex are not yet defined, suggestions for the targets of the electron flow include the conversion of the sulfur of cysteine (formally S0) to the sulfide (S2−) present in Fe/S clusters and/or the reductive coupling of two [2Fe-2S] clusters to form a [4Fe-4S] cluster (13). This nondissociative electron transfer process might also rationalize how anamorsin regulates cell survival/death mechanisms in human cells (35) and why a stable Dre2-Tah18 interaction is essential for yeast viability (16). Indeed, the disruption of the stable interaction between anamorsin and Ndor1 might provoke the interruption of the electron flow between the two proteins within the cell and, as a result of that, its essential function for cellular survival is abolished and consequently cell death mechanisms might be activated.
Materials and Methods
Protein Production.
A detailed procedure of cloning and protein production of the FMN-binding domain of Ndor1 is reported in SI Materials and Methods. The DNA encoding CIAPIN1-single of anamorsin (205–312 aa) was amplified by PCR from the Gateway pEntr-TEV-d-Topo vector containing full-length anamorsin and inserted into the same vector. Full-length anamorsin, CIAPIN1-single, and the N-terminal domain of anamorsin were expressed, purified, and chemically reconstituted following a previously reported procedure (18).
X-Ray Crystallography.
Crystals of the FMN-binding domain of Ndor1 (1–161 aa) were obtained using the vapor diffusion technique at 20 °C from 1.3-mM protein solutions containing 0.1 M 2-(N-morpholino)ethanesulfonic acid (Mes) (pH 6.5), 0.2 M ammonium sulfate, and 30% (vol/vol) PEG MME 5000. The data collection was carried out at PROXIMA1 beamline (SOLEIL, Paris). The dataset was collected at 100 K, using a wavelength of 1.02 Å, and the crystals used for data collection were cryo-cooled using 25% (vol/vol) ethylene glycol in the mother liquor. Data analysis is reported in SI Materials and Methods.
NMR.
Standard 1H-detected triple-resonance NMR experiments for backbone resonance assignment were recorded on 0.5- to 1-mM 13C, 15N-labeled samples at 298 K ([2Fe-2S]-anamorsin and [2Fe-2S]-CIAPIN1-single) and at 308 K (FMN-Ndor1, 1–174 aa), using a Bruker AVANCE 500 MHz spectrometer. 13C-detected protonless NMR experiments (26) [CBCACO-in phase antiphase (IPAP), CACO-IPAP, CON-IPAP, CBCACON-IPAP, and CBCANCO-IPAP] acquired on a Bruker AVANCE 700 spectrometer, equipped with a cryogenically cooled probehead optimized for 13C-direct detection, were also used for sequence-specific resonance assignment (N, C′, Cα, and Cβ) of [2Fe-2S]-anamorsin and [2Fe-2S]-CIAPIN1-single. 13C-direct–detected NMR experiments (26) acquired on 13C,15N-labeled [2Fe-2S]-anamorsin allowed us to obtain the backbone resonance assignment of all linker residues, with the exception of three (173–175). To identify backbone amide resonances affected by paramagnetic relaxation effects, 1H-15N HSQC, edited by a 1H inversion recovery and observed in the antiphase component (IR-HSQC-AP), were collected, at 500 MHz, using a 512 × 100 data point matrix, collected over a 25 × 40 ppm spectral region. A total of 2,048 scans per fid were acquired, using inversion recovery, inept transfer, and recycle delays of 50 ms, 0.83 ms, and 55 ms, respectively. Fast spin relaxation of paramagnetic signals, however, decreases dramatically the efficiency of coherence transfer and prevents resonance assignment via the standard protocols that rely on 1H- or 13C-direct–detected triple resonance experiments. NMR experiments tailored to assign resonances affected by the presence of a paramagnetic center were therefore performed on a Bruker AVANCE 500 or on a Bruker AVANCE 700, specifically modifying HNCA, HNCO, CBCACONH, and 13C-detected CON, CC-COSY, CACO, IR-CACO-AP, and CBCACO pulse sequences (see SI Materials and Methods for details). All NMR data were processed using the Topspin software package and were analyzed with the program CARA (36). Secondary structure analysis has been performed by TALOS+ (37). The secondary structure propensity was determined from the chemical shifts following a previously described approach (38), with random-coil reference chemical shift values corrected for primary sequence, temperature, and pH effects.
Titrations of 15N-labeled FMN-Ndor1 (oxidized or in the FMNH2 state) with unlabeled apo- or oxidized [2Fe-2S]-anamorsin, the N-terminal domain, and oxidized [2Fe-2S]-CIAPIN1-single were performed to follow chemical shift changes in 1H-15N HSQC maps. Reversed titrations were performed between 15N-labeled or 13C,15N-labeled [2Fe-2S]-anamorsin or [2Fe-2S]-CIAPIN1-single and unlabeled FMN-Ndor1, both proteins in the oxidized state, and chemical shift changes followed by 1H-15N HSQC and 13C-direct detection experiments (CON, CACO, and CBCACO) after addition of increasing amounts of the unlabeled partner.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Programmi di Ricerca di Rilevante Interesse Nazionale (PRIN) (2009FAKHZT_001), BIO-NMR (Contract 261863), WeNMR (Contract 261572), Ministero dell'Istruzione, dell'Università e della Ricerca-Fondo per gli Investimenti della Ricerca di Base (MIUR-FIRB) PROTEOMICA (RBRN07BMCT) and Ente Cassa di Risparmio for financial support. This work was also supported by Instruct [part of the European Strategy Forum on Research Infrastructures (ESFRI)] and by national member subscriptions. Specifically, we thank the European Union ESFRI Instruct Core Centre Centro di Risonanze Magnetiche (Italy).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4H2D).
2Deceased July 7, 2012.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302378110/-/DCSupplemental.
References
- 1.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 2.Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24(8):398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460(7257):831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 4.Meyer J. Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J Biol Inorg Chem. 2008;13(2):157–170. doi: 10.1007/s00775-007-0318-7. [DOI] [PubMed] [Google Scholar]
- 5.Qi W, Cowan JA. Structural, mechanistic and coordination chemistry of relevance to the biosynthesis of iron-sulfur and related iron cofactors. Coord Chem Rev. 2011;255(7–8):688–699. doi: 10.1016/j.ccr.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreini C, Banci L, Bertini I, Elmi S, Rosato A. Non-heme iron through the three domains of life. Proteins. 2007;67(2):317–324. doi: 10.1002/prot.21324. [DOI] [PubMed] [Google Scholar]
- 7.Lill R, Mühlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: Mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 8.Fontecave M, Ollagnier-de-Choudens S. Iron-sulfur cluster biosynthesis in bacteria: Mechanisms of cluster assembly and transfer. Arch Biochem Biophys. 2008;474(2):226–237. doi: 10.1016/j.abb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Mansy SS, Cowan JA. Iron-sulfur cluster biosynthesis: Toward an understanding of cellular machinery and molecular mechanism. Acc Chem Res. 2004;37(9):719–725. doi: 10.1021/ar0301781. [DOI] [PubMed] [Google Scholar]
- 10.Sharma AK, Pallesen LJ, Spang RJ, Walden WE. Cytosolic iron-sulfur cluster assembly (CIA) system: Factors, mechanism, and relevance to cellular iron regulation. J Biol Chem. 2010;285(35):26745–26751. doi: 10.1074/jbc.R110.122218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lill R, et al. Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes. Biochim Biophys Acta. 2006;1763(7):652–667. doi: 10.1016/j.bbamcr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol Cell Biol. 2008;28(18):5569–5582. doi: 10.1128/MCB.00642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netz DJ, et al. Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat Chem Biol. 2010;6(10):758–765. doi: 10.1038/nchembio.432. [DOI] [PubMed] [Google Scholar]
- 14.Murataliev MB, Feyereisen R, Walker FA. Electron transfer by diflavin reductases. Biochim Biophys Acta. 2004;1698(1):1–26. doi: 10.1016/j.bbapap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Vernis L, et al. A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast. PLoS ONE. 2009;4(2):e4376. doi: 10.1371/journal.pone.0004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soler N, et al. Interaction between the reductase Tah18 and highly conserved Fe-S containing Dre2 C-terminus is essential for yeast viability. Mol Microbiol. 2011;82(1):54–67. doi: 10.1111/j.1365-2958.2011.07788.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. Investigation of in vivo diferric tyrosyl radical formation in Saccharomyces cerevisiae Rnr2 protein: Requirement of Rnr4 and contribution of Grx3/4 AND Dre2 proteins. J Biol Chem. 2011;286(48):41499–41509. doi: 10.1074/jbc.M111.294074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banci L, et al. Anamorsin is a [2Fe-2S] cluster-containing substrate of the Mia40-dependent mitochondrial protein trapping machinery. Chem Biol. 2011;18(6):794–804. doi: 10.1016/j.chembiol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Soler N, et al. A S-adenosylmethionine methyltransferase-like domain within the essential, Fe-S-containing yeast protein Dre2. FEBS J. 2012;279(12):2108–2119. doi: 10.1111/j.1742-4658.2012.08597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia C, et al. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc Natl Acad Sci USA. 2011;108(33):13486–13491. doi: 10.1073/pnas.1106632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q, et al. Crystal structure of the FMN-binding domain of human cytochrome P450 reductase at 1.93 A resolution. Protein Sci. 1999;8(2):298–306. doi: 10.1110/ps.8.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machonkin TE, Westler WM, Markley JL. Strategy for the study of paramagnetic proteins with slow electronic relaxation rates by nmr spectroscopy: Application to oxidized human [2Fe-2S] ferredoxin. J Am Chem Soc. 2004;126(17):5413–5426. doi: 10.1021/ja037077i. [DOI] [PubMed] [Google Scholar]
- 23.Machonkin TE, Westler WM, Markley JL. (13)C[(13)C] 2D NMR: A novel strategy for the study of paramagnetic proteins with slow electronic relaxation rates. J Am Chem Soc. 2002;124(13):3204–3205. doi: 10.1021/ja017733j. [DOI] [PubMed] [Google Scholar]
- 24.Bertini I, Lee YM, Luchinat C, Piccioli M, Poggi L. Locating the metal ion in calcium-binding proteins by using cerium(III) as a probe. ChemBioChem. 2001;2(7–8):550–558. doi: 10.1002/1439-7633(20010803)2:7/8<550::AID-CBIC550>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Balayssac S, Jiménez B, Piccioli M. Assignment strategy for fast relaxing signals: Complete aminoacid identification in thulium substituted calbindin D 9K. J Biomol NMR. 2006;34(2):63–73. doi: 10.1007/s10858-005-5359-z. [DOI] [PubMed] [Google Scholar]
- 26.Bermel W, et al. 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Reson Spectrosc. 2006;48(1):25–45. [Google Scholar]
- 27.Finn RD, et al. Determination of the redox potentials and electron transfer properties of the FAD- and FMN-binding domains of the human oxidoreductase NR1. Eur J Biochem. 2003;270(6):1164–1175. doi: 10.1046/j.1432-1033.2003.03474.x. [DOI] [PubMed] [Google Scholar]
- 28.Bashir Q, Scanu S, Ubbink M. Dynamics in electron transfer protein complexes. FEBS J. 2011;278(9):1391–1400. doi: 10.1111/j.1742-4658.2011.08062.x. [DOI] [PubMed] [Google Scholar]
- 29.Ubbink M. The courtship of proteins: Understanding the encounter complex. FEBS Lett. 2009;583(7):1060–1066. doi: 10.1016/j.febslet.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 30.Laursen T, Jensen K, Møller BL. Conformational changes of the NADPH-dependent cytochrome P450 reductase in the course of electron transfer to cytochromes P450. Biochim Biophys Acta. 2011;1814(1):132–138. doi: 10.1016/j.bbapap.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Vincent B, et al. The closed and compact domain organization of the 70-kDa human cytochrome P450 reductase in its oxidized state as revealed by NMR. J Mol Biol. 2012;420(4–5):296–309. doi: 10.1016/j.jmb.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Pudney CR, et al. Kinetic and spectroscopic probes of motions and catalysis in the cytochrome P450 reductase family of enzymes. FEBS J. 2012;279(9):1534–1544. doi: 10.1111/j.1742-4658.2011.08442.x. [DOI] [PubMed] [Google Scholar]
- 33.Ellis J, et al. Domain motion in cytochrome P450 reductase: Conformational equilibria revealed by NMR and small-angle x-ray scattering. J Biol Chem. 2009;284(52):36628–36637. doi: 10.1074/jbc.M109.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay S, et al. Nature of the energy landscape for gated electron transfer in a dynamic redox protein. J Am Chem Soc. 2010;132(28):9738–9745. doi: 10.1021/ja1016206. [DOI] [PubMed] [Google Scholar]
- 35.Shibayama H, et al. Identification of a cytokine-induced antiapoptotic molecule anamorsin essential for definitive hematopoiesis. J Exp Med. 2004;199(4):581–592. doi: 10.1084/jem.20031858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller R. The Computer Aided Resonance Assignment Tutorial. Goldau, Switzerland: CANTINA Verlag; 2004. [Google Scholar]
- 37.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berjanskii M, Wishart DS. NMR: Prediction of protein flexibility. Nat Protoc. 2006;1(2):683–688. doi: 10.1038/nprot.2006.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.