Abstract
Despite recent insights gained from the effects of targeted deletion of the Finkel-Biskis-Jinkins osteosarcoma oncogene (c-fos), Spleen focus-forming virus (SFFV) proviral integration 1 (PU.1), microphthalmia-associated transcription factor, NF-κB, and nuclear factor of activated cells cytoplasmic 1 (NFATc1) transcription factor genes, the mechanism underlying transcription factors specifying osteoclast (OC) lineage commitment from monocyte/macrophage remains unclear. To characterize the mechanism by which transcription factors regulate OC lineage commitment, we mapped the critical cis-regulatory element in the promoter of cathepsin K (Ctsk), which is expressed specifically in OCs, and found that CCAAT/enhancer binding protein α (C/EBPα) is the critical cis-regulatory element binding protein. Our results indicate that C/EBPα is highly expressed in pre- OCs and OCs. The combined presence of macrophage colony-stimulating factor and receptor activator of NF-κB ligand significantly induces high C/EBPα expression. Furthermore, C/EBPα−/− newborn mice exhibited impaired osteoclastogenesis, and a severe osteopetrotic phenotype, but unaffected monocyte/macrophage development. Impaired osteoclastogenesis of C/EBPα−/− mouse bone marrow cells can be rescued by c-fos overexpression. Ectopic expression of C/EBPα in mouse bone marrow cells and monocyte/macrophage cells, in the absence of receptor activator of NF-κB ligand, induces expression of receptor activator of NF-κB, c-fos, Nfatc1, and Ctsk, and it reprograms monocyte/macrophage cells to OC-like cells. Our results demonstrate that C/EBPα directly up-regulates c-fos expression. C/EBPα+/− mice exhibit an increase in bone density compared with C/EBPα+/+ controls. These discoveries establish C/EBPα as the key transcriptional regulator of OC lineage commitment, providing a unique therapeutic target for diseases of excessive bone resorption, such as osteoporosis and arthritis.
Keywords: cell lineage commitment, gene expression regulation, osteoclast differentiation, bone homeostasis, osteopetrosis phenotype
Osteoclasts (OCs), bone-resorbing cells, play a key role in normal bone remodeling and in many diseases, such as osteopetrosis, osteoporosis, arthritis, periodontal disease, and certain bone metastases. OCs develop from monocytic precursors from the hematopoietic lineage. Spleen focus-forming virus (SFFV) proviral integration 1 (PU.1) can induce the expression of the macrophage colony-stimulating factor (M-CSF) receptor and is critical for monocyte/macrophage lineage commitment (1). On receptor activator of NF-κB ligand (RANKL) stimulation and immunoreceptor tyrosine-based activation motif (ITAM) activation, OC precursors undergo further differentiation to mononuclear OCs (2). RANKL specifically and potently induces nuclear factor of activated T cells cytoplasmic 1 (NFATc1), which is a master regulator of OC differentiation, through both the TNF receptor-associated factor 6 (TRAF6)–NF-κB (p105n) pathway and the Finkel-Biskis-Jinkins osteosarcoma oncogene (c-Fos) pathway (3–7). This robust induction of NFATc1 is based on an autoamplifying mechanism effected through persistent calcium signal-mediated activation of NFATc1 (NFATc1 binds to NFAT binding sites on its own promoter, constituting a positive feedback loop) (7). In the nucleus, NFATc1 cooperates with other transcription factors, such as activator protein-1, PU.1, cAMP-responsive element binding protein, and microphthalmia-associated transcription factor, to induce various OC-specific genes (7, 8). NFATc1, together with other transcription factors, such as c-fos and NF-κB, drives osteoclastogenesis (2, 5). Peroxisome proliferator-activated receptor γ (PPARγ) is also reported to be important for OC differentiation (9). Although much has been revealed pertaining to the regulation of terminal differentiation of OCs, the cascade of transcription factors that specifies OC lineage commitment from monocytes/macrophages remains unclear (10). Indeed, a long-standing challenge remaining in the field is to define the mechanism by which M-CSF acts with RANKL to promote OC differentiation, whereas it promotes macrophage differentiation when acting alone (10), and to define the factor that regulates OC lineage commitment.
Cathepsin K (Ctsk) is a lysosomal cysteine protease that is abundantly and selectively expressed in OCs (11).The specific expression of the Ctsk in OCs, as well as its enzymatic properties, implies that it has a key role in normal bone remodeling and in pathological processes, such as osteoporosis, osteoarthritis, and pycnodysostosis (12). Because expression of Ctsk is OC-specific and induced by RANKL stimulation, we characterized the Ctsk critical cis-regulatory elements (CCREs) to identify the transcription factor(s) that modulate OC-specific gene (e.g., Ctsk) expression and to determine which factor(s) enable monocytes to commit to the OC lineage after RANKL stimulation.
Our research revealed that CCAAT/enhancer binding protein α (C/EBPα) regulates OC cell lineage commitment and differentiation. C/EBPα is a key molecular determinant in myeloid lineage commitment (13, 14), which drives myeloid differentiation through activation of myeloid target genes and through inhibition of the cell cycle through interactions with regulatory proteins (15, 16). We characterized C/EBPα as the binding protein of the OC gene Ctsk CCRE. We found that the deletion of C/EBPα in KO mice results in severely blocked osteoclastogenesis and that the overexpression of c-fos rescues osteoclastogenesis when C/EBPα is knocked out. Forced expression of C/EBPα, in the absence of RANKL, induces expression of receptor activator of NF-κB (RANK) and OC genes and reprograms the monocyte/macrophage cell line to OC-like cells. These discoveries establish C/EBPα as the key regulator of OC lineage commitment.
Results
Identification of Mouse Ctsk CCRE and Characterization of CCRE DNA Binding Protein.
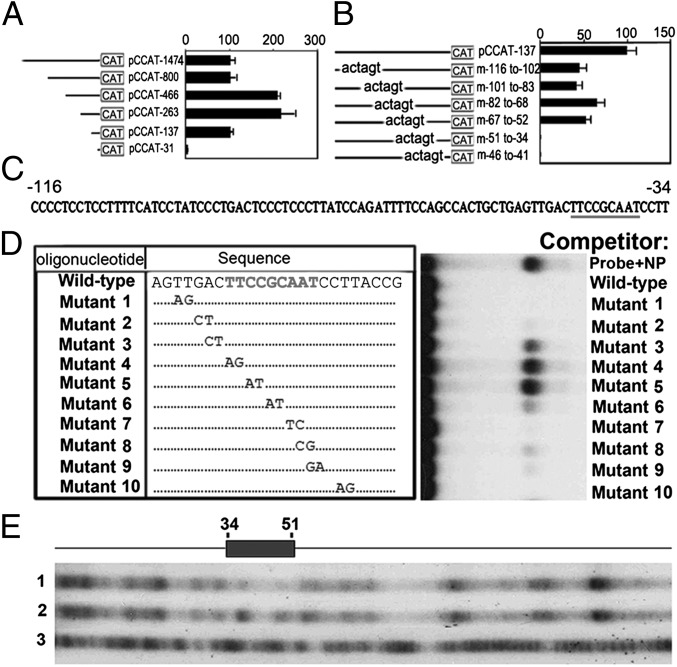
To understand the transcription factors that modulate OC gene expression and OC differentiation, we characterized the Ctsk CCREs. Our results show that RANKL stimulates Ctsk gene promoter activity and indicate the importance of RANKL in combination with the CCRE (Fig. 1A). To analyze the −137 to −31 region identified as containing a potential CCRE further, we examined the effect of internal deletions within the promoter-CAT fusion gene (pCCAT)-137 construct on Ctsk activity and found that internal deletions of the sequence between −51 and −34 or between −46 and −41 resulted in the total loss of Ctsk promoter activity compared with the complete pCCAT-137 construct (Fig. 1B). Thus, we determined that the sequence between −51 and −34 contains a Ctsk CCRE (Fig. 1C). We performed a gel mobility shift experiment using the 32P-labeled WT Ctsk oligonucleotide probe in nuclear extracts prepared from mouse OC precursor cell line RAW264.7 cells induced with RANKL, with and without a 100-fold molar excess of unlabeled WT or 10 different mutant oligonucleotides. We found that a 100-fold molar excess of mutants 3 and 6 partially competes, whereas mutants 4 and 5 are completely unable to compete, for the DNA–protein complex, indicating that this core sequence of nucleotides is essential for DNA–protein interaction (Fig. 1D). We analyzed this sequence using transcription factor binding site database searches and found that it is a C/EBPα binding site (TTCCGCAAT). This indicates that C/EBPα may regulate the CCRE. We also performed DNase I footprint analysis and found that the −51 to −34 region, which contains the C/EBPα binding site, was protected (Fig. 1E). To confirm that C/EBPα binds to this CCRE recognition site, we performed supershift mobility assays using a panel of specific antibodies against different C/EBP family members (Fig. S1). The bound proteins only produced supershifted complexes in the presence of C/EBPα antibody, indicating that C/EBPα is involved in the formation of this DNA–protein complex (Fig. S1). Together, these data indicate that C/EBPα may be a major component of the OC-specific complex that regulates the Ctsk CCRE.
Fig. 1.
Identification of Ctsk CCRE and CCRE DNA binding protein as C/EBPα. (A) Activity of deletion mutants of the pCCAT construct was measured in RANKL-induced RAW64.7 cells. Schematic representation of each reporter construct is shown. Values are relative to the activity obtained with the largest promoter fragment (pCCAT-1474). CAT activities are normalized with the cotransfected β-gal activity. Experimental data are reported as mean ± SD of triplicate independent samples (n = 5, repeated three times). (B and C) Summary of site-specific mutagenesis of the Ctsk promoter binding site from −116 to −34 and characterization of the CCRE as a C/EBPα site. (D) Gel mobility shift experiment using a 32P-labeled −53 to −30 WT Ctsk promoter oligonucleotide probe in nuclear extracts prepared from RANKL-induced RAW264.7 cells, with and without 100-fold molar excess of unlabeled WT or mutant oligonucleotides as described on the left. NP, nuclear protein. (E) DNase I footprint assays identify a binding site between −34 to −51 in the −137 to −31 mouse Ctsk promoter. The protected region is indicated by a shaded box, which contains a C/EBPα site. DNase I digestion of the naked DNA incubated with (lane 1) and without (lane 2) RANKL-induced RAW264.7 cell nuclear extracts is shown. G + A Maxam–Gilbert reaction of the −137 to −31 promoter fragment is shown in lane 3.
C/EBPα Is Expressed in Pre-OCs and OCs and Is Induced by RANKL.
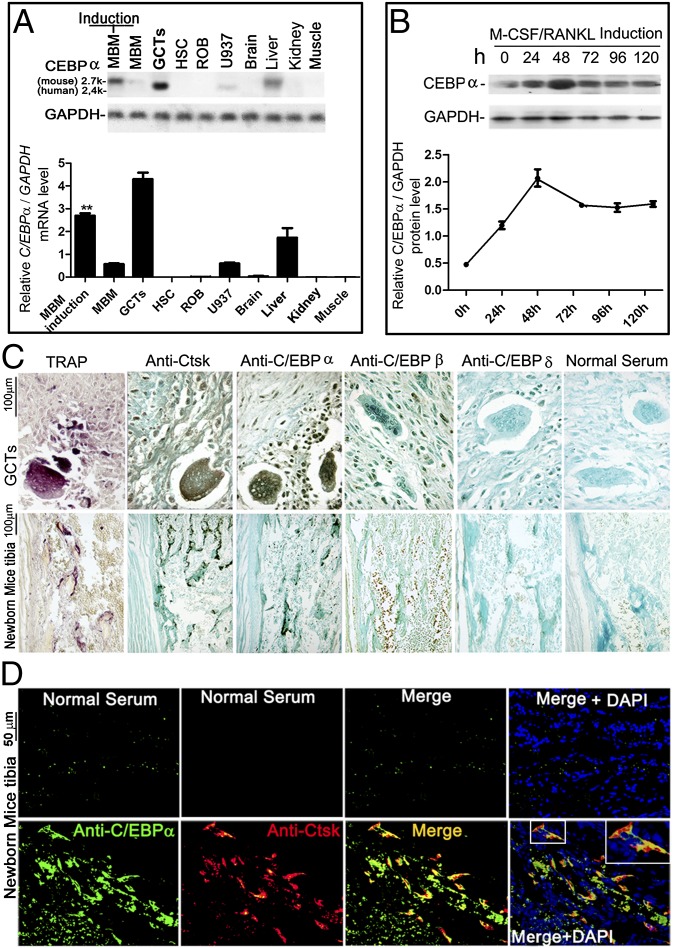
We next examined the tissue and cellular distribution of C/EBPα mRNA by performing a Northern hybridization assay. Giant cell tumors (GCTs) of bone contain human stromal cells (hSCs), OC-like precursors, and OC-like giant cells. Because OC-like giant cells from GCTs of bone are effectively the same as OCs in bone, we used GCTs and hSCs obtained as previously described (11). In addition, RANKL-induced mouse bone marrow (MBM), uninduced MBM, rat osteoblast, and a human macrophage cell line (U-937 cells) were used along with C/EBPα+/+ mouse tissues. The human C/EBPα transcript (2.4 kb) and mouse C/EBPα transcript (2.7 kb) used as C/EBPα-cDNA probes for this Northern blot assay are highly specific to C/EBPα. As shown in Fig. 2A, C/EBPα (2.7 kb) was highly expressed in RANKL-induced MBM and C/EBPα+/+ liver tissue. C/EBPα (2.4 kb) was prominently expressed in GCTs of bone (which contain hSCs, OC-like precursors, and OC-like giant cells) but was not expressed in hSCs (Fig. 2A). Furthermore, there was very low C/EBPα expression in U-937 cells, MBM, and mouse kidney and brain tissue. Expression of C/EBPα could not be detected in other tissues in these conditions, but this does not exclude a role for C/EBPα in those tissues. In order to characterize C/EBPα expression during OC differentiation, we performed Western blot analysis of C/EBPα expression in MBM cultured with M-CSF (20 ng/mL) alone for 2 d and then stimulated with M-CSF (10 ng/mL)/RANKL (10 ng/mL) for 0–120 h. We found that C/EBPα expression more than doubled after 24 h of stimulation. Interestingly, C/EBPα expression peaked at 48 h but retained high expression thereafter (Fig. 2B). The dramatic increase in C/EBPα expression early during OC differentiation indicates that C/EBPα may play a critical role in the regulation of OC commitment and OC differentiation. To determine the expression of Ctsk and C/EBP family members (i.e., C/EBPα, C/EBPβ, C/EBPδ) in situ, OC cells were subjected to immunohistochemical staining (17). We found strong C/EBPα expression in Ctsk-positive multinucleated OCs and mononuclear pre-OCs in GCTs and C/EBPα+/+ tibiae in a similar pattern of expression as Ctsk (Fig. 2C). In contrast, C/EBPβ was weakly expressed in TNF receptor-associated protein 1 (TRAP)-positive mononuclear pre-OCs, and C/EBPδ expression was not detected in GCTs or C/EBPα+/+ tibiae (Fig. 2C). Immunofluorescence staining of C/EBPα+/+ tibiae also revealed that C/EBPα colocalized with Ctsk (Fig. 2D). The pattern of C/EBPα expression indicates a possible critical function of C/EBPα in OC cell lineage commitment and differentiation.
Fig. 2.
C/EBPα is expressed in pre-OCs and OCs, and it is induced by RANKL. (A) Northern blot hybridization using a C/EBPα-specific probe in RANKL-induced and uninduced C/EBPα+/+ MBM, GCTs of bone, hSCs, rat osteoblast (ROB), a human macrophage cell line (U-937 cells), and C/EBPα+/+ mouse tissues. (B) Time-course Western blot analysis of C/EBPα expression in C/EBPα+/+ MBM cultured with M-CSF (20 ng/mL) alone for 2 d and then stimulated with M-CSF (10 ng/mL)/RANKL (10 ng/mL) for 0–120 h. (C) TRAP staining and immunostaining for Ctsk, C/EBPα, C/EBPβ, and C/EBPδ in GCTs of bone and C/EBPα+/+ tibiae sections. Normal serum is shown as a control. (D) Immunofluorescence staining of C/EBPα (green) and Ctsk (red) in C/EBPα+/+ tibiae indicates significant overlap, visualized as a yellow area in the merged image (n > 9). Inset is the magnified image of the boxed area.
CEBPα−/− Newborn Mice Exhibited a Severe Osteopetrotic Phenotype Due to Impaired Osteoclastogenesis.
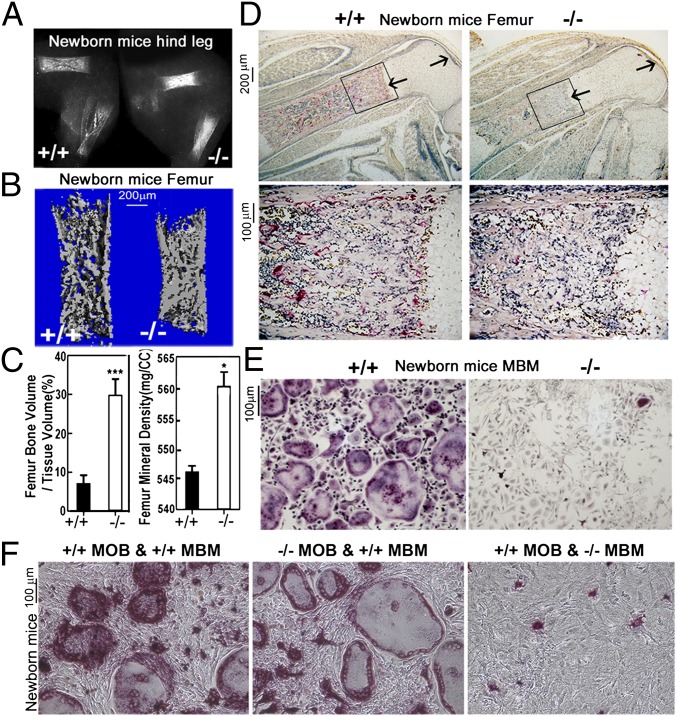
X-ray analyses revealed that C/EBPα−/− mice have dramatically increased bone density compared with C/EBPα+/+ mice (Fig. 3A), indicating that C/EBPα−/− mice have an osteopetrotic phenotype. This is supported by microcomputed tomography analysis, which showed significantly increased bone volume and bone mineral density in C/EBPα−/− femora compared with C/EBPα+/+ femora (Fig. 3 B and C). In order to analyze the role of C/EBPα in OCs further, we examined whether a null mutation in C/EBPα alleles affects the differentiation of OCs (17). Our histochemical stains revealed that C/EBPα−/− mice tibiae have long growth plates and a dramatic reduction in TRAP+ OCs compared with C/EBPα+/+ controls (Fig. 3D). Furthermore, TRAP stains demonstrated that TRAP+ multinucleated OCs were not formed in RANKL-induced MBM culture from C/EBPα−/− mice compared with C/EBPα+/+ controls (Fig. 3E). These data confirm that C/EBPα−/− mice are osteopetrotic and indicate that this phenotype is mainly due to defective osteoclastogenesis. In order to determine if C/EBPα−/− osteoblasts contribute to the defective osteoclastogenesis observed in C/EBPα−/− mice, we used a coculture system and TRAP stain as we have previously described (17). We determined that a coculture of calvarial mouse osteoblasts (MOBs) derived from C/EBPα+/+ mice with MBM from C/EBPα−/− mice is unable to rescue osteoclastogenesis (compared with C/EBPα+/+ MOBs with C/EBPα+/+ MBM) (Fig. 3F). Coculture of C/EBPα−/− MOBs with C/EBPα+/+ MBM revealed that mutant osteoblasts sustain osteoclastogenesis (Fig. 3F), indicating that osteoblasts lacking C/EBPα do not contribute to the defective osteoclastogenesis in C/EBPα−/− mice.
Fig. 3.
Increased bone mass and defective osteoclastogenesis in C/EBPα−/− mice in vivo and in vitro. (A) Radiographic analysis of femora and tibiae indicates osteopetrosis in C/EBPα−/− newborn mice compared with C/EBPα+/+ newborn mice (n = 5, repeated three times). (B) Three-dimensional microcomputed tomography images of C/EBPα+/+ and C/EBPα−/− newborn mice femora (n = 5, repeated three times). (C) Quantification of bone volume/tissue volume and bone mineral density of C/EBPα+/+ and C/EBPα−/− newborn mice femora. *P < 0.05; ***P < 0.001. (D) Histological sections of C/EBPα−/− newborn mice tibiae have an extended growth plates (arrows) and a dramatic reduction in TRAP+ OCs compared with C/EBPα+/+ controls (n > 50). Boxed areas (Upper) are magnified (Lower). The histological sections of the same area of C/EBPα−/− newborn mice also show dramatically decreased TRAP+ OCs. (E) TRAP stain of RANKL-induced C/EBPα+/+ and C/EBPα−/− MBM. (F) TRAP stain of cocultures with MOBs and MBM obtained from C/EBPα−/− and C/EBPα+/+ mice.
C/EBPα KO Reduces the Expression of OC Differentiation Regulator and Marker Genes.
To determine the mechanism of C/EBPα function in OC commitment and differentiation, we performed real-time (RT) quantitative PCR (qPCR) analysis of gene expression in C/EBPα+/+ and C/EBPα−/− MBM cells cultured with M-CSF/RANKL to generate OC-like cells (Table S1) (12, 17, 18). The RT-qPCR results (Fig. S2) suggest that C/EBPα is important for the induction of OC-specific genes and that C/EBPα may suppress macrophage-specific genes. These results were confirmed by Western blot analysis of C/EBPα+/+ and C/EBPα−/− MBM cells-derived OC-like cells, which showed that Ctsk, c-fos, and Nfatc1 decreased in C/EBPα−/− cells compared with control (Fig. 4A). Additionally, there was a significant difference in the protein level of PU.1 in C/EBPα+/+ and C/EBPα−/− MBM cells OC-like cells (Fig. 4 A and B). Time-course Western blot analysis revealed that there are no major changes in the expression of NF-κB inhibitor, inhibitor of κBα (IκBα), and MAPK 14 (p38) or their phosphorylated forms (p-IκBα and p-p38) in C/EBPα−/− MBM cells cultured with M-CSF/RANKL for 0–60 min, compared with the C/EBPα+/+ control (Fig. 4C). These data indicate that the role of C/EBPα in OCs does not involve the IκBα or p38 signaling pathways but that it may directly regulate the c-fos arm of the RANKL signaling pathways to promote osteoclastogenesis.
Fig. 4.
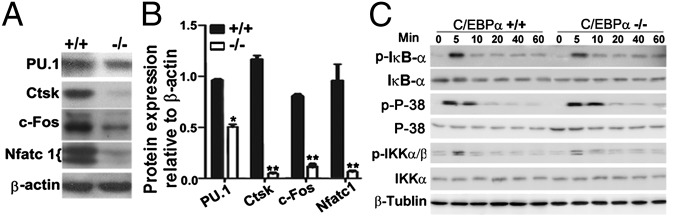
C/EBPα KO reduces the expression of key OC regulators and marker genes. Western blot (A) and quantification of expression (B) of PU.1, Ctsk, c-Fos, and Nfatc1 in C/EBPα+/+ and C/EBPα−/− MBM cells cultured with M-CSF (20 ng/mL) alone for 2 d and then stimulated with M-CSF (10 ng/mL)/RANKL (10 ng/mL) for 5 d to generate OC-like cells. *P < 0.05; **P < 0.01. (C) Time-course Western blot analysis of IκBα and p38 and their phosphorylated forms (p-IκBα and p-p38) in C/EBPα+/+ and C/EBPα−/− MBM cells cultured with M-CSF (20 ng/mL) alone for 2 d and then stimulated with M-CSF (10 ng/mL)/RANKL (10 ng/mL) for 0–60 min.
Both C/EBPα−/− and C/EBPα+/− Mice Exhibit an Increase in Trabecular Bone Numbers.
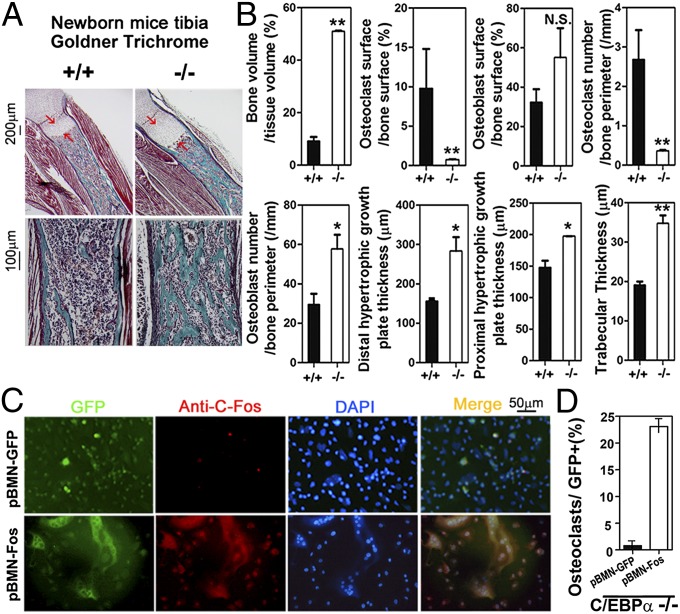
To analyze the role of C/EBPα in bone homeostasis further, we stained C/EBPα+/+ and C/EBPα−/− tibiae sections with Goldner’s trichrome stain and performed histomorphometric analysis (using Bioquant software; BIOQUANT Image Analysis Corporation). Compared with C/EBPα+/+ controls, C/EBPα−/− tibiae have growth plates with an extended zone of growth plate and a dramatic increase in mineralized tissue, which indicates osteopetrosis (Fig. 5A). Histomorphometric analysis revealed that KO of C/EBPα results in increased bone volume, distal and proximal hypertrophic growth plate thickness, and trabecular thickness (Fig. 5B). Our analyses also revealed that the ratio of OC surface to bone surface and the number of OCs per bone perimeter dramatically decreased in C/EBPα−/− tibiae compared with C/EBPα+/+ tibiae (Fig. 5B), further confirming the important role of C/EBPα in osteoclastogenesis. To determine the importance of the C/EBPα dose in mature animals, we analyzed the bone phenotype in C/EBPα+/− mature mice.
Fig. 5.
C/EBPα−/− mice exhibit a dramatic increase in trabecular bone number, and C/EBPα−/− impaired osteoclastogenesis is rescued by ectopic expression of c-fos. (A) Compared with C/EBPα+/+ controls, C/EBPα−/− tibiae have extended growth plates (arrows) and a dramatic increase in mineralized tissue (bright blue regions), which indicates osteopetrosis (n = 3, repeated three times). (B) Histomorphometric analysis of tibiae from C/EBPα+/+ and C/EBPα−/− mice. *P < 0.05; **P < 0.01. N.S., not significant. (C and D) Impaired OC differentiation in C/EBPα−/− MBM cells cultured with M-CSF/RANKL can be rescued by ectopic expression of c-fos using pBMN–c-fos compared with control virus (pBMN-GFP). ***P < 0.001.
Femora from heterozygous C/EBPα+/− mice (8 mo of age) exhibit an increase in bone density and an increase in trabecular bone compared with C/EBPα+/+ controls (Fig. S3). Goldner’s trichrome stain and histomorphometric analysis indicate that C/EBPα+/− femora have an increase in bone volume/tissue volume and trabecular number and a decrease in OC surface/bone surface and OC number/bone perimeter (Figs. S4 and S5).
C/EBPα−/− Impaired Osteoclastogenesis Is Rescued by Ectopic Expression of c-fos.
In order to confirm if C/EBPα regulates osteoclastogenesis through the c-fos arm of the RANKL signaling pathway, we next investigated whether ectopic expression of c-fos using retrovirus-mediated gene transfer could rescue the impaired OC differentiation caused by C/EBPα KO. MBM cells were stimulated with M-CSF/RANKL for OC differentiation analysis. These cultured C/EBPα−/− MBM cells were transduced by pBMN–c-fos or the control retrovirus pBMN-GFP (Fig. 5 C and D). Compared with the control, the number of multinucleated OCs present increased more than 20-fold when c-fos was overexpressed in cultured C/EBPα−/− myeloid cells (Fig. 5 C and D). This indicates that impaired OC differentiation caused by C/EBPα KO can be rescued by ectopic expression of c-fos.
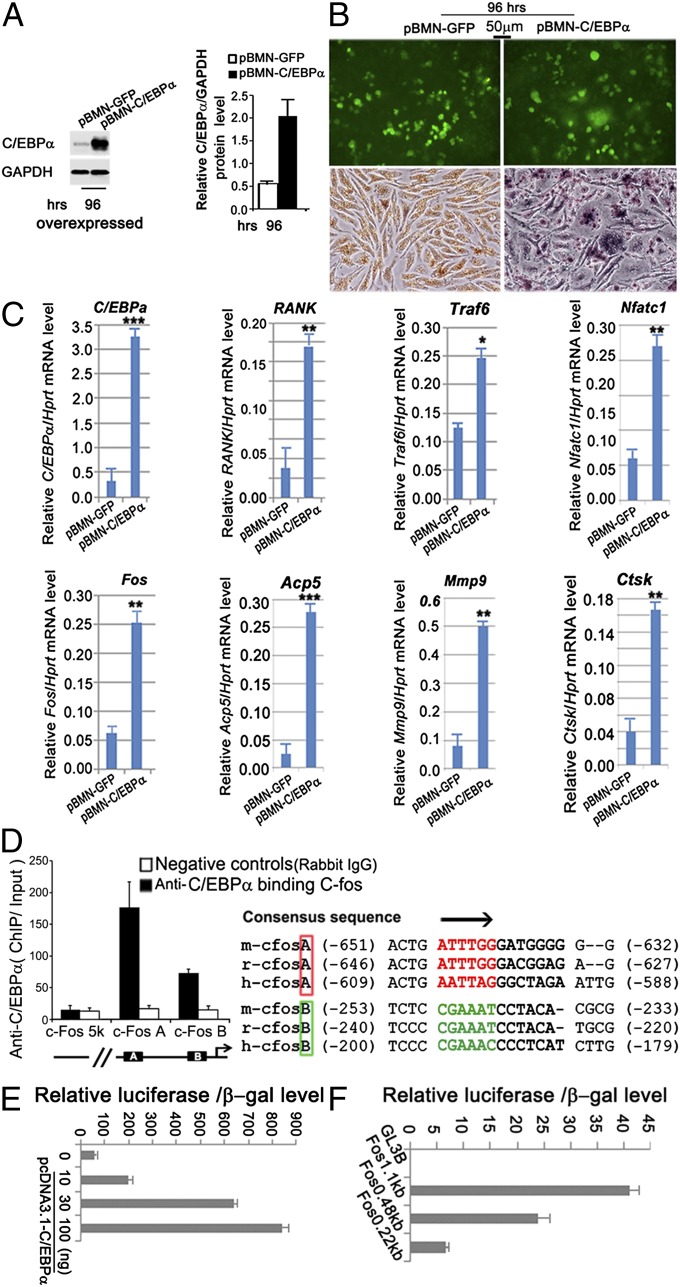
C/EBPα Induces Osteoclastogenesis in the Absence of RANKL and Activates the c-fos Promoter.
To determine the mechanism of C/EBPα function in OC differentiation and gene expression further, we forced expression of C/EBPα in MBM cells by stimulating MBM with M-CSF alone (Fig. 6). To express C/EBPα in MBM ectopically, we constructed a retrovirus vector (pBMN-C/EBPα) that was engineered to express both C/EBPα and GFP. Then, MBM was transduced with the C/EBPα-expressing virus (pBMN-C/EBPα) or with a control virus (pBMN-GFP) as described (3). Western blot analysis demonstrates the effective retrovirus-mediated overexpression of C/EBPα in MBM stimulated with M-CSF alone for 96 h (Fig. 6A). The expression of C/EBPα increased significantly after 96 h of M-CSF stimulation (Fig. 6A). At the 96-h time point, there is a surprising marked increase in the number of TRAP+ multinucleated OCs when C/EBPα is overexpressed compared with the control group even in the absence of RANKL stimulation. This underscores the important role of C/EBPα in osteoclastogenesis (Fig. 6B). Through qPCR, it was determined that the expression of markers common for macrophages and OCs (e.g., RANK, Traf6), key OC genes (e.g., Nfatc1, c-fos, acid phosphatase 5 (Acp5), matrix metallopeptidase 9 (Mmp9), Ctsk), and C/EBPα increased in the pBMN-C/EBPα transfected group (Fig. 6C). These data indicate that C/EBPα overexpression in the absence of RANKL stimulation can induce the formation of TRAP+ multinucleated cells (Fig. 6 B and C). Because our results showed that overexpression of c-Fos can rescue the OC defect of C/EBPα−/− cells (Fig. 5C), it is important to determine if the c-fos promoter has C/EBPα binding sites. Accordingly, we performed ChIP assays with MBM stimulated with RANKL to generate mature OCs and found that C/EBPα directly binds regulatory sequences on the c-fos promoters (Fig. 6D) and activates c-fos expression (Fig. 6E). The truncated c-fos promoters were induced by C/EBPα, indicating the presence of two C/EBPα binding sites (Fig. 6F).
Fig. 6.
C/EBPα induces osteoclastogenesis in the absence of RANKL and activates the c-fos promoter. (A) Western blot and quantification of forced expression of C/EBPα in C/EBPα+/+ MBM stimulated with 20 ng/mL M-CSF for 24 h and then transduced with pBMN-C/EBPα for 96 h. Compared with the control group, C/EBPα expression increased significantly 96 h after cells were transduced with pBMN-C/EBPα. (B) GFP expression and TRAP expression in C/EBPα+/+ MBM in which C/EBPα is overexpressed (pBMN-C/EBPα) compared with the control (pBMN-GFP) after 96 h of stimulation with M-CSF (20 ng/mL) alone. (C) qPCR analysis in MBM cultured in M-CSF alone and transduced with pBMN-C/EBPα (+) or with the vector control pBMN-GFP for 96 h. *P < 0.05; **P < 0.01; ***P < 0.001. (D) ChIP analysis of C/EBPα binding to the c-fosA, c-fosB, or upstream promoter region [c-fos (−5 Kb) as a negative control] in MBM stimulated with RANKL/M-CSF to generate mature OCs. (E) Consensus sequence alignment of putative C/EBPα binding sites of mouse, rat, and human c-fos promoter regions. C/EBPα activates c-Fos expression. (F) Truncated c-fos promoters were induced by C/EBPα. Luciferase reporter assays with truncated c-fos promoters indicated the presence of two C/EBPα binding sites (GL3B, control vector).
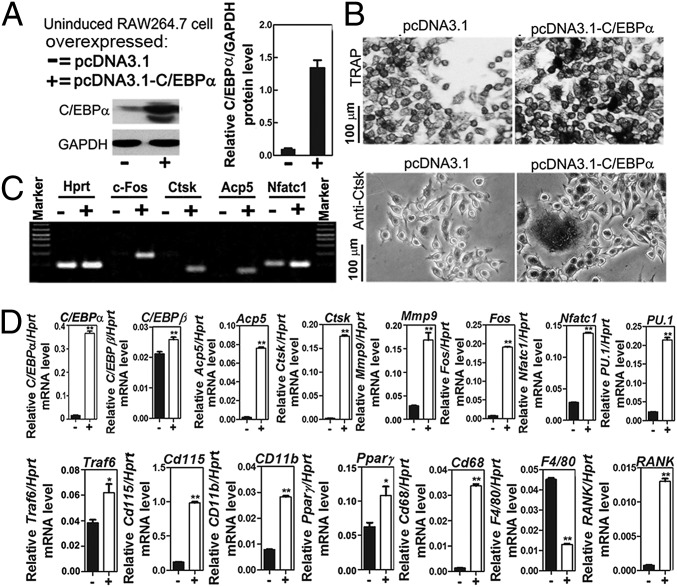
Ectopic Expression of C/EBPα Reprograms the Monocyte/Macrophage Cell Line to OC-Like Cells in the Absence of RANKL.
To question whether ectopic expression of C/EBPα can reprogram the monocyte/macrophage cell line RAW264.7 cells to OC-like cells in the absence of RANKL, we ectopically expressed C/EBPα in RAW264.7 cells by using pcDNA3.1-C/EBPα or the vector control pcDNA3.1 (Fig. 7 A–D). Western blot analysis demonstrated the effective overexpression of C/EBPα by the plasmid stable transfection approach (Fig. 7A). Similarly, TRAP staining revealed that TRAP+ OCs developed when C/EBPα was overexpressed in the absence of RANKL stimulation (Fig. 7B). In addition, Ctsk immunostaining demonstrated that C/EBPα overexpression in the absence of RANKL stimulation still resulted in the expression of the OC marker gene Ctsk (Fig. 7B). C/EBPα overexpression reduced the expression of F4/80, which is a macrophage marker gene. Semi-qPCR of c-fos, Ctsk, Acp5, and Nfatc1 also demonstrated that C/EBPα overexpression increases the expression of key OC genes even in the absence of RANKL (Fig. 7C). We found that C/EBPα overexpression increased the expression of genes important for OC lineage commitment and differentiation (e.g., Ctsk, PU.1, c-fos, CD115, Traf6, CD11b, Acp5, Nfatc1, Mmp9, Pparγ, CD68, RANK) (9) (Fig. 7D) through qPCR analysis of gene expression. Expression of C/EBPβ increased slightly when C/EBPα was overexpressed in uninduced RAW264.7 cells (Fig. 7D).
Fig. 7.
Ectopic expression of C/EBPα reprograms the monocyte/macrophage cell line to OC-like cells in the absence of RANKL. (A) Western blot of C/EBPα expression in RAW264.7 cells (not stimulated with M-CSF/RANKL) transfected with pcDNA3.1-C/EBPα (+) or with the vector control pcDNA3.1 (−). (B) TRAP and Ctsk immunostaining in uninduced RAW264.7 cells in which C/EBPα is overexpressed [pcDNA3.1-C/EBPα (+)] compared with the control [pcDNA3.1 (−)]. Semi-qPCR (C) and qPCR (D) analysis (25 cycles, Hprt served as a loading control) of genes important for monocytes/macrophages and OCs in uninduced RAW264.7 cells transfected with pcDNA3.1-C/EBPα (+) or with the vector control pcDNA3.1 (−).*P < 0.05; **P < 0.01.
Discussion
In this study, we demonstrated that C/EBPα expression is essential for OC lineage commitment but that it may not be essential for macrophage differentiation. Importantly, forced expression of C/EBPα in MBM and RAW264.7 cells induced the expression of OC marker genes in the absence of RANKL stimulation (Figs. 6 and 7), which demonstrates that high expression of C/EBPα is sufficient for OC lineage commitment. This study filled the gap in our understanding of how transcription factor(s) specify OC lineage commitment from monocyte/macrophage cells.
C/EBPα Expression Is Essential for OC Lineage Commitment but May Not Be Essential for Macrophage Differentiation.
We sought insight into the mechanism underlying C/EBPα’s role in OC differentiation. Specifically, it is important to clarify if C/EBPα expression is important for OC lineage commitment alone or if it is also important for monocyte/macrophage differentiation because OCs are multinucleated cells derived from hematopoietic precursors of the monocyte/macrophage series. Feng et al. (19) discovered that the combined expression of PU.1 and C/EBPα is sufficient to activate a myeloid program in fibroblasts, which are derived from mesenchymal stem cells, and to induce a macrophage-like phenotype. It has also been shown that enforced expression of C/EBPα and C/EBPβ in B cells leads to their rapid and efficient reprogramming into macrophages (20). Di Tullio et al. (21) recently clarified that this C/EBPα-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation. These studies indicate that C/EBPα is important for monocyte/macrophage differentiation. It has been determined that the loss of C/EBPα gene expression in hematopoietic progenitor cells results in a block in differentiation at the common myeloid progenitor and granulocyte/macrophage progenitor stage of development (22, 23), indicating that C/EBPα expression is important for the development of macrophage CFUs. There is some controversy regarding the requirement of C/EBPα for macrophage development (22, 23). Heath et al. (23) demonstrated that there was a defect in macrophage development in mice that received transplants of C/EBPα−/− fetal liver cells and that C/EBPα−/− fetal liver cells had decreased M-CSF receptor (Cd115). In contrast, Zhang et al. (22) determined that circulating blood and hematopoietic organs from C/EBPα−/− mice did not lack monocytes or macrophages and that there was no defect in the M-CSF receptor. Consistent with the study by Zhang et al. (22), our analysis of C/EBPα−/− fetal liver cells, C/EBPα−/− tibiae, and MBM-derived monocyte/macrophage cells indicates that C/EBPα is not essential for macrophage differentiation (Figs. S6–S8). These contrasting results are likely due to differences in the experimental systems utilized, such as C/EBPα−/− mice and mice transplanted with C/EBPα−/− fetal liver cells.
C/EBPα Is a Major Regulator of OC Lineage Commitment but Is Not Sufficient for Terminal OC Differentiation.
C-fos functions downstream of RANKL signaling and plays an essential function in the developmental stage from monocyte/macrophage precursors to mature OCs (5). C-fos is also important for the expression of Nfactc1, which is a regulator of terminal differentiation of OCs (3). Importantly, forced expression of C/EBPα, even in the absence of RANKL stimulation, induced the expression of these key OC transcription factors (e.g., c-fos, Nfatc1) and PU.1 (24) (Fig. 6). Our data indicate that C/EBPα may directly regulate c-fos to promote osteoclastogenesis. We determined that the c-fos promoter has two C/EBPα binding sites (Fig. 6D) and that C/EBPα may activate the c-fos promoter through the two C/EBPα binding sites (Fig 6 E and F). Notably, our results indicate that C/EBPα modulates both OC lineage commitment and terminal OC differentiation. Indeed, C/EBPα binding sites are present at both the c-fos promoter and the Ctsk promoter, the latter of which is a gene expressed by committed OCs. In addition, ectopic expression of C/EBPα induces upregulation of both early and late OC markers. Thus, these findings demonstrate that high expression of C/EBPα alone is sufficient for OC lineage commitment and that C/EBPα also modulates terminal OC differentiation.
Potential Mechanism Underlying C/EBPα Is Induced by RANKL.
How C/EBPα is induced by RANKL is unclear. However, NF-κB, which is induced by RANKL, regulates C/EBPα expression in myeloid cells. It is thus possible that RANKL induces NF-κB expression, which, in turn, activates C/EBPα expression. It was noted that C/EBPα overexpression largely increased RANK (RANKL receptor) expression (Figs. 6C and 7D). Thus, another way that C/EBPα may be induced by RANKL (ligand) is through a self-amplification pathway. We suspect that C/EBPα upregulates the expression of receptor RANK, which allows for increased binding of RANKL and triggers the cascade of transcription factors specifying OC lineage commitment.
Importantly, our research indicates that a threshold level of C/EBPα expression is an important component in the mechanism by which M-CSF acts with RANKL to promote OC differentiation, whereas it promotes macrophage differentiation when it acts alone. Thus, this study provides essential insight into the long-standing question of how RANKL promotes OC differentiation. These findings expand the existing view of the role of C/EBPα in bone homeostasis beyond its role in lineage allocation of mesenchymal stem cells and macrophage cells to include a direct and specific role in OC lineage commitment from monocyte/macrophage cells. C/EBPα may be an ideal target for treating osteolytic diseases.
Materials and Methods
Ctsk Promoter CAT Constructs.
A Ctsk pCCAT containing a 1,505-bp XbaI/EcoRI fragment (−1,474 to −31, numbered relative to the cap site) of the mouse Ctsk 5′-flanking region was used as a starting point for deletion analysis. The sizes of series of 5′ deletions generated were approximated by electrophoresis in a 3:1 NuSieve-agarose gel (Lonza) or, in selected cases, by DNA sequence analysis with the dideoxy chain termination method.
Forced Expression.
We used a retrovirus system to express c-fos ectopically, as described previously (17), in C/EBPα−/− myeloid cells cultured with M-CSF/RANKL to induce osteoclastogenesis. We similarly used a retrovirus system to express C/EBPα ectopically in RANKL-induced C/EBPα+/+ MBM as described previously (17). C/EBPα was also overexpressed in uninduced RAW264.7 cells by stable transfection.
Statistical Analysis and Data Quantification Analysis.
Experimental data are reported as mean ± SD of triplicate independent samples. Data were analyzed with the two-tailed Student’s t test. P values <0.05 were considered significant. Data quantification analyses were performed using the National Institutes of Health ImageJ program as described (12, 17) (*P < 0.05 and **P < 0.01 throughout the paper). Additional details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ms. Christie Paulson for her excellent assistance with our manuscript and Dr. Jay M. McDonald for his extensive reading and discussion of our manuscript. We also thank Dr. Yihong Wan for the generous gift of c-fos promoter luciferase constructs, Dr. Gretchen J. Darlington (Baylor College of Medicine, Houston) for the generous gift of C/EBPα+/− mice, and Matthew McConnell for his assistance with bioinformatics analysis. We appreciate the assistance of the Center for Metabolic Bone Disease at the University of Alabama at Birmingham (Grant P30 AR046031). We are also grateful for assistance from the Small Animal Phenotyping Core, Metabolism Core, and Neuroscience Molecular Detection Core Laboratory at the University of Alabama at Birmingham (Grant P30 NS0474666). This work was supported by National Institutes of Health Grants AR-44741 and AR-055307 (both to Y.-P.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211383110/-/DCSupplemental.
References
- 1.Valledor AF, Borràs FE, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63(4):405–417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- 2.Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13(4):234. doi: 10.1186/ar3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 4.Iotsova V, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 5.Grigoriadis AE, et al. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 6.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):235. doi: 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:E13–E18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 8.Tondravi MM, et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386(6620):81–84. doi: 10.1038/386081a0. [DOI] [PubMed] [Google Scholar]
- 9.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13(12):1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SM, et al. Genetics and genomics of osteoclast differentiation: Integrating cell signaling pathways and gene networks. Crit Rev Eukaryot Gene Expr. 2006;16(3):253–277. doi: 10.1615/critreveukargeneexpr.v16.i3.40. [DOI] [PubMed] [Google Scholar]
- 11.Li YP, et al. Cloning and complete coding sequence of a novel human cathepsin expressed in giant cells of osteoclastomas. J Bone Miner Res. 1995;10(8):1197–1202. doi: 10.1002/jbmr.5650100809. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet. 2007;16(4):410–423. doi: 10.1093/hmg/ddl474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepers H, et al. Reintroduction of C/EBPalpha in leukemic CD34+ stem/progenitor cells impairs self-renewal and partially restores myelopoiesis. Blood. 2007;110(4):1317–1325. doi: 10.1182/blood-2006-10-052175. [DOI] [PubMed] [Google Scholar]
- 14.Cammenga J, et al. Induction of C/EBPalpha activity alters gene expression and differentiation of human CD34+ cells. Blood. 2003;101(6):2206–2214. doi: 10.1182/blood-2002-05-1546. [DOI] [PubMed] [Google Scholar]
- 15.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26(47):6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 16.Koleva RI, et al. C/EBPα and DEK coordinately regulate myeloid differentiation. Blood. 2012;119(21):4878–4888. doi: 10.1182/blood-2011-10-383083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21(14):1803–1816. doi: 10.1101/gad.1544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23(4):447–451. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 19.Feng R, et al. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105(16):6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117(5):663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 21.Di Tullio A, et al. CCAAT/enhancer binding protein alpha (C/EBP(alpha))-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation. Proc Natl Acad Sci USA. 2011;108(41):17016–17021. doi: 10.1073/pnas.1112169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang DE, et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94(2):569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath V, et al. C/EBPalpha deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood. 2004;104(6):1639–1647. doi: 10.1182/blood-2003-11-3963. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.