Abstract
Ocean acidification affects a wide diversity of marine organisms and is of particular concern for vulnerable larval stages critical to population replenishment and connectivity. Whereas it is well known that ocean acidification will negatively affect a range of calcareous taxa, the study of fishes is more limited in both depth of understanding and diversity of study species. We used new 3D microcomputed tomography to conduct in situ analysis of the impact of ocean acidification on otolith (ear stone) size and density of larval cobia (Rachycentron canadum), a large, economically important, pantropical fish species that shares many life history traits with a diversity of high-value, tropical pelagic fishes. We show that 2,100 μatm partial pressure of carbon dioxide (pCO2) significantly increased not only otolith size (up to 49% greater volume and 58% greater relative mass) but also otolith density (6% higher). Estimated relative mass in 800 μatm pCO2 treatments was 14% greater, and there was a similar but nonsignificant trend for otolith size. Using a modeling approach, we demonstrate that these changes could affect auditory sensitivity including a ∼50% increase in hearing range at 2,100 μatm pCO2, which may alter the perception of auditory information by larval cobia in a high-CO2 ocean. Our results indicate that ocean acidification has a graded effect on cobia otoliths, with the potential to substantially influence the dispersal, survival, and recruitment of a pelagic fish species. These results have important implications for population maintenance/replenishment, connectivity, and conservation efforts for other valuable fish stocks that are already being deleteriously impacted by overfishing.
Present day atmospheric CO2 concentration is higher than at any point in the past 800,000 y (1), driving unprecedented anthropogenic ocean acidification in pelagic (2) and coastal environments (3). Future climate scenarios project further decline in ocean pH (4–6) at a rate of change faster than any experienced in the last 300 million years (7). Although ocean acidification is known to influence a diversity of marine organisms (8), it is a particular concern for vulnerable larval stages critical to population replenishment and connectivity (9). Recently, the impact of ocean acidification on the larval stages of invertebrate and vertebrate marine species has attracted increased attention; however, experiments on larval fishes raised under projected ocean acidification scenarios have produced mixed results (10, 11). The days- to month-long pelagic larval period is an ecologically vital ontogenetic phase in marine fishes because it constitutes the primary mode of dispersal in many species (9) and represents the life stage most susceptible to mortality (12). During this phase, the sensory abilities of larval fishes are important determinants of survival (13) and ultimately influence the persistence of viable populations. Therefore, the study of ocean acidification impacts on sensory function in fishes is of critical importance to our understanding of the cumulative effect of ocean acidification on fish populations.
To date, ocean acidification impacts on the sensory function of larval fishes have been documented in small, demersal study species through tests of olfactory discrimination (14–16), and to a more limited extent, behavioral response to visual (17) and auditory stimulus (18). Although otoliths (ear stones) are an important part of the auditory and vestibular sense organs in fishes (19), their formation under ocean acidification conditions has received limited attention. Previous studies of larval fish otoliths have identified consistent ocean acidification effects across some species, but have been constrained by the use of 2D measures of size, which limits further analysis of sensory consequences and the ability to examine the full extent of ocean acidification impacts (20–22). With this in mind, we used high-resolution microcomputed tomography (micro-CT) to measure the 3D size and density of otoliths in fish larvae raised under acidified conditions (Fig. 1). This approach provided a more complete perspective of ocean acidification impacts on otoliths and enabled modeling of the sensory consequences of those effects.
Fig. 1.
Lateral view micro-CT imagery of a 22-d posthatch larval cobia head. Three-dimensional data were filtered to produce imagery of (A) the complete skeletal structure of the cobia skull and (B) only more dense material, such as otoliths (marked with arrow).
The study species we used, Rachycentron canadum (cobia), is one of the largest and most widely distributed tropical species studied to date and is also of significant ecological and economic value (23, 24). It is a eurytopic top predator and the target of recreational and commercial fisheries throughout a nearly circumglobal distribution in the continental shelf waters of tropical to warm temperate regions (23, 24). Global fishery landings were approximately 11,000 tons in the year 2000 and aquaculture production had a global value over USD 36 million in 2004 (25). Cobia life history traits are shared by several high-value, pelagic, tropical fishes; thus their use in these experiments provides a useful perspective of possible ocean acidification impacts to other pelagic species of high ecological and economic value.
Results and Discussion
The sagittal and lapillar otoliths of larval cobia raised for 20 d in acidified conditions expected for the years 2100 and 2300 [800 and 2,100 μatm partial pressure of carbon dioxide (pCO2), respectively] (5, 6) differed significantly from otoliths of larvae raised under control conditions (300 μatm pCO2; Table 1). Otoliths from larvae raised in seawater at 2,100 μatm pCO2 had significantly greater volume, surface area, and density relative to controls (Fig. 2 A–C; see Table 2 for statistical summary). There was also a significant decrease in the surface area to volume ratio (SA:V) of otoliths in both elevated-CO2 treatments, as well as a significant increase in the estimated relative mass of sagittal otoliths under both elevated-CO2 treatments (Fig. 2 D and E). Otoliths from larvae raised in seawater at 800 μatm pCO2 exhibited a trend of increased volume and surface area, but these patterns were not significant. Relative density of 800 μatm pCO2 treatment otoliths was also not significantly different from controls. No treatment effect was detected for the standard length (SL) of larvae at any treatment level (P = 0.809, n = 4). These results are unique direct measurements of otolith volume, surface area, and density in larval fish and expand upon previous reports of increased otolith size measured using 2D microscopy techniques (10, 20–22).
Table 1.
Summary of water chemistry results
| Treatment | Temperature,°C | pH, total scale | TA, μmol⋅kg−1 | pCO2, μatm |
| Control | 27.0 (±0.1) | 8.13 (±0.01) | 2291 (±32) | 305 (±8) |
| Year 2100 | 26.9 (±0.1) | 7.79 (±0.02) | 2291 (±37) | 796 (±37) |
| Year 2300 | 27.0 (±0.1) | 7.40 (±0.03) | 2285 (±34) | 2123 (±113) |
Temperature, pH, and total alkalinity measured during larval rearing and mean pCO2 calculated with the software CO2SYS (26). Values are means (±SEM).
Fig. 2.
Change in larval cobia otoliths as a result of increased pCO2. When raised in seawater with 300 μatm, 800 μatm, or 2,100 μatm pCO2 (white, gray, and black bars and symbols, respectively), larvae in the highest CO2 treatment had lapillar and sagittal otoliths with up to (A) 49% greater volume, (B) 37% greater surface area, (C) 6% greater relative density, (D) 19% lower surface area to volume ratio (SA:V), and (E) 58% greater relative mass. The 800 μatm treatment only had a significant effect on otolith SA:V and the relative mass of sagittal otoliths. Within each otolith type, bars or symbols not sharing a letter are significantly different (P < 0.05, n = 4 per treatment). Values are (A and B) adjusted means (±SEM) and (C–E) means (±SEM).
Table 2.
Summary of statistical information
| Otolith | Test | Variable | F value | P value |
| Lapillus | ANCOVA | Volume | 22.16 | 0.001 |
| Surface area | 22.67 | <0.001 | ||
| ANOVA | SA:V | 36.82 | <0.001 | |
| Relative mass | 34.37 | <0.001 | ||
| Sagitta | ANCOVA | Volume | 21.43 | 0.001 |
| Surface area | 5.12 | 0.037 | ||
| ANOVA | SA:V | 1,275.38 | <0.001 | |
| Relative mass | 78.76 | <0.001 | ||
| Combined | ANOVA | Relative density | 14.26 | 0.002 |
Results of analysis of covariance (ANCOVA) and analysis of variance (ANOVA) of tank mean otolith data. Larval standard length was a continuous covariate in all ANCOVAs, and resulting adjusted mean surface area and volume were used in ANOVA procedures for surface area to volume ratio (SA:V) and relative mass. ANOVA procedures for relative density included a single value from each fish, determined across all three otolith pairs (sagittal, lapillar, and asterisci). n = 4 per treatment.
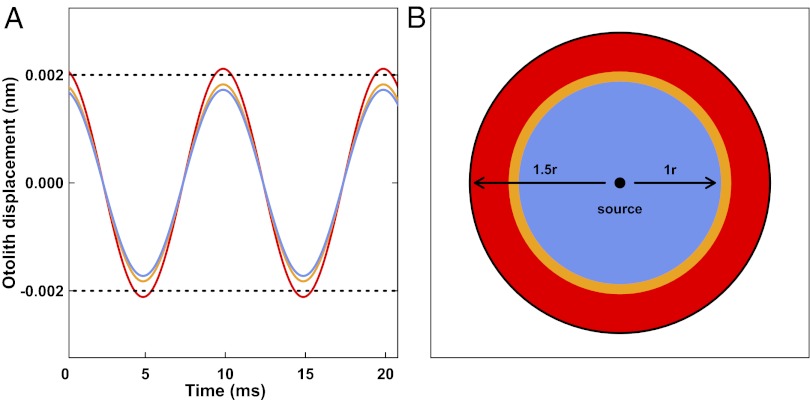
Alteration of otolith size, density, and mass has direct impacts on otolith mechanics and influences sensory function (27–29). To simulate the mechanics of CO2-altered otoliths, we applied the size and relative density data from sagittal otoliths in our experiment to a mathematical model of otolith motion in response to an 0.8-nm amplitude sinusoidal acoustic wave (27, 28). Our simulation demonstrated that when subjected to the same sound stimulus, the estimated CO2-driven increase in relative otolith mass results in an increased displacement amplitude compared with control otoliths (Fig. 3A). Increased otolith displacement amplitude would enable larvae developing in high-CO2 water to detect sounds that fish in low-CO2 water cannot detect. For otolith displacement to reach the hearing threshold that was attained by control otoliths in response to a sound amplitude of 1 nm, 800 μatm pCO2 treatment otoliths required 5% less sound amplitude (0.95 nm) and 2,100 μatm pCO2 treatment otoliths required nearly 20% less sound amplitude (0.80 nm). As sound amplitude decreases with distance from the source (13), heightened auditory sensitivity leads to detection of sounds at a greater distance from the source. We calculated the relative hearing ranges for larval fish with the auditory sensitivities of high-CO2 (0.80-nm sound amplitude threshold), intermediate-CO2 (0.95-nm threshold), and control otoliths (1-nm threshold) from our mathematical model assuming cylindrical spreading of sound (13) and determined that the more massive otoliths from high-CO2 larvae produced ∼50% greater hearing range compared with control larvae, whereas otoliths from intermediate-CO2 larvae produced ∼10% greater hearing range (Fig. 3B).
Fig. 3.
Simulated otolith displacement amplitude and hearing range for larval cobia under elevated-pCO2 conditions. (A) When exposed to a simulated 0.8-nm amplitude 100 Hz sound wave, otoliths at 2,100 μatm pCO2 (red line) had greater otolith displacement than those at 800 μatm pCO2 (orange line) or controls (300 μatm pCO2, blue line), thereby reaching the hearing threshold (dotted horizontal line) when 800 μatm and control otoliths did not. (B) Assuming loss of sound amplitude by cylindrical spreading (13), 300 μatm pCO2 fish (blue) had hearing range r and 800 μatm pCO2 fish (orange) had a 10% greater range, but those at 2,100 μatm pCO2 (red) had 50% greater range due to the lower sound amplitude necessary for threshold otolith displacement.
Increased auditory or vestibular sensitivity has important implications for the utilization of these sensory functions by fishes: it could influence a fish’s ability to navigate to a desired habitat, detect predators or prey, perceive changes in water turbulence or current speeds, or maintain proper kinesthetic awareness. These changes would be most relevant near the periphery of hearing ability, such as at distance from a sound source or when otolith displacement amplitude approaches the threshold for detection. Altered sensory ability could prove to be beneficial or detrimental, depending on how a fish perceives this increased sensitivity. Improved detection of useful auditory information (e.g., distant nearshore sounds) would be advantageous to navigating coastal fishes; however, increased sensitivity to disruptive background noise (e.g., sea state) may mask useful auditory information. The need for auditory or vestibular sensitivity may also be life history specific. Many bottom dwelling fish species possess large otoliths relative to their body size, which may indicate an ecological need for high auditory and vestibular sensitivity (28). In contrast, highly mobile pelagic species often possess small otoliths relative to their body size, implying less sensitivity (28). Because these traits have likely evolved to suit the particular ecological needs of a species, benthic species may find increased otolith mass advantageous, whereas such changes may be detrimental for pelagic species. Of course, it is also conceivable that increased otolith size could impinge upon the closely associated sensory hair cells of the macula and be detrimental to the function of the otolith organ regardless of species. These effects should apply to fishes of all ages; however, younger fish have less sensitive hearing ability (30) and any sensory advantage or disadvantage during the larval stage could be particularly influential to survival, with cascading effects on recruitment, population connectivity, and stock replenishment. Although ocean acidification is typically considered a future threat, these implications already may influence the dispersal and distribution of fishes currently developing in high-CO2 water in habitats such as fjords (31) and upwelling zones (3).
The mechanistic cause of increased otolith size with ocean acidification has not been determined empirically, but has been attributed to the physiological response of fish to high environmental CO2 (i.e., HCO3− retention) (32), which likely causes an increase in the aragonite saturation state of the endolymph fluid surrounding the otoliths (20). This physiological mechanism is sustained for the duration of high-CO2 exposure (32), therefore it can be assumed that effects on otoliths will persist with age. Additionally, ocean acidification is known to alter neurological function in fishes (11) and there is evidence for neurological control of otolith mineralization (33). Therefore, CO2-induced neurological disruption may indirectly contribute to increased otolith size and density, either by changing the chemical composition of endolymph fluid or by altering neurologically controlled expression of genes that influence the crystalline or lattice structure of otoliths (33). The results of either mechanism of change have important implications for the function of otoliths as sense organs, but there are also implications for their use as tools for fisheries biology research and conservation. Fisheries oceanographers and ecologists rely on otoliths to study fisheries stocks and the early life dynamics of fishes, often using the widths of daily otolith increments as a proxy for daily somatic growth (34). This method depends on a consistent correlation between otolith growth and somatic growth, but an increase in otolith size without a corresponding increase in somatic growth disrupts this relationship and may confound the use of this technique under variable CO2 conditions. Also, otoliths formed in high-CO2 water may have a different mineralogical composition, thereby interfering with stock identification methodologies such as those using otolith microchemistry analysis (35). Similar to the ecological effects discussed above, present day occurrence of high-CO2 water in fjords (31) and upwelling zones (3) makes this a current problem, and may already influence the interpretation of data collected using these techniques.
Our results indicate a graded impact of ocean acidification on cobia otoliths, similar to previously reported effects on 2D otolith surface area under identical treatment conditions (22). This is evident in the end-of-century 800 μatm pCO2 acidification treatment, where effects on otolith size followed a similar but nonsignificant trend. This is a potentially optimistic result, indicating some resistance to acidification and suggesting that under near-future scenarios these impacts may be most relevant in habitats already experiencing high pCO2 levels. However, the trend for larger otoliths with increased CO2 still produced an ∼10% increase in hearing range and it is not yet clear at what point these effects will become ecologically significant. Empirically, it is also unclear if natural exposure to variable environmental conditions leads to preadaptation, and thus resistance, to acidification in fishes (36). Because cobia is eurytopic, inhabiting environments ranging from epipelagic to estuarine waters, this may affect the intensity of their response to ocean acidification, whereas species restricted to more constant environments (e.g., entirely pelagic species) may respond differently to similar acidification scenarios.
It is widely accepted that the impact of ocean acidification on marine organisms varies along a gradient from obvious to subtle effects. Our observation of CO2-induced increases in otolith size and relative density is an unexpected subtle effect with important implications for the sensory abilities of fishes. Whether these sensory changes are ultimately positive or negative will depend on the species, but they have the potential to influence the survival, dispersal, and recruitment of a diversity of marine fishes, with subsequent population consequences. Because many ecologically and economically important species have characteristics similar to cobia, such population changes are expected to produce substantial ecological and economic effects. These results contribute to a fuller understanding of the complex suite of direct and indirect ocean acidification effects on fishes as well as the broader ecological and economic consequences that may challenge fishery populations and conservation efforts in the future.
Materials and Methods
Study System.
Cobia (R. canadum) is a highly mobile marine fish that reaches sizes >1.5 m and over 60 kg (23, 24). Pelagic spawning occurs during warmer months (25–30 °C) and planktonic larvae hatch at ∼3 mm SL, undergo flexion at 5–10 mm SL, and develop via a gradual transition into the juvenile stage within 30 d at 15–30 mm SL (23). Cobia eggs and larvae for this experiment were produced at the University of Miami Experimental Hatchery from a population of 10 F1-generation broodstock (six females, four males). Eggs were collected and allowed to hatch and develop until 2 d posthatch (dph), then stocked into 12 replicated 400 L flow-through experimental tanks at a density of 9–10 larvae L−1 and raised according to established methods (22, 37). Treatments were applied upon stocking and reached full effect within 24 h. Larvae were sampled at 22 dph, preserved in 95% ethanol, and the SL of each larva was measured to the nearest 0.1 mm using digital calipers (MC0006; Avenger). All live animal use was conducted with approval of the University of Miami Institutional Animal Care and Use Committee (Protocol 09-088 ad 2).
Water Chemistry.
Treatments represented ocean acidification scenarios for the years 2100 (800 μatm pCO2) (6) and 2300 (2,100 μatm pCO2) (5), but also are found presently in fjords (31) and upwelling zones (Table 1) (3). Seawater carbonate chemistry was manipulated via the addition of equimolar HCl and NaHCO3 before introduction into tanks (38). Tank pH was monitored daily using a handheld pH meter (pH 11; Oakton) and Ross Electrode (Orion 9102BWNP; Thermo Scientific) calibrated daily with Tris buffer. Water samples were collected every 5 d in 250 mL polyethylene terephthalate (PET) bottles and fixed with 100 μL of saturated mercuric chloride. Total alkalinity (TA) and total pH (pHT) were measured using automated Gran titration checked for accuracy with Dickson standards (Scripps Institution of Oceanography, La Jolla, CA). The software CO2SYS was used to solve the carbonate system using the two measured parameters (pHT and TA) (26). Temperature and dissolved oxygen were measured with a combination meter (550A; YSI) twice and once d−1, respectively, and salinity was measured once daily using a refractometer (RHS-10 ATC; Premium Aquatics). See Table 1 for summary of water chemistry results.
Micro-CT Procedures.
Three larvae per tank (12 per treatment) were randomly selected and individually scanned in the micro-CT scanner (Skyscan 1174v2, 13-μm resolution, 0.3° step, 180° total rotation). X-ray attenuation was standardized across scans using hydroxy-apatite bone mineral density (BMD) standards. Two-dimensional X-ray images were reconstructed into 3D image stacks using NRecon (v1.6.6.0) (Bruker-microCT) and analyzed using CTan (v1.12.4.3) (Bruker-microCT). Gray-scale X-ray images were thresholded to isolate regions of interest (ROIs) containing otoliths. Mean BMD was recorded from within these regions, 3D volumes were interpolated across image stacks using a “shrink-wrap” function, and both volume and surface area were measured. Relative density was determined by comparing the mean X-ray attenuation coefficients from micro-CT scans between control and treatment otoliths.
Mathematical Modeling.
The mathematical model adapted to simulate otolith displacement is based on an elliptical otolith oscillating in response to a plane sinusoidal wave (27, 28). Otolith displacement, Δx, relative to the closely associated bed of sensory hair cells (macula) is described by
 |
where
and ax is amplitude of water particle displacement, ω is angular frequency of the wave (ω = 2πv, v is frequency), m is otolith mass in milligrams, ρe is density of the endolymph fluid (assumed to be 1,000 mg⋅cm−3), V is otolith volume in cubic centimeters, t is time in seconds, γx is a coefficient of friction (γx = 0.0029535 × 106m0.6356), kx is a stiffness coefficient of hair cell bundles (kx = 21.2652 × 106m0.6356), and δx is the apparent additional mass of an object moving irregularly in liquid (δx = 0.0241m) (27, 28). Control otolith density was set at a standard value of 2,900 mg⋅cm−3 (27) and used along with micro-CT volume and relative density data to calculate simulated otolith density and mass.
We designated a water particle displacement amplitude (ax) of 1.0 nm at 100 Hz as the threshold sound level for control fish. This is reported to be the behavioral threshold for detection of particle motion in some adult fishes without gas bladders (39) and is a frequency representative of sounds produced by coastal fishes (40). This was chosen in contrast to thresholds measured using the pressure component of sound and neuronal response, which may not accurately describe the detection of particle motion (39) and can underestimate behavioral response thresholds (13). Because thresholds have been shown to decrease with age (30), 1.0 nm is likely a conservative estimate for a larval fish. The otolith displacement magnitude (Δx) needed for auditory detection (hearing threshold) was obtained by calculating control otolith displacement (∼0.002 nm) under modeled conditions. Modeling was then repeated with particle displacement reduced to 0.8 nm, where the simulated control otolith no longer reached the hearing threshold but the 2,100 μatm pCO2 treatment otolith did. Hearing range was calculated assuming cylindrical spreading of sound with distance (r) from the source and amplitude decreasing as 1/(√r) (13).
Data Analysis.
Data were analyzed in SYSTAT software (version 11; SYSTAT) using tank means, following verification of normality and homoscedasticity by Shapiro–Wilk and Bartlett tests. Data from the right or left sagittal and lapillar otoliths were randomly selected from each fish to test volume and surface area using analysis of covariance (ANCOVA), for which surface area or volume was the response variable, pCO2 a fixed factor, and SL the continuous covariate. Adjusted means of surface area and volume data calculated during ANCOVA were subsequently used to produce SA:V and relative mass data, using mean mass of control treatments as a reference. Relative densities of all otoliths were calculated in reference to mean control treatment otolith density. Larval SL, relative otolith density, SA:V, and relative mass were tested using analysis of variance (ANOVA), with each as the response variable and pCO2 as a fixed factor. SA:V and relative otolith mass data were log transformed to correct for lack of homoscedasticity. See Table 2 for summary statistics.
Acknowledgments
We thank D. Gledhill and L. Jewett of the National Oceanic and Atmospheric Administration’s Ocean Acidification Program for providing the micro-CT scanner; T. Capo, D. Benetti, C. Langdon, E. Bryant, and K. Ternus for facility use and logistical assistance; M. Grosell, J. Dallman, Z. Lu, and E. Staaterman for discussions; and the University of Miami Experimental Hatchery. This study was supported by grants from the National Science Foundation GK-12 program, University of Miami Maytag Ichthyology Chair, Guy Harvey Ocean Foundation, Florida Sea Grant, International Light Tackle Tournament Association, Manasquan River Marlin and Tuna Club, and the Yamaha Contender/Miami Billfish Tournament.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lüthi D, et al. High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature. 2008;453(7193):379–382. doi: 10.1038/nature06949. [DOI] [PubMed] [Google Scholar]
- 2.Byrne RH, Mecking S, Feely RA, Liu X. Direct observations of basin-wide acidification of the North Pacific Ocean. Geophys Res Lett. 2010;37(2):L02601. [Google Scholar]
- 3.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science. 2008;320(5882):1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 4.Caldeira K, Wickett ME. Oceanography: Anthropogenic carbon and ocean pH. Nature. 2003;425(6956):365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 5.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:C09S04. [Google Scholar]
- 6.Meehl G, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge: Cambridge Univ Press; 2007. pp. 748–845. [Google Scholar]
- 7.Hönisch B, et al. The geological record of ocean acidification. Science. 2012;335(6072):1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- 8.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett. 2010;13(11):1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 9.Cowen R, Sponaugle S. Larval dispersal and marine population connectivity. Annu Rev Mar Sci. 2009;1:433–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 10.Munday PL, Gagliano M, Donelson JM, Dixson DL, Thorrold SR. Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol Prog Ser. 2011;423:211–221. [Google Scholar]
- 11.Nilsson GE, et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang. 2012;2(3):201–204. [Google Scholar]
- 12.Houde E. Patterns and trends in larval-stage growth and mortality of teleost fish. J Fish Biol. 1997;51(Supplement A):52–83. [Google Scholar]
- 13.Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv Mar Biol. 2006;51:143–196. doi: 10.1016/S0065-2881(06)51003-X. [DOI] [PubMed] [Google Scholar]
- 14.Munday PL, et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA. 2009;106(6):1848–1852. doi: 10.1073/pnas.0809996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munday PL, et al. Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA. 2010;107(29):12930–12934. doi: 10.1073/pnas.1004519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixson DL, Munday PL, Jones GP. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett. 2010;13(1):68–75. doi: 10.1111/j.1461-0248.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari MCO, et al. Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct Ecol. 2012;26:553–558. [Google Scholar]
- 18.Simpson SD, et al. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett. 2011;7(6):917–920. doi: 10.1098/rsbl.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popper AN, Ramcharitar J, Campana SE. Why otoliths? Insights from inner ear physiology and fisheries biology. Mar Freshw Res. 2005;56(5):497–504. [Google Scholar]
- 20.Checkley DM, Jr, et al. Elevated CO2 enhances otolith growth in young fish. Science. 2009;324(5935):1683. doi: 10.1126/science.1169806. [DOI] [PubMed] [Google Scholar]
- 21.Munday PL, Hernaman V, Dixson DL, Thorrold SR. Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosciences. 2011;8:1631–1641. [Google Scholar]
- 22.Bignami S, Sponaugle S, Cowen RK. 2013. Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Global Change Biol 19(4):996–1006. [DOI] [PubMed]
- 23.Shaffer RV, Nakamura EL. 1989. Synopsis of biological data on the cobia Rachycentron canadum (Pisces: Rachycentridae). NOAA Tech Rep NMFS 82 [FAO Fish Syn 153] (NOAA, Silver Spring, MD)
- 24.Fry G, Griffiths S. Population dynamics and stock status of cobia, Rachycentron canadum, caught in Australian recreational and commercial coastal fisheries. Fish Manag Ecol. 2010;17:231–239. [Google Scholar]
- 25.Kaiser JB, Holt JG. 2007. in: FAO Fisheries and Aquaculture Department. Rome. Updated May 23, 2007. Available at http://www.fao.org/fishery/culturedspecies/Rachycentron_canadum/en. Accessed February 25, 2013.
- 26.Lewis E, Wallace D. Program Developed for CO2 System Calculations. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 1998. [Google Scholar]
- 27.Lychakov DV, Rebane YT. Fish otolith mass asymmetry: Morphometry and influence on acoustic functionality. Hear Res. 2005;201(1-2):55–69. doi: 10.1016/j.heares.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Lychakov DV, Rebane YT. Otolith regularities. Hear Res. 2000;143(1-2):83–102. doi: 10.1016/s0378-5955(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 29.Kondrachuk AV. Mass and mechanical sensitivity of otoliths. Adv Space Res. 2003;32(8):1521–1526. doi: 10.1016/S0273-1177(03)90390-5. [DOI] [PubMed] [Google Scholar]
- 30.Kenyon TN. Ontogenetic changes in the auditory sensitivity of damselfishes (pomacentridae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1996;179(4):553–561. [Google Scholar]
- 31.Thomsen J, et al. Calcifying invertebrates succeed in a naturally CO2 enriched coastal habitat but are threatened by high levels of future acidification. Biogeosciences Discuss. 2010;7:5119–5156. [Google Scholar]
- 32.Claiborne JB, Heisler N. Acid-base regulation and ion transfers in the carp (Cyprinus carpio): pH compensation during graded long- and short-term environmental hypercapnia, and the effect of bicarbonate infusion. J Exp Biol. 1986;126:41–61. doi: 10.1242/jeb.126.1.41. [DOI] [PubMed] [Google Scholar]
- 33.Anken RH. On the role of the central nervous system in regulating the mineralisation of inner-ear otoliths of fish. Protoplasma. 2006;229(2-4):205–208. doi: 10.1007/s00709-006-0219-6. [DOI] [PubMed] [Google Scholar]
- 34.Sponaugle S. In: Tropical Fish Otoliths: Information for Assessment, Management and Ecology. Reviews: Methods and Technologies in Fish Biology and Fisheries. Green BS, Mapstone BD, Carlos G, Begg GA, editors. New York: Springer; 2009. pp. 93–132. [Google Scholar]
- 35.Thorrold SR, Swearer S. In: Tropical Fish Otoliths: Information for Assessment, Management and Ecology. Reviews: Methods and Technologies in Fish Biology and Fisheries. Green BS, Mapstone BD, Carlos G, Begg GA, editors. New York: Springer; 2009. pp. 249–295. [Google Scholar]
- 36.Munday PL, Jones GP, Pratchett MS, Williams AJ. Climate change and the future for coral reef fishes. Fish Fish. 2008;9(3):261–285. [Google Scholar]
- 37.Benetti DD, et al. Intensive larval husbandry and fingerling production of cobia Rachycentron canadum. Aquaculture. 2008;281(1-4):22–27. [Google Scholar]
- 38.Gattuso J-P. 2010. in Guide to Best Practices for Ocean Acidification Research and Data Reporting, eds Riebesell U, Fabry V, Hannson L, Gattuso J-P (Publications Office of the European Union, Luxembourg), pp 44–52.
- 39.Fay RR, Megela Simmons A. In: Comparative Hearing: Fish and Amphibians. Fay RR, Popper AN, editors. New York: Springer; 1999. pp. 269–318. [Google Scholar]
- 40.Fish MP, Mowbray WH. Sounds of Western North Atlantic Fishes. A Reference File of Biological Underwater Sounds. Baltimore: The Johns Hopkins University Press; 1970. [Google Scholar]