Abstract
Hypoxia, or low oxygen tension, is a major regulator of tumor development and aggressiveness. However, how cancer cells adapt to hypoxia and communicate with their surrounding microenvironment during tumor development remain important questions. Here, we show that secreted vesicles with exosome characteristics mediate hypoxia-dependent intercellular signaling of the highly malignant brain tumor glioblastoma multiforme (GBM). In vitro hypoxia experiments with glioma cells and studies with patient materials reveal the enrichment in exosomes of hypoxia-regulated mRNAs and proteins (e.g., matrix metalloproteinases, IL-8, PDGFs, caveolin 1, and lysyl oxidase), several of which were associated with poor glioma patient prognosis. We show that exosomes derived from GBM cells grown at hypoxic compared with normoxic conditions are potent inducers of angiogenesis ex vivo and in vitro through phenotypic modulation of endothelial cells. Interestingly, endothelial cells were programmed by GBM cell-derived hypoxic exosomes to secrete several potent growth factors and cytokines and to stimulate pericyte PI3K/AKT signaling activation and migration. Moreover, exosomes derived from hypoxic compared with normoxic conditions showed increased autocrine, promigratory activation of GBM cells. These findings were correlated with significantly enhanced induction by hypoxic compared with normoxic exosomes of tumor vascularization, pericyte vessel coverage, GBM cell proliferation, as well as decreased tumor hypoxia in a mouse xenograft model. We conclude that the proteome and mRNA profiles of exosome vesicles closely reflect the oxygenation status of donor glioma cells and patient tumors, and that the exosomal pathway constitutes a potentially targetable driver of hypoxia-dependent intercellular signaling during tumor development.
Keywords: biomarker, blood vessels, CNS
Considerable interest in the cancer field is focused on the specific characteristics of the tumor microenvironment and how these phenomena depend on intercellular communication of malignant and nonmalignant cells of the host. Low oxygen tension, or hypoxia has emerged as a specific and general feature of the microenvironment of malignant tumors. Tumor hypoxia induces adaptive mechanisms that rely on phenotypic modulation of stromal cells that serve to promote the survival and dissemination of malignant cells (1–5). It has become generally accepted that cancer progression is driven by hypoxic signaling, and the expression of hypoxia-related markers has been correlated with poor patient outcome in several tumor types, which partly may relate to increased resistance to radiotherapy and chemotherapy (6).
The hypoxic response of cancer cells involves the regulation of a large number of cytokines, growth factors, and proteases resulting in the induction of, e.g., angiogenesis and remodeling of the extracellular matrix (7–9). Hypoxia-induced, proangiogenic proteins provide new targets in the treatment of a growing number of tumor types (10, 11). However, from clinical studies, it has become clear that successful cancer treatment targeted at hypoxic rescue pathways requires a better understanding of the complex network of intercellular communication that shapes the tumor microenvironment (12).
Recent studies point at a hitherto unknown role of exosomes [also known as microvesicles or extracellular vesicles (EVs)] as important signaling entities in the cross-talk between various cell types. Interestingly, exosomes can carry complex biological information consisting of mRNAs, miRNAs, as well as soluble and transmembrane proteins between cells (13–20). Here, we have investigated the potential role of exosome vesicles in hypoxia-dependent intercellular signaling in glioblastoma multiforme (GBM), i.e., highly aggressive brain tumors characterized by profound hypoxia (21–23).
Results
Molecular Profile of Hypoxic Exosomes Reflects the Hypoxic Response of GBM Donor Cells and GBM Patient Tumors.
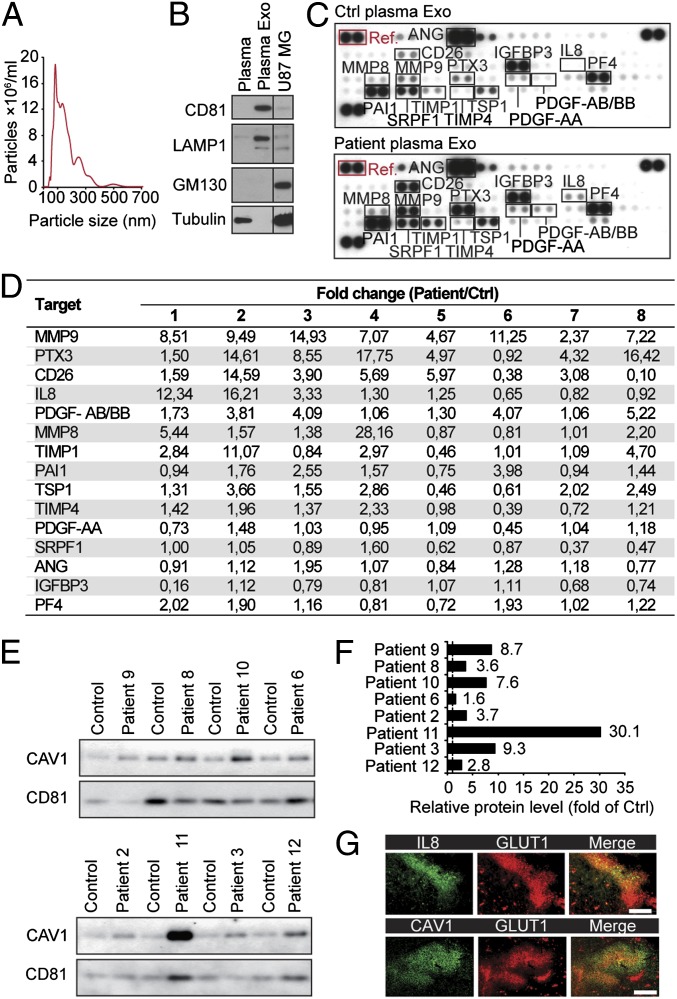
In our search for biomarkers of GBM, we set out to investigate EVs isolated from plasma of GBM patients and matched control subjects (Fig. S1A). Nanoparticle tracking analysis of isolated plasma EVs showed a size distribution consistent with exosome vesicles (approximately 50–200 nm; Fig. 1A) that were highly enriched in exosomal markers and depleted of the cytoskeletal protein tubulin and the cis-Golgi marker GM130 compared with whole plasma and GBM cell lysates (Fig. 1B). Because hypoxia is a characteristic feature of GBM and malignant tumors in general (1–5), we decided to study hypoxia-associated proteins in patient exosomes. Exosomes from GBM patients compared with sex- and age-matched controls were enriched in several hypoxia regulated proteins known to have important roles in GBM pathology, most notably matrix metalloproteinase 9 (MMP9), pentraxin 3 (PTX3), IL8, PDGF-AB/AA, CD26 (also known as dipeptidyl-peptidase-4), and plasminogen activator inhibitor 1 (PAI1) (Fig. 1 C and D and Fig. S1 B–D). Independent studies have suggested that caveolin 1 (CAV1), i.e., a protein associated with membrane raft domains, is overexpressed in GBM cells and tumors compared with normal astrocytes and human brain tissue (24–27). Further, CAV1 was shown to be hypoxia regulated and to be present in plasma exosomes of melanoma patients (28, 29). Interestingly, we found that in all cases (n = 8), CAV1 was enriched in exosomes from GBM patients compared with matched controls (Fig. 1 E and F). We found that hypoxic regions of GBM tumors, as determined by glucose transporter 1 (GLUT1) staining, displayed enhanced expression of CAV1. Further, coassociation with GLUT1 expression in patient tumors was demonstrated with the selected protein, IL8 (Fig. 1G).
Fig. 1.
GBM patient plasma exosomes have increased levels of hypoxia-regulated proteins involved in tumor development. (A) Nanoparticle-sized distribution profile of GBM patient-derived exosomes indicates an average diameter of 148 ± 79 nm. (B) GBM patient whole plasma, plasma exosomes (Plasma Exo), and U87 MG cell lysates were analyzed for the indicated proteins by Western blotting. (C) Antibody array analyses of Exo from GBM patients (Patient plasma Exo) and matched control subjects (Ctrl plasma Exo). Shown is array data from a representative patient-control experiment. (D) Fold change of relative protein levels (normalized to array reference marked with red box in C) in the respective patient-control pairs (1–8). (E and F) Exo isolated from GBM patients and matched control subjects were analyzed for caveolin 1 (CAV1) by Western blotting (n = 8) with CD81 as loading control. (G) Hypoxic regions of patient GBM tumors display increased expression of IL8 and CAV1. (Scale bar: 100 μm.)
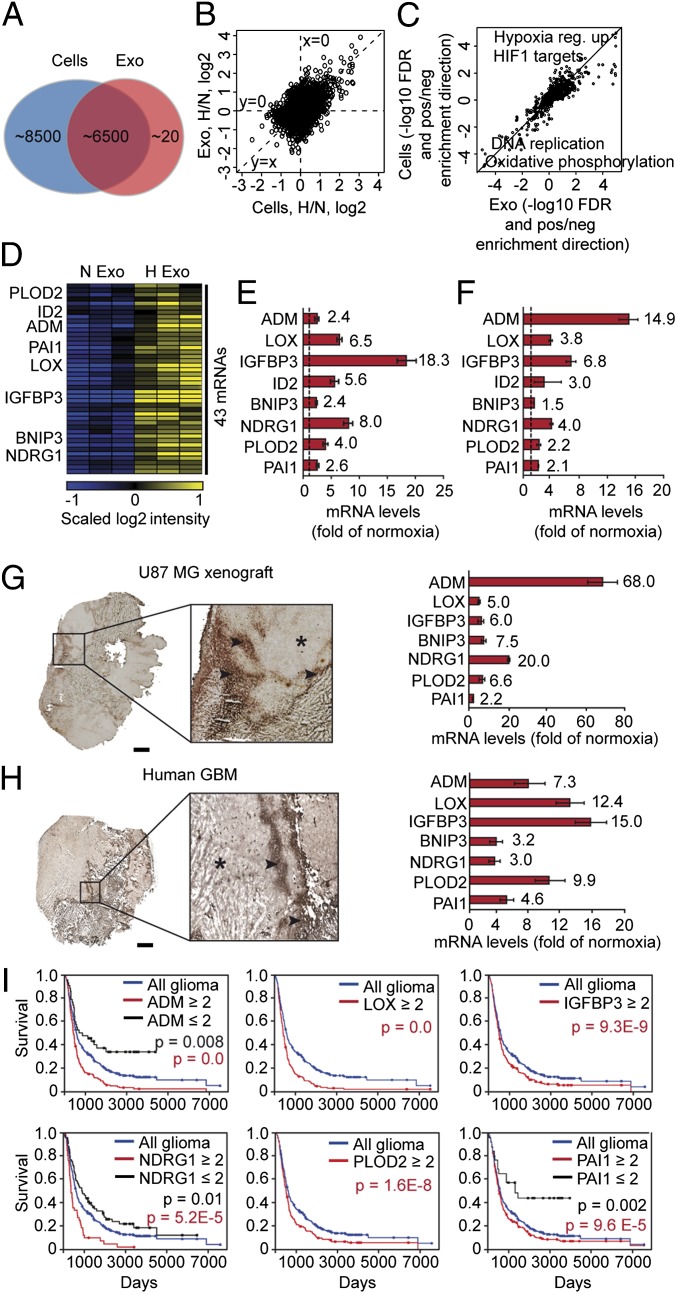
The systemic release of several hypoxia-associated proteins with tumor promoting activities through exosomes prompted further studies on the potential role of exosomes in hypoxia-dependent intercellular signaling. We initially performed comprehensive transcriptome analyses comparing hypoxic and normoxic exosomes with their respective donor cells. We chose to study the well characterized U87 MG cell line established from a GBM patient (30). GBM cells produce significant amounts of exosomes (16, 31–34), and as shown here, the gross composition of exosomes was not affected by the oxygenation status of donor GBM cells (Fig. S2). Our results from gene expression analyses revealed that almost half of the transcripts detected in cells (approximately 15,000) were significantly expressed also in exosomes (approximately 6,500) (Fig. 2A). Interestingly, we found that the gene expression profile of hypoxic exosomes mirrors that of GBM donor cells (Fig. 2B). Gene set enrichment analysis (35) showed the up-regulation of validated gene expression themes relating to hypoxia, and the down-regulation of processes such as DNA replication and oxidative phosphorylation, which are known to be part of the hypoxic response (Fig. 2C).
Fig. 2.
The hypoxic transcriptional profile of exosomes reflects the hypoxic signaling response of GBM cells and tumors. (A) Number of transcripts detected by microarray analysis in GBM cell-derived exosomes and GBM cells. (B) Ratios of hypoxia/normoxia intensities plotted as log2 scale for cells and exosomes. (C) False discovery rates (−log10 space) relating to gene set enrichment analysis. Positive value, up-regulation in hypoxia; negative value, down-regulation in hypoxia of a specific gene set. (D) Heat map of 43 transcripts with significantly higher expression levels in exosomes secreted by hypoxic compared with normoxic GBM cells. (E) Validation of hypoxic induction of indicated mRNAs in exosomes by qRT-PCR. Data are presented as fold increase in hypoxic compared with normoxic exosomes ± SD and are representative of three independent experiments. Values beside bars indicate fold change in hypoxic exosomes. (F) Similar experiment as in E performed with GBM cells. (G and H) Identification of the hypoxic exosome gene expression profile in U87 MG GBM xenografts and GBM patient tumors. Laser-capture microdissection of hypoxic (stars) and normoxic (arrowheads) tumor regions was performed as described in SI Materials and Methods and analyzed for the expression of indicated transcripts by qRT-PCR. (Scale bar: 2 mm.) (I) Kaplan–Meier survival curves of hypoxia-regulated transcripts, as indicated. Blue lines, median expression level of all gliomas (n = 343). Black lines, expression ≤ twofold compared with median. Red lines, expression ≥ twofold compared with median.
We identified 43 exosome resident transcripts that were significantly induced by hypoxia (P ≤ 0.05, as determined by Student’s t test) (Fig. 2D); see Table S1 for a complete list of exosome transcripts significantly up- or down-regulated by hypoxia. Hypoxic induction of eight exosomal transcripts related to tumor development, i.e., adrenomedullin (ADM), lysyl oxidase (LOX), IGF binding protein (IGFPB) 3, inhibitor of DNA binding 2, B-cell lymphoma (BCL)2/adenovirus E18 19-kDa interacting protein 3 (BNIP3), N-myelocytomatosis viral related oncogene (myc) downstream regulated 1 (NDRG1), procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 (PLOD2), and PAI1, was validated in independent experiments by quantitative RT-PCR (qRT-PCR) (Fig. 2E). This set of mRNAs was up-regulated also in hypoxic GBM cells (Fig. 2F), which strengthens the conclusion that the hypoxic exosome transcriptome mirrors that of donor cells. This conclusion was corroborated by experiments with additional, patient-derived GBM cell lines (U118 MG and LN18). These cells also secreted EVs with exosome characteristics, and hypoxic regulation of several mRNAs was reflected by their corresponding exosomes (Fig. S3).
We next used laser-capture microdissection of hypoxic and normoxic tumor regions to investigate whether the identified transcripts were associated with hypoxia also in vivo. Frozen tumor sections were stained for GLUT1 to define hypoxic areas (Fig. 2 G and H, Left). The validity of this approach was supported by the induction of GLUT1 and VEGFA mRNAs, i.e., well-known hypoxia-induced transcripts, in hypoxic compared with normoxic tumor regions (Fig. S4). Our data clearly showed that hypoxia-induced, exosome resident mRNAs are substantially up-regulated also in hypoxic regions of GBM mouse xenografts (Fig. 2G, Right) as well as of GBM patient tumors (Fig. 2H, Right). Further, high tumor expression of the identified transcripts was associated with significantly worse prognosis in glioma patients [Fig. 2I; data were retrieved from the Repository of Molecular Brain Neoplasia Data (REMBRANDT) public database of the National Cancer Institute]. These data support the notion that the exosomal molecular signature reflects the hypoxic status and aggressiveness of glioma tumors.
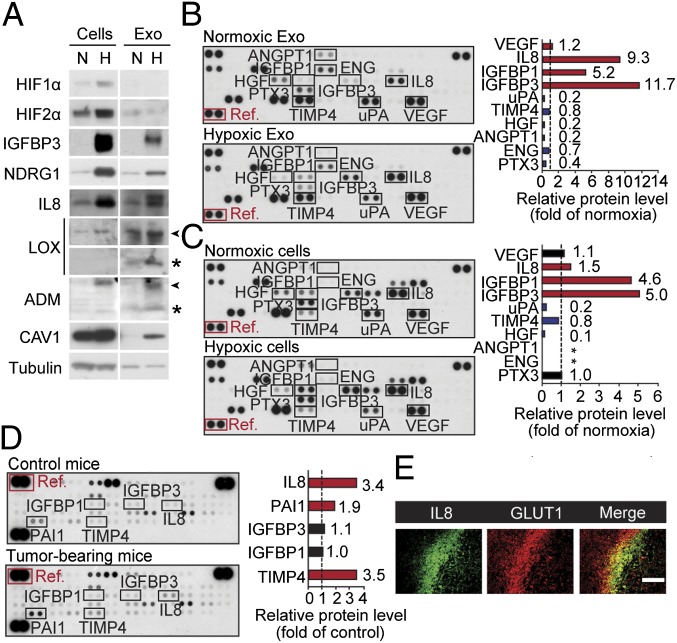
We found that several exosome components shown to be hypoxia-regulated at the mRNA level, i.e., IGFBP3, NDRG1, LOX, and ADM (Fig. 2), were induced also at the protein level (Fig. 3A). Further studies revealed that several proteins with established roles in tumor development are associated with exosomes; importantly, some of these components were substantially induced by hypoxia (Fig. 3 B and C), e.g., IL8, IGFBP1, and IGFBP3 (approximately 9-, 5-, and 12-fold induction, respectively, compared with normoxic exosomes). The most hypoxia up-regulated proteins in exosomes from array experiments, IL8 and IGFBP3, were validated by Western blotting (Fig. 3A). As shown in Table S2, the majority of proteins expressed in GBM cells were also present in exosomes. Notably, FGF2 and IL1β, which are known to exit cells through an unconventional secretory pathway (36), were virtually absent in exosomes. Importantly, the regulation of hypoxia-related proteins in exosomes mirrored that of donor cells. This correlation was true also for the other GBM cell lines, U118 MG and LN18 (Fig. S3).
Fig. 3.
Hypoxic regulation of the GBM cell and exosome angiogenesis-related proteome. (A) Equal amount of total protein from normoxic (N) or hypoxic (H) GBM cells and corresponding exosomes (Exo) were analyzed for the indicated proteins and tubulin (loading control) by Western blotting. Arrowheads and stars denote proforms and mature forms of proteins, respectively. GBM Exo (B) and cells (C) from N and H conditions were subjected to human angiogenesis protein antibody array analyses. (B and C Left) Array data from a representative experiment. (B and C Right) Data are representative of at least three independent experiments and represent fold change of relative protein levels (normalized to array reference marked with red box) in H compared with N samples. Stars indicate protein expression below detection level. (D) Circulating Exo were isolated from plasma of tumor-free control mice and GBM tumor-bearing mice, and equal amounts of total protein were analyzed by angiogenesis antibody arrays. (Left) Array data from a representative experiment. (Right) Data are representative of at least three independent experiments and represent fold change of relative protein levels (normalized to array reference marked with red box) in Exo from GBM tumor-bearing mice compared with control mice. (E) Hypoxic regions of GBM xenografts display increased IL8 protein levels. (Scale bar: 100 μm.)
We next analyzed exosomes isolated from plasma of tumor-free control and GBM tumor-bearing mice. IL8 is a well-established, hypoxia-responsive factor and has been suggested to have a role in the development of aggressive gliomas (37, 38). Interestingly, we typically found increased levels of IL8 in exosomes from GBM tumor-bearing mice (approximately 3.4-fold compared with control mice; Fig. 3D), and IL8 was associated with hypoxic regions of GBM xenografts (Fig. 3E). These results are consistent with our finding of IL8 enrichment in exosomes from GBM patients vs. controls (Fig. 1C and Fig. S1) and in hypoxic vs. normoxic exosomes from GBM cells in vitro (Fig. 3A). Moreover, CAV1 showed a corresponding enrichment in hypoxic compared with normoxic exosomes in vitro (Fig. 3A) and in GBM patient compared with control exosomes (Fig. 1 E and F). Together, these studies indicate that hypoxic GBM cells secrete exosomes enriched in several proteins implicated in tumor aggressiveness and that the molecular profile of exosome vesicles largely reflects the oxygenation status of GBM cells and patient tumors.
Exosomes Mediate Hypoxia-Dependent Paracrine Stimulation of Angiogenesis and Autocrine Activation of GBM Cells.
Our findings on the hypoxic regulation of exosome-associated effector molecules motivated further studies on the functional role of exosomes in hypoxia-dependent cross-talk between malignant cells and cells of the tumor stroma. GBM cell-derived exosomes were efficiently internalized by endothelial cells (ECs) (Fig. S5 A and B), and we found indications of direct membrane vesicle transfer from GBM cells into ECs in live confocal microscopy coculture experiments (Movie S1). Remarkably, exosomes derived from hypoxic compared with normoxic GBM cells were found to substantially induce microvascular sprouting (Fig. 4 A and B). Consistent with these ex vivo angiogenesis data, hypoxic exosomes compared with normoxic ones were significantly more potent at stimulating tube forming capacity of primary human umbilical vein ECs (HUVECs) (Fig. 4 C and D). These results were corroborated by experiments with primary human brain microvascular ECs (HBMECs) (Fig. S5E). Proliferation and survival at hypoxic stress conditions were significantly promoted by hypoxic exosomes both in HUVECs and HBMECs, although some effects were demonstrated also with normoxic exosomes (Fig. 4 E and F and Fig. S5 F and G). Moreover, we could show that exosomes isolated from the plasma of GBM patients (n = 4) in all cases significantly stimulated EC proliferation and survival (Fig. S5 H and I). On a mechanistic level, GBM cell-derived exosomes were shown to activate several cell surface receptors known to elicit an angiogenic response, i.e., EGFR, ephrin type A receptor 2 (EPHA2), and vascular endothelial growth factor receptor 2 (VEGFR2) (Fig. S6 A and B). Receptor kinase activation converged on major intracellular kinase pathways, i.e., ERK1/2 MAPK, PI3K/AKT, and focal adhesion kinase (FAK) in ECs (Fig. S6 C and D). We further found evidence of increased autocrine stimulation of GBM cell migration by hypoxic, compared with normoxic, exosomes (Fig. 4G). Collectively, these data indicate a direct involvement of exosomes in hypoxia-mediated autocrine signaling and in paracrine stimulation of EC function and angiogenesis.
Fig. 4.
Role of exosomes in hypoxia-dependent cross-talk between malignant cells and vascular cells. (A) Mouse aortas were incubated in the absence (Ctrl) or presence of exosomes (Exo) (25 µg/mL) derived from normoxic (N) or hypoxic (H) GBM cells for 24 h and then embedded in Matrigel overlaid with medium supplemented with 2% mouse serum ± Exo. Shown are representative photomicrographs of microvessels at day 7. (B) Quantitative analysis of microvessel number (Left) and length (Right) presented as the mean ± SD, n = 6 aortas per group. *P < 0.05. (C) HUVECs were cultured for 24 h in the absence (Ctrl) or presence of Exo (10 µg/mL) and then grown on Matrigel for 20 h. Shown are representative photomicrographs of EC tubes from the different treatment groups. (Scale bar: 500 µm.) (D) Quantitative analysis of tubes/microscopic field presented as the mean ± SD, n = 6 per group. *P < 0.05. (E) HUVECs were cultured in the presence of varying concentrations of Exo derived from N or H GBM cells for a total period of 72 h and assessed for proliferation by [3H]-thymidine incorporation. (F) HUVECs were cultured in the presence of varying concentrations of Exo derived from N or H GBM cells for 48 h and assessed for cell death by 7-AAD staining. (G) Hypoxia potentiates autocrine stimulation by Exo. GBM cells were assessed for transwell migration over a period of 6 h in the absence (Ctrl) or presence of Exo (50 µg/mL) derived from of N or H GBM cells. (E–G) Data are presented as fold of untreated, control cells and are the mean ± SD, n ≥ 6 per group. *P < 0.05. (H) Primary HBVPs were assessed for transwell migration over a period of 6 h in serum-free medium (Ctrl) or in the different media as indicated. EC preconditioning with H GBM cell Exo significantly increased paracrine stimulation of pericyte migration (compare EC CM and EC + Exo CM). (I) Pericytes were analyzed for proliferative activity with the different treatments, as indicated. EC Exo preconditioning significantly increased pericyte proliferation, and the activity was in the soluble, vesicle-free CM fraction (compare EC + Exo CM and EC + Exo CM Sol). (J and K) Similar experiment as in H and I, respectively, with GBM cells; EC Exo preconditioning significantly increased paracrine stimulation of GBM cell migration (J) and proliferation (K). (H–K) Data are presented as fold of untreated, control cells, and are the mean ± SD, n ≥ 6 per group. *P < 0.05.
ECs Preconditioned with Hypoxic Exosomes Exhibit Enhanced Paracrine Stimulation of Pericytes and GBM Cells.
ECs exert paracrine effects on pericytes, which contribute to GBM development through stabilization of the proliferative vasculature that may counter act the effect of antiangiogenic treatment (39, 40). We next tested the idea that ECs can be programmed by GBM cell-derived exosomes toward enhanced paracrine stimulation of pericytes, specifically in the context of hypoxia. As initial support of this idea, the soluble, exosome-free fraction of conditioned medium from ECs stimulated with GBM cell-derived exosomes (EC + Exo CM Sol) compared with conditioned medium from untreated ECs (EC CM) contained enhanced levels of several potent growth factors and cytokines (Fig. S7 A and B). In accordance with the functional data (Fig. 4 A–F and Fig. S5), some effects were seen also with normoxic exosomes, although hypoxic exosomes in many cases were more potent (Fig. S7 A and B). Medium from exosome-treated ECs was shown to trigger PI3K/AKT signaling in primary human brain vascular pericytes (HBVPs; Fig. S7 C and D). In line with previous studies on EC-mediated, paracrine activation of pericytes (39), EC conditioned medium per se was shown to stimulate pericyte migration (Fig. 4H; EC CM). Direct addition of exosomes, neither in the context of unconditioned medium (Exo) nor when added to EC conditioned medium (EC CM + Exo), did not significantly increase pericyte migratory activity (Fig. 4H). This result could not be explained by deficient uptake, because pericytes were shown to internalize exosomes at significant levels (Fig. S5 C and D). Interestingly, paracrine stimulation of pericyte migration was substantially potentiated by preconditioning of EC with GBM cell-derived exosomes (Fig. 4H; EC + Exo CM). We found similar exosome preconditioning effects in ECs with regard to paracrine stimulation of pericyte proliferation (Fig. 4I), and GBM cell migration and proliferation (Fig. 4 J and K). Importantly, the stimulatory activity was unperturbed when ECs were preconditioned with exosomes, followed by depletion of exosomes from conditioned medium before addition to pericytes and GBM cells (Fig. 4 I–K; EC + Exo CM Sol). Together, these results show that GBM cell-derived exosomes activate ECs to exert increased paracrine stimulation of pericytes and GBM cells.
Hypoxic Exosomes Accelerate GBM Tumor Growth and Angiogenesis.
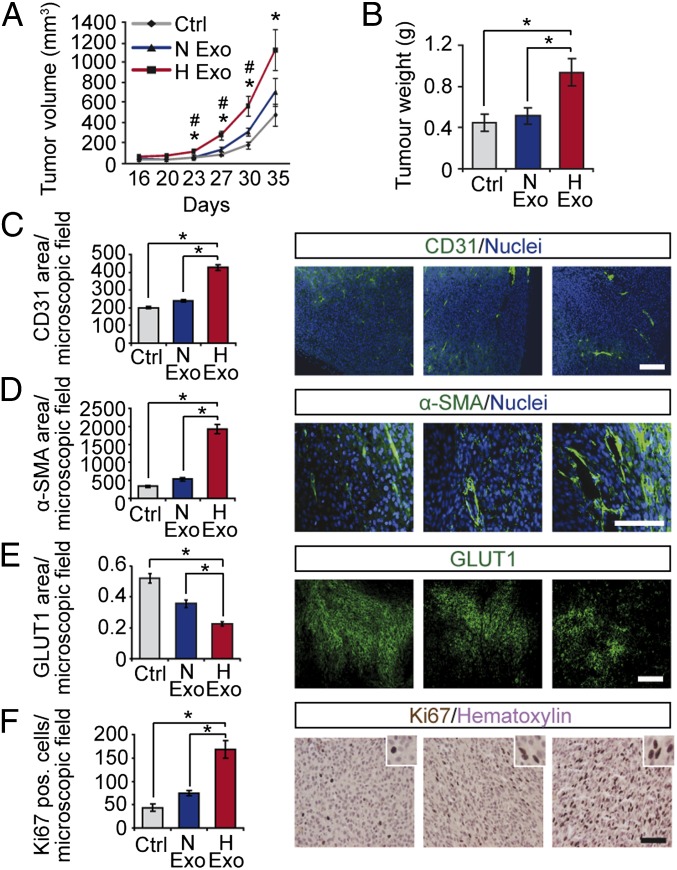
To investigate whether the above findings hold true in vivo, we next established a mouse GBM xenograft model (Fig. 5). During early lag phase, we found no significant effect of exosomes on tumor growth; interestingly, hypoxic exosomes were shown to substantially accelerate tumor expansion at later time points, resulting in an almost threefold increase in final tumor volume and 2.5-fold increase in final tumor weight compared with control tumors (Fig. 5 A and B). Although normoxic exosomes showed a tendency to stimulate tumor growth, hypoxic exosomes were significantly more potent (Fig. 5 A and B). Histological examination suggested that tumors grown in the presence of exosomes exhibited enhanced vascularisation compared with control tumors (Fig. S8). This notion was evidenced by immunofluorescent stainings for ECs, showing that tumor vascularisation was significantly increased by hypoxic exosomes compared with normoxic exosomes and untreated control (Fig. 5C). Remarkably, pericyte staining was increased by almost fourfold in tumors with hypoxic exosomes compared with normoxic exosomes and control tumors (Fig. 5D), indicating enhanced tumor vessel pericyte coverage. In support of these data, tumors with hypoxic exosomes compared with normoxic exosomes and untreated controls showed substantially decreased areas of hypoxia (Fig. 5E) and increased tumor cell proliferation (Fig. 5F).
Fig. 5.
Hypoxic induction of exosome-mediated stimulation of tumor development. Human GBM xenografts were established with or without exosomes (1 μg/mL) from normoxic (N Exo) or hypoxic (H Exo) GBM cells. Tumor volumes at the indicated time points (A) and final tumor weights (B) were determined. Data are presented as the mean ± SEM, *P < 0.05 compared with untreated Control; #P < 0.05 compared with N Exo tumors (n = 5). Tumors were analyzed by immunofluorescence microscopy for vascular density (C), pericyte coverage (D), hypoxic area (E), and proliferation (F). (Scale bars: 100 µm.) Results are the mean ± SEM, *P < 0.05.
Discussion
Tumor cells release diffusible factors that reshape the tumor microenvironment (41), e.g., by creating a distinct phenotype of the tumor endothelium compared with that of normal vasculature. Progress in isolating and identifying these factors contributed to a new era in cancer therapeutics development. So far, most of these strategies are based on targeting one or a few factors, most importantly VEGF. The complexity of the hypoxic signaling response in the tumor microenvironment (1–3, 7–12) clearly needs to be better defined to develop more rational therapies. Here, we provide evidence that exosome vesicles constitute a potent mediator of hypoxia-dependent intercellular communication between malignant and vascular cells, i.e., ECs and pericytes, suggesting an important role of exosomes in hypoxia-driven, phenotypic alteration of the tumor vasculature. Another significant finding of our work is that exosome vesicles from hypoxic conditions reflect the signaling status of hypoxic GBM cells and patient tumors.
Exosomes may participate in intercellular signaling at several levels, e.g., via protein ligands in the exosome membrane that activate signaling receptors and downstream kinases, and through the transfer of miRNAs, mRNAs, and signaling receptors to recipient cells (13–20). Our data show hypoxic regulation of a multitude of exosome-associated proteins and mRNAs and the activation of several signaling receptors and intracellular kinases in target cells. These results support the notion that hypoxic exosomes mediate a proangiogenic response through the concerted action of hundreds or even thousands of effector molecules. Previous studies have suggested that hypoxic compared with normoxic cells produce higher amounts of exosomes (42), and that more than half of the secreted proteome from hypoxic carcinoma cells may be associated with exosomes (43), providing quantitative data to support a complex role of exosomes in the hypoxic response. Strategies aimed at targeting this system should thus focus on general mechanisms of exosome-dependent intercellular communication, e.g., exosome uptake by recipient cells, exosome formation, and exosome stability in the extracellular milieu, rather than on single exosome constituents. Interestingly, recent studies have shown that the tumorigenic and prometastatic functions of exosomes could be efficiently counteracted by inhibition of the small GTPase Rab27a (44, 45), which has been shown to be involved in exosome formation (46).
We were initially intrigued by the finding that the mRNA expression signature observed in exosomes closely mirrors the transcriptome of donor cells. Previous studies with GBM cells (15) suggested specific sorting of mRNAs into exosomes. One possible explanation of these discrepancies is differences in normalization algorithms used for microarray hybridizations. The assumption that most transcripts are equally expressed in the compared RNA extractions from cells and exosomes, respectively, would result in data that favor the conclusion of higher expression of selected transcripts in exosomes compared with cells. Future studies in additional systems will have to clarify whether specific selection mechanisms operate during intracellular cargo loading into exosomes.
Numerous molecular markers, e.g., GLUT1, carbonic anhydrase 9 (CAIX), and hypoxia inducible factor (HIF), have been shown to correlate with hypoxia; however, the clinical utility of such markers is complicated by considerable intratumoral heterogeneity of hypoxia. Although our studies with GBM patient exosomes are limited in number, the presented data suggest the interesting possibility that the exosomal molecular signature consisting of, e.g., CAV1, IL8, PDGFs and MMPs, could provide a noninvasive, biomarker profile that reflects hypoxic signaling of GBM tumors.
In summary, our data suggest that exosomes constitute a potentially targetable mediator of hypoxia-driven tumor development, and that the exosomal molecular signature may serve as a noninvasive biomarker to assess the oxygenation status and aggressiveness of malignant tumors.
Materials and Methods
Detailed descriptions of reagents, cell lines and primary cells, exosome isolation, fluorescence and electron microscopy, immunoblotting, gene expression, in vitro and ex vivo functional experiments, and animal studies are listed in SI Materials and Methods.
Clinical Samples.
Tumor biopsy specimens were obtained from patients with GBM (World Health Organization grade IV) at the Department of Neurosurgery, Skåne University Hospital, Lund. Blood samples from GBM patients were collected before brain tumor surgery. Tumor and blood samples were collected with informed consent according to Protocol H15 642/2008 approved by the Lund University Regional Ethics Board, Lund, Sweden.
Exosome Isolation.
Exosomes were isolated by differential centrifugation as described in SI Materials and Methods.
Membrane-Based Antibody Arrays.
Total protein from cells and exosomes was analyzed by using human antibody arrays (R&D Systems) as described in SI Materials and Methods.
Gene Expression Microarray Analysis.
Microarray experiments were performed at Swegene Center for Integrative Biology at Lund University Genomics Center, Sweden, by using the Illumina HumanHT-12 v3 Expression BeadChip as described in SI Materials and Methods.
Quantitative Real-Time PCR.
cDNA synthesis was performed by using SuperScript III First-Strand Synthesis System (Life Technologies) and used for PCR based on SYBR Green I chemistry (Sigma) in an ABI PRISM 7900 HT machine (Applied Biosystems) as described in SI Materials and Methods.
Exosome Uptake.
Exosomes were labeled with PKH67 Green Fluorescent labeling kit (Sigma) as recommended by the manufacturer, and uptake was determined by flow cytometry and fluorescence microscopy as described in SI Materials and Methods.
Cell Proliferation Assay.
Cell proliferation was assessed by the [3H]-thymidine incorporation assay as described in SI Materials and Methods.
Survival Assay.
Cell survival was determined by the 7-amino-actinomycin D (7-AAD) and MTT assays as described in SI Materials and Methods.
Cell Migration Assay.
Cell migration was studied by the transwell assay as described in SI Materials and Methods.
Matrigel Tube Formation Assay.
EC tube formation on growth factor-reduced BD Matrigel (BD Bioscience) was determined as described in SI Materials and Methods.
Ex Vivo Mouse Aortic Ring Sprouting Assay.
Microvessel sprouting from nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mouse thoracic aortas in BD Matrigel was determined as described in SI Materials and Methods.
Laser-Capture Microdissection.
RNA from normoxic and hypoxic tumor regions was isolated by laser-capture microdissection for qRT-PCR analysis as described in SI Materials and Methods.
Animal Xenograft Tumor Model.
The experimental setup was approved by the Ethical Committee for Animal Research at Lund University in Malmö/Lund, Sweden. Eight-week-old female NOD/SCID mice were inoculated via s.c. injection on the dorsal region with U87 MG cells (2.5 × 106 in 150 µLof PBS) with or without normoxic or hypoxic exosomes (1 µg/mL). Processing of tumors after 5 wk of incubation were performed as described in SI Materials and Methods.
Statistical Analysis.
Statistical significance was evaluated with Student’s t test. A P value of <0.05 was considered statistically significant. Data shown from antibody array and Western blotting experiments are representative of at least two independent experiments.
Supplementary Material
Acknowledgments
We thank the members of the Neurosurgery and Oncology departments (Lund University) for many helpful discussions. This work was supported by grants from the Swedish Cancer Fund; the Swedish Research Council; the Swedish Society of Medicine; the Physiographic Society (Lund); the Gunnar Nilsson Foundation, the Anna Lisa and Sven Eric Lundgren Foundation, and the Kamprad Foundation; Skåne University Hospital donation funds; and governmental funding for clinical research in national health services (ALF).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE45301).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220998110/-/DCSupplemental.
References
- 1.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 3.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans SM, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10(24):8177–8184. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 5.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29(2):285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toustrup K, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71(17):5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 7.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 8.Liao D, Johnson RS. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26(2):281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 9.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: Risky liaisons, or not? Nat Rev Clin Oncol. 2011;8(7):393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 13.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 15.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 16.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: Implications in health and disease. J Cell Biol. 2008;183(7):1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 19.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 20.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Kaur B, et al. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncol. 2005;7(2):134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 23.Marotta D, et al. In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res. 2011;71(3):779–789. doi: 10.1158/0008-5472.CAN-10-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallinen SL, et al. Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res. 2000;60(23):6617–6622. [PubMed] [Google Scholar]
- 25.Abulrob A, et al. Interactions of EGFR and caveolin-1 in human glioblastoma cells: Evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene. 2004;23(41):6967–6979. doi: 10.1038/sj.onc.1207911. [DOI] [PubMed] [Google Scholar]
- 26.Cassoni P, et al. Caveolin-1 expression is variably displayed in astroglial-derived tumors and absent in oligodendrogliomas: Concrete premises for a new reliable diagnostic marker in gliomas. Am J Surg Pathol. 2007;31(5):760–769. doi: 10.1097/01.pas.0000213433.14740.5d. [DOI] [PubMed] [Google Scholar]
- 27.Parat MO, Riggins GJ. Caveolin-1, caveolae, and glioblastoma. Neuro-oncol. 2012;14(6):679–688. doi: 10.1093/neuonc/nos079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci USA. 2012;109(13):4892–4897. doi: 10.1073/pnas.1112129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark MJ, et al. U87MG decoded: The genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010;6(1):e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 32.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonyak MA, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA. 2011;108(12):4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson KJ, et al. Hypoxia triggers a pro-angiogenic pathway involving cancer cell derived microvesicles and PAR-2 mediated HB-EGF signaling in endothelial cells. Proc Natl Acad Sci USA. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickel W. The unconventional secretory machinery of fibroblast growth factor 2. Traffic. 2011;12(7):799–805. doi: 10.1111/j.1600-0854.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- 37.Rajaraman P, et al. Genome-wide association study of glioma and meta-analysis. Hum Genet. 2012;131(12):1877–1888. doi: 10.1007/s00439-012-1212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncol. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118(10):2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zetter BR. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980;285(5759):41–43. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]
- 42.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JE, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bobrie A, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 46.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.