Abstract
Pheromone orientation in moths is an exemplar of olfactory acuity. To avoid heterospecific mating, males respond to female-produced blends with high specificity and temporal resolution. A finely tuned sensory to projection neuron network secures specificity, and this network is thought to assess pheromone quality continually during orientation. We tested whether male moths do indeed evaluate each pheromone encounter and surprisingly found that male European corn borer moths instead generalize across successive encounters. Although initially highly ratio specific, once “locked on” to the pheromone plume the acceptable ratio can vary widely, and even unattractive blends can become attractive. We further found that this “mental shortcut” may be a consequence of the fact that sensory neurons exposed to frequent encounters do not reliably encode blend ratios. Neurons tuned to either of the two pheromone components adapt differentially in plumes containing the preferred blend ratio (97:3) and cause the olfactory sensory signal to “evolve,” even in narrowly tuned pheromonal circuits. However, apparently the brain interprets these shifting signals as invariant “gestalts.” Generalization in pheromone perception may mitigate stabilizing selection and allow introgression between sympatric strains, such as in the European corn borer, that otherwise appear isolated by pheromonal differences. Generalization may also be important in responses to general odorants, as circuits underlying these display vast sensitivity differences, complex interactions, and temporal intricacies.
Keywords: blend recognition, odor processing, sensory constraints, evolution, sexual selection
In moths, pheromones dominate mate finding. Female-produced pheromones generally consist of a blend of two to several long-chain derivatives of fatty acids. The composition and ratio of these components create species-specific blends, and males are narrowly tuned to these to reduce the chance of heterospecific matings. Because of its high specificity, moth pheromone communication has been important in understanding species recognition and the evolutionary processes underlying signal evolution (1). In addition, owing to its simplicity, moth pheromone communication has been a prime model for odor-mediated behavior, as well as olfactory detection, processing, and blend recognition (2–6). In the current models, male moths respond to successive encounters of pheromone filaments within a plume by surging upwind. Flights slowly revert to casting (flying across the wind line without upwind progress) if sufficient time (generally less than a second) has passed since the last contact with such “whiffs” of pheromone (3, 4).
Moths are exquisitely sensitive to the odor plume’s fine-scale structure (3, 4) and can resolve the composition of a blend on a millisecond timescale, both behaviorally and physiologically. Male Heliothis virescens perceived pheromone and antagonist as “separate” if strands were encountered only 1 ms apart (2). Similarly, only simultaneous arrival of pheromone components triggered the response of blend-sensitive projection neurons in the brain (4). Furthermore, the complete blend has been viewed as dictating all responses from activation to mating, with the natural ratio having the lowest threshold for inducing all behaviors, from activation, wing fanning, taking flight, locking onto the plume, and landing (7). An emergent property of studies on moth pheromone orientation is that blend ratios are equally important from activation to source finding (7). In line with this, it is thought that males continuously assess quality during reiterative encounters with pheromone filaments in spatiotemporally dynamic plumes.
The crambid moth Ostrinia nubilalis is a species well known for its narrow tuning to a binary blend of female produced (Z)-11-tetradecenyl acetate (Z11) and (E)-11-tetradecenyl acetate (E11) (8, 9). Two strains exist, the Z and E strains, which produce and prefer opposite ratios of Z11 and E11, 97:3 and 1:99 Z11:E11, respectively. This has made this species a model for neurophysiological and evolutionary studies on blend production and preference (5, 6, 10). However, despite the reportedly high pheromone specificity of males, strains interbreed in the field, as sympatric populations may consist of up to 15% of hybrids (11, 12). Here, we investigate how pheromone communication, an exemplar of olfactory specificity, may break down and mitigate purifying selection. Implications for our understanding of olfactory coding and preference in general are also considered.
Results and Discussion
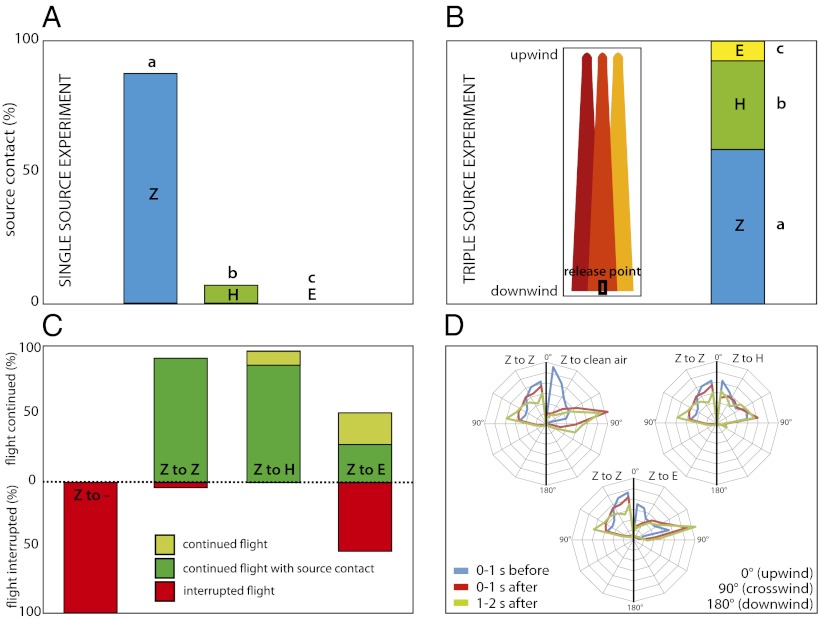
In a laminar flow wind tunnel (Fig. 1), we confirmed that Z males are highly specific to their own pheromone. Nearly 100% of the Z males flew to a source of their own blend (Fig. 2A), only 10% flew to the intermediate blend [hybrid (H) blend, 50:50 Z11:E11], and the E lure did not induce any upwind flight (30-µg septum load, Kruskal–Wallis rank test, P = 0.021). This supports wind tunnel studies that show that O. nubilalis males are attracted to ratios close to those produced by females of their own strain (9).
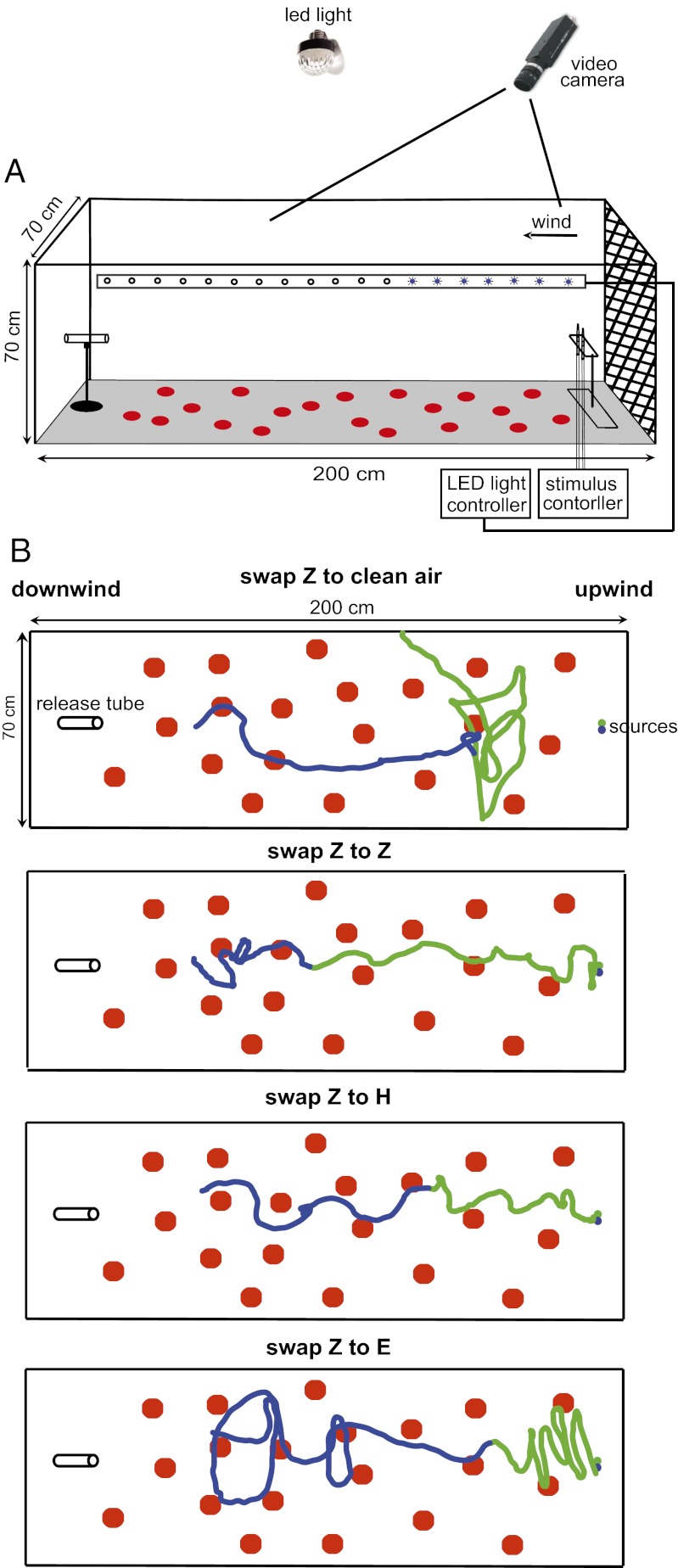
Fig. 1.
(A) Laminar flow wind tunnel of 70 × 70 × 200 cm was used in the behavioral assays. Males were released 180 cm downwind from a platform at 30-cm height. The camera was placed at a 20° angle toward downwind and recorded the upwind 150-cm section of flights. Visual markers were provided on the wind tunnel floor. An array of 850-nm LEDs along the side of the wind tunnel demarcated the transition point of one plume into another (swapped plume experiments). For further details, see Materials and Methods. (B) Sample traces of male O. nubilalis flying in pheromone plumes of which the ratio was swapped during flight. Text above each sample trace indicates the new ratio to which the plume was swapped.
Fig. 2.
(A) Behavioral response of Ostrinia nubilalis males to individual plumes in a wind tunnel. n = 30 for each treatment. Different letters above the bars indicate significant differences. (B) (Left) Schematic of partially overlapping plumes (three upwind lures at 10 cm apart). (Right) Distribution of landing of males on three lures in overlapping plumes. Different letters besides the bars indicate significant differences. (C) Percentage disrupted and continued flights after source swapping (new source indicated in bar, Z = Z blend, H = H blend, E = E blend, − = clean air). N values: 33 (Z/−), 67 (Z/Z), 33 (Z/H), 116 (Z/E). (D) Distribution in percentage of track angles before and after the swap (N values as in C). For easy comparison, each diagram depicts in the left half the response of male moths to a Z blend-to-Z blend swap. Swaps from Z to E or clean air did not differ for the first second after the swap (average, 74° and 78°, respectively; P = 0.33). However, in contrast with moths transiting into the E blend, those transiting into clean air continued to increase their average track angle (from 78° to 89°; P = 2.2 E-16).
Thus, where both strains are sympatric, cross-strain attraction and hybridization should be very rare for such narrowly tuned species. Surprisingly, however, in the field, rates of hybridization, which produces viable individuals with intermediate pheromone production and preference (8), can be as high as 15% (11–13). This is hard to explain on the basis of the high specificity demonstrated in numerous wind tunnel studies, including ours. We hypothesized that, in the field, close proximity of calling females of different strains might cause pheromone plumes to overlap and lower through some mechanism the specificity of males. To test this, we exposed males in a laminar flow wind tunnel (Fig. 1A) to partially overlapping pheromone plumes, such as could occur in nature at high population densities. In a choice between three partially overlapping pheromone sources (Z blend, H blend, and E blend; Fig. 2B), Z males indeed lowered their specificity, with 58%, 38%, and 4% of the males landing on Z, H, and E lures, respectively (Fig. 2B, χ2 test, P = 0.0069, n = 90). The position of the lures (left–center–right: Z–H–E, E–Z–H, or H–E–Z) had no significant effect (χ2 = 7.32, df = 4, P = 0.12). After taking flight, males apparently relax their specificity.
The decrease in specificity likely occurs once a male engages in sustained upwind flight (locks onto the plume). To test this, we created plumes that transitioned seamlessly from one blend into another (see sample tracks; Fig. 1B). Once a male locked onto the plume, sources were swapped. All Z males casted as soon as they flew from a Z-blend plume into clean air, which shows that filaments of Z-blend pheromone did not linger in the wind tunnel after the swap (Fig. 2C). In contrast, male moths continued upwind without interruption when blends were swapped from a Z blend to a Z blend, as also shown by their track angles (Fig. 2D).
How did switching to a new blend affect upwind flight? Surprisingly, flights were undisturbed by a swap from the Z blend to the H blend (Figs. 2C and 1B). In fact, the track angle and source finding rates were indistinguishable to the Z-blend swap (Fig. 2D). Moreover, when Z males commenced in the Z blend, but entered an E-blend plume, 52% continued upwind, with 28% of these males landing on the source of the E blend. The remaining 48% of the males ceased upwind flight (Fig. 2C). This is also reflected in the track angle. After transiting into clean air, moth projected their flights more crosswind. However, the average track angles did not change after swapping to the Z blend or to the H blend (one-way ANOVA, P = 0.48 and 0.35, respectively). Although swapping from the Z blend to the E blend increased the moth’s average track angle (from 61° to 74°, P < 0.0001), it was subsequently kept constant over time, resulting in a significant portion of source finding. These findings are remarkable, as in one-choice trials the H blend induced orientation in only 10% of the males, whereas the E blend never induced any response (Fig. 2A). This demonstrates that, once males have locked onto the plume, they are substantially less attuned to blend quality, which likely explains the occurrence of hybrids in nature. “Locking onto the plume” as a decisive step in moth orientation is also implied in other studies (e.g., refs. 14 and 15).
Pheromone orientation in moths is a striking example of narrow bandwidth quality tuning with high temporal resolution. It is therefore surprising to find relaxed specificity during orientation. We asked whether this was a consequence of neural constraints of blend quality processing in dynamic plumes. We challenged pheromone-sensitive antennal olfactory sensory neurons (OSNs) to a train of Z-blend puffs over a 3-log stimulus loads over time, the lower two of which induce sustained upwind flight and source finding in O. nubilalis. In O. nubilalis, the OSNs sensitive to Z11 and E11 are colocalized in the same sensillum (8). Accordingly, responses to pulse trains were scored simultaneously. We stimulated with pulse trains of 1 s on/off and 200 ms on/off (see Fig. 3 for sample traces and dose–response curves of the E and Z neuron). Each train was preceded by a single Z11 and E11 puff to establish baseline sensitivity. After the pulse train, a single E11 stimulation was given (further details in Fig. 4).
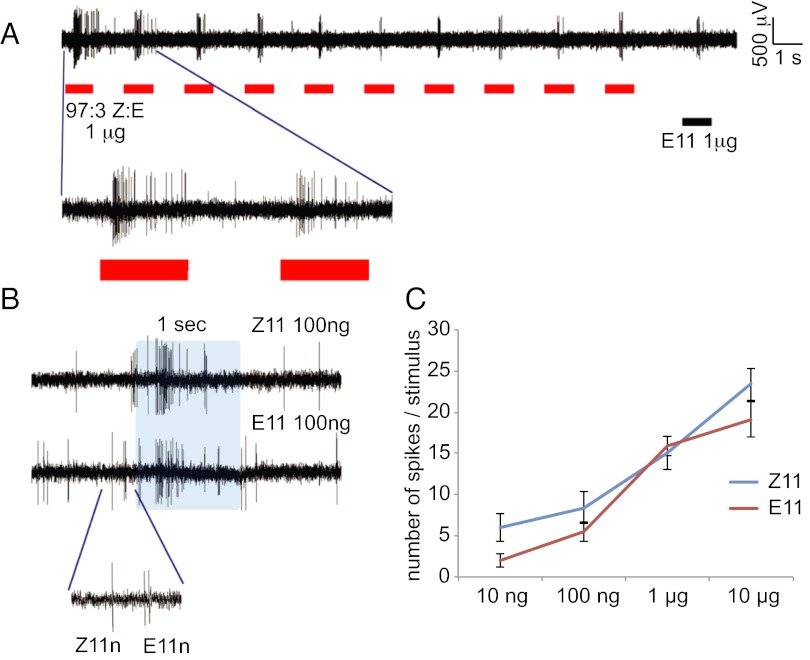
Fig. 3.
(A) Sample trace of a stimulus train stimulation with 1 μg of 97:3 Z11:E11. (B) Enlargement of a stimulation with 100-ng stimulus load of Z11 and E11. (C) Dose–response curves of Z11 and E11 sensory neurons to 500-ms stimulations.
Fig. 4.
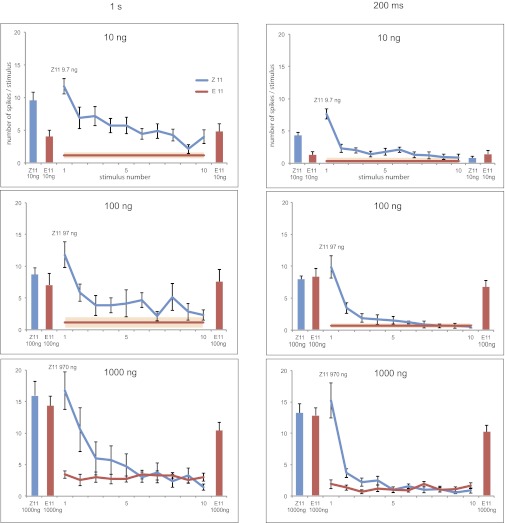
Sensory neuron responses to Z-blend pulse trains across 3-log stimulus loads. Each panel represents the response of the two neurons to Z11, followed in 30 s by E11 at equal dose (of Z11 in the blend). After 1 min, males were exposed to a train of 10 Z-blend pulses at a rate of either 1 s on/1 s off, or 200 ms on/200 ms off. The E neuron spike frequency to 100 ng of Z blend and lower was very low, and averaged across the spike train (horizontal bar with an SE envelope). In addition, we verified whether the E neuron was adapted by stimulating with an E11 puff directly following the pulse train at a higher stimulus load (equal to Z11 in the blend). Finally, in the 10-ng, 200-ms series, we stimulated with Z11 after the stimulation train. The Z neuron showed a similar (lack of) response to the posttrain Z11 pulse as to the last stimulation within the train (P < 0.001, two-tailed t test), which excludes head space depletion as a contributing factor. Stimulus quantity in physiological and behavioral experiments was simulated using stimulus pipettes loaded with 1 μg of Z11-14:OAc and showed no difference in the total amount released between the two setups (1.88 ± 0.32 ng and 2.16 ± 0.49 ng, respectively; 5-min collection, collection on superQ, analysis with GC-MS). At equal stimulus load of E11 and Z11, spike rate and adaptation of E and Z neurons was similar (Fig. S2). N values: 1-s pulse trains, n = 7, and 200-ms pulse trains, n = 9, for each dose. Error bars represent SEM. The response of the Z neuron to the first and last stimulation in the train were significantly different in each case at P ≤ 0.001.
If blend detection was unaffected by dynamic stimulation, the ratio of spikes from the two neurons during trains of odor pulses should remain relatively constant. However, repetitive stimulation of the OSNs with the Z blend resulted in greater adaptation of Z over E neurons, including at source dosages to which males readily flew upwind (Fig. 4 A and B; see also Figs. S1 and S2 for further details). The spike frequency of Z neurons decreased with reiterative stimulations, and at the highest stimulus load neurons stopped responding within 10 stimulations. In contrast, the E neuron did not adapt to stimulus loads that cause upwind flight in the Z strain (Fig. 4). The observed differential adaptation rates of E and Z neurons seems to be caused by the excess (more than 30 times) of Z11 over E11, which is supported by the fact that at 1-µg blend dose the E-neuron started to adapt (P = 0.07, 0.13, respectively, for 1-s and 200-ms pulse trains; unpaired two-tailed t test; Fig. 4). In studies with the moth Agrotis segetum, cessation of upwind flight at excessively high concentrations correlated with sensory adaptation of one of the pheromone-sensitive neurons (16). In our study, higher stimulus loads (1 µg) also did not induce upwind flight, and adaptation of sensory neurons may underlie this process. However, our results indicate that also at behaviorally relevant stimulus loads males continued to fly upwind, despite the apparent decline in spike frequency of the Z11 neuron.
The finding that male O. nubilalis fly upwind along plumes that shift in quality contrasts with the prevailing model of blend detection, in which pheromone encounters are evaluated throughout orientation for their exact match with the natural blend. We found that dynamic stimulation of OSNs, as occurs within a turbulent plume, can affect ratio coding in the periphery. This would likely affect accurate assessment of blend quality from filament-to-filament at more central level, neurons of which linearly increase spike frequency with concentration (Fig. S3). Adaptation-induced shifts in the odor “images” apparently induce male O. nubilalis to broaden its ratio preference and base its behavior in part on previous assessments of blend quality. Despite adaptation, however, moths should still be able to detect large changes in ratio. The unadapted E neuron increased spiking at the end of a pulse train when stimulated with a dose of E11 similar to the Z content in the blend (Fig. 3), the increase in dose being similar to a swap from the Z blend to the E blend. Such a sudden increase in the E neuron’s spiking rate could indicate a change in blend to the moth, despite adaptation of the Z neuron. This may have caused the increased track angle and decreased source finding after a switch from a Z blend to an E blend. A strategy that permits some deviation from the natural ratio after initial blend recognition may be favored in small brains with limited processing power (17).
Our findings are particularly surprising given that, in wind tunnels, O. nubilalis displays a high selectivity for blend ratio compared with some other moths, yet in the field the two pheromone strains often cross (11–13). Our results suggest that hybridization rates may be a function of population density and the likelihood of overlapping plumes. O. nubilalis aggregates and mates in grassy edges surrounding fields of its host plant maize (18), which would enhance the probability of plume overlap and hybridization. Several other prezygotic isolation barriers such as voltinism (13), host plants (19), and male pheromones (20) can reduce hybridization, but generalization of the kind observed here could result in scenarios in which off-blends may become permissible. This may lessen stabilizing selection and create opportunities for pheromones to evolve, depending on the suite of selection forces a population is experiencing (21). Interestingly, field populations of the sibling taxon Ostrinia scapulalis (22), which has an identical pheromone polymorphism as O. nubilalis, were found in apparent Hardy–Weinberg equilibrium (23), although such situations have not been reported for O. nubilalis (24).
Whether other moth species relax their specificity to blend ratio during orientation deserves further study. Most studies have scored activation, followed by navigation along the plume, and ultimately source contact, using an unvarying source of the natural pheromone blend or blend variants (7), rather than presenting a choice between two kinds of lures initially encountered in overlapping plumes (25). Our findings with O. nubilalis indicate that, although the natural ratio of the blend is crucial for initializing a response, at later orientational stages constraints of neural processing may expand the response window to a wider range of initially unattractive ratios. A higher selectivity in early stages of the orientation process is also implied in other studies (14, 15). Although we found a compelling correlation with sensory input, more central processing factors, not accounted for here, may also contribute to behavioral generalization.
Male moths are attuned to rather simple pheromone blends. In contrast, host odors used by host searching insects typically involve a multitude of compounds that are sorted through an ensemble of different OSN types (26). These OSNs generally differ by many log magnitudes in their neuronal sensitivities (27) and display temporal and mixture interaction complexities in their response (28), although nonlinear transformation of sensory input, such as has been observed in Drosophila neural circuits (e.g., refs. 29 and 30), may buffer systems against peripheral adaptation. The occurrence of behavioral generalization, and neurophysiological correlates in more diverse and complex nonpheromonal circuits needs further study.
Materials and Methods
Insects.
Origin.
The Z-strain colony of O. nubilalis was established from 50 females collected at UV light at the edge of a cornfield in Kéty (Tolna, Hungary; August 2004). Moths were reared on a semiartificial diet at 25 °C, 70% relative humidity (RH) with a 17:7 light/dark cycle. The pheromone identity of the culture was verified by gas chromatographic analysis (GC) (HP5890 using a SP2340 column; 30 m × 0.32 mm; film thickness, 0.2 µm; splitless mode; carrier gas helium; 60 °C for 1 min, 10 °C/min to 120 °C, 5 °C/min to 220 °C) of 10 ovipositor tips from 1- to 3-d-old virgin females concentrated to 2 µL of n-hexane. Retention times of synthetic samples were used for identification of E11- and Z11-14OAc.
Experimental animals.
Pupae were sexed and held in different climate rooms. The males had access to a 5% (vol/vol) honey solution. Experimental animals were 1–4 d old.
Wind Tunnel.
The wind tunnel (70 × 70 × 200 cm (31), ) was set with an airflow of 40 cm⋅s−1, a 2-lx [white light-emitting diode (LED) light, top mounted and separated by a diffusing cloth], 19 °C, and 60–75% RH. Incoming air passed through a rack (63 × 90 × 45 cm) holding 24 cylinders of 4 kg of activated charcoal each (Camfil). A laminar airflow was created by a 70 × 70 cm stainless-steel fine mesh (50 mesh) mounted between the charcoal filter and the flight section of the wind tunnel. To provide visual feedback cues during orientation, 20 solid red circles of 10-cm diameter (Ø) were placed randomly in a nonoverlapping pattern on the floor (Fig. 1) Approximately 3 h before the tests, experimental males were moved singly into a glass tube (12.5 × 2.5 cm). Both sides of the tube were closed with netting. Tubes were placed in a closed box, and moved into the wind tunnel room. At the start of an experiment, a tube was placed on a holder (180 cm from the source, 30 cm above the floor), and the netting was removed. Males were released individually. The behavioral experiments were conducted 1–3 h into scotophase.
Odor Sources.
Three different binary blends of Z11 and E11 were used in all experiments: Z blend, 97:3 Z11:E11; E blend, 1:99 Z11:E11; H blend, 50:50 Z11:E11. The purity of the compounds was verified by GC-MS (Z11, 99.7:0.3%; E11, 0.05:99.95%; courtesy of M. Bengtsson, Swedish University of Agricultural Sciences, Alnarp, Sweden). Rubber septum sources were prepared by adding 30 μg of the different synthetic blends in 10 μL of redistilled hexane into the well of the septum (PheroNet). Septa were held in a fume hood overnight before use.
For experiments using swapped plumes, the pheromone was loaded onto a filter paper disk and placed in a Pasteur pipette. Ten microliters of redistilled hexane containing 10 ng of a binary blend was loaded onto a 12.7-mm-Ø filter paper (S&S Antibiotic-Assay disk; Sigma-Aldrich) 1 h before a test and used for 1 h of experiments maximally. Pipettes were vented 1 h before experiments to remove hexane and excess pheromone. Because the two pheromone components are stereoisomers with identical vapor pressures, the volatilization rates of both compounds should be the same and directly correlated with stimulus load on the filter paper (27).
Single- and Three-Choice Tests.
In the one-choice experiment, single males were presented with a single binary blend (either Z, E, or H) for 3 min. Ten males per d were tested for each source (total 30 males per d). This was repeated over 3 different days (total, 90 males). The order of the sources was changed on each experimental day.
In the three-choice experiment, septa with the three different binary blends were positioned horizontally, 10 cm apart. “Smoke” from three septa positioned as above and loaded with titanium tetrachloride formed a mixed plume at the tunnel’s halfway point. A total of 98 males was tested over 3 experimental days (38, 30, 30 males, respectively). The position of the sources was changed each day.
For the single-choice experiments we used a Kruskal–Wallis rank test followed by a Ryan multicomparison test for proportions. For the triple-choice experiments we used a 3 × 2 χ2 test followed by a Ryan multicomparison test for proportions.
Swapped Source Experiment.
Two pipettes were positioned 5 mm apart with their tips pointing upward. The pipettes were connected to a stimulus controller (CS 55; Syntech, Kirchzarten, Germany), with a continuous 0.6 L⋅min−1 airflow. The stimulus controller allowed switching the flow between two outlets each connected with a different stimulus pipette. Loaded and unused pipettes were placed under the same continuous airflow to avoid changing doses during the swap.
After males locked onto a Z-blend plume emerging from a Z-blend pipette, the flow was switched to the other pipette containing either no-odor (negative control, n = 33), the Z blend (positive control, n = 67), or an alternative blend (E blend, n = 116; or H blend, n = 33). Titanium tetrachloride source-generated “smoke” demonstrated that there was no clean-air junction due to swapping of the plumes. A row of 940-nm LEDs set to flash sequentially along the side of the wind tunnel synchronously with the wind flow was used to mark accurately when the odor source was switched and when it reached a flying male. The accuracy was verified using the negative control (Z to clean air swap).
Video Recording and Flight Track Analysis.
The wind tunnel setup was equipped with a 45CSHRX-12 CCD camera (RFconcepts Limited) equipped with a 760-nm high-pass filter. The camera out fed into a AC/DC converter (Canopus ADVC-55; Grass Valley), which was then fed into a PC for recording of the signal. The camera was mounted 60 cm above the wind tunnel top and 20 cm from the upwind end of the wind tunnel under a 20° angle (facing downwind), which provided a view of roughly 80 cm × 150 cm in the flight plane of moths. Moths appeared as light dots on a dark background through reflection of 850-nm IR light from two light sources (RFconcepts Limited) placed at the downwind end of the wind tunnel. Video records of flying moths were tracked every 0.04 s using GraphClick 3.0 (Arizona), and coordinates of flying male moths were converted to actual positions using a custom-made conversion program in Visual Basic, which corrected for parallax and perspective errors. We analyzed tracks records separately in three bins of 1 s: 0–1 s preceding the switch, and 0–1 s/1–2 s after the switch. As the data did not deviate from normality (Kolmogorov–Smirnov test), they were statistically compared using a one-way ANOVA, followed by a Tukey honestly significant difference test to sort among differences between means.
Single Sensillum Recordings.
A male moth was restrained in a plastic micropipette tip, with its head protruding from the aperture. A tungsten wire was inserted into the abdomen as a reference electrode. The head was immobilized with dental wax (Surgident periphery wax; Heraeus Kulzer), and one antenna was fixed on a microscope glass slide using double-sided sticky tape. An electrolytically sharpened recording electrode was inserted at the base of a sensillum under a light microscope (Olympus BX51WI) at 500× magnification. Synthetic odors were diluted in redistilled n-hexane. Solutions of Z11 (purity, 99.93%) and E11 (purity, 99.99%) and a 97:3 blend of these components were prepared volumetrically with redistilled n-hexane and diluted to 1 ng, 10 ng, 100 ng, and 1 µg/µL concentrations. Ten microliters of solution were applied on a 12.7-mm-Ø filter paper disk (Schleicher & Schuell), and the disk was inserted into a Pasteur’s pipette. Pipettes were prepared 1 h before experiments and ventilated for 30 s at 5 mL/s to get rid of solvent and excess pheromone. A charcoal-filtered, humidified air stream (1 L⋅min−1) was directed continuously over the antenna via a glass tube (20 cm length × 6 mm i.d.), positioned ∼2 cm from the antenna. Two stimulation-train protocols were used with Z-blend dosages of 10 ng, 100 ng, 1 µg, either 10 cycles of 200 ms on, 200 ms off, or 10 cycles of 1 s on, 1 s off. Pulse trains were followed by a single stimulation with E11 or Z11. Preceding a stimulation train, the antenna was stimulated with a either a 1-s or a 200-ms (depending on the protocol) puff of Z11, followed 1 min later by a puff of E11. Stimulation followed 1 min after this treatment. The tip of the recording electrode was inserted into the base of a pheromone-sensitive sensillum trichodea in the antennae between antennal segment 3–6 using a micromanipulator (Märzhauser PM-10). The electrode was connected to an AC/DC 10× gain probe (Syntech). When extracellular contact was established, the antenna was stimulated and the activity of the neurons before, during, and after stimulation was recorded. The signal was amplified, digitized (IDAC-4 USB; Syntech), and visualized using a PC with AutoSpike 3.7 software (Syntech). Spikes were counted manually. The response of OSNs was expressed as the number of spikes during the stimulation period after stimulus onset minus the number of spikes before stimulus onset (which represents the spontaneous activity of the neuron). A Student t test (two-tailed, homoscedastic) was used for statistical analyses.
Supplementary Material
Acknowledgments
Zita Kárpáti-Bátri and J. Kárpáti provided technical support. Two anonymous reviewers provided valuable comments that helped improve the manuscript considerably. We acknowledge the following sources for funding: Carl Trygger Stiftelse and Marie Curie Grant IEF-255193/Hungarian Scientific Research Fund PD 1041310 for support of Z.K., Accordo di Programma (Trento Province, Italy) and Stiftelse Lantbruksforskning H1156188 (Sweden) to M.T., Fysiografen (Royal Academy of Natural Sciences, Medicine and Technology in Lund) for support for R.T.C., and Linnaeus Grant IC-E3 to the Division of Chemical Ecology, and Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (2007-1491) for support of T.D.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216145110/-/DCSupplemental.
References
- 1.Löfstedt C. Moth pheromone genetics and evolution. Phyl Trans Roy Soc B. 1993;340:167–177. [Google Scholar]
- 2.Baker T, Fadamiro H. Moth uses fine tuning for odour resolution. Nature. 1998;393:530. [Google Scholar]
- 3.Mafra-Neto A, Cardé RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 4.Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain’s olfactory code. Nature. 2001;410(6827):466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- 5.Kárpáti Z, Dekker T, Hansson BS. Reversed functional topology in the antennal lobe of the male European corn borer. J Exp Biol. 2008;211(Pt 17):2841–2848. doi: 10.1242/jeb.017319. [DOI] [PubMed] [Google Scholar]
- 6.Kárpáti Z, Olsson S, Hansson BS, Dekker T. Inheritance of central neuroanatomy and physiology related to pheromone preference in the male European corn borer. BMC Evol Biol. 2010;10:286. doi: 10.1186/1471-2148-10-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linn CE, Jr, Campbell MG, Roelofs WL. Pheromone components and active spaces: What do moths smell and where do they smell it? Science. 1987;237(4815):650–652. doi: 10.1126/science.237.4815.650. [DOI] [PubMed] [Google Scholar]
- 8.Roelofs W, et al. Sex pheromone production and perception in European corn borer moths is determined by both autosomal and sex-linked genes. Proc Natl Acad Sci USA. 1987;84(21):7585–7589. doi: 10.1073/pnas.84.21.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linn CE, Young MS, Gendle M, Glover TJ, Roelofs WL. Sex pheromone blend discrimination in two races and hybrids of the European corn borer moth, Ostrinia nubilalis. Physiol Entomol. 1997;22(3):212–223. [Google Scholar]
- 10.Lassance JM, et al. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature. 2010;466(7305):486–489. doi: 10.1038/nature09058. [DOI] [PubMed] [Google Scholar]
- 11.Klun JA, Huettel MD. Genetic regulation of sex pheromone production and response. J Chem Ecol. 1988;14(11):2047–2061. doi: 10.1007/BF01014249. [DOI] [PubMed] [Google Scholar]
- 12.Dopman EB, Robbins PS, Seaman A. Components of reproductive isolation between North American pheromone strains of the European corn borer. Evolution. 2010;64(4):881–902. doi: 10.1111/j.1558-5646.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roelofs WL, Du JW, Tang XH, Robbins PS, Eckenrode CJ. Three European corn borer populations in New York based on sex pheromones and voltinism. J Chem Ecol. 1985;11(7):829–836. doi: 10.1007/BF01012071. [DOI] [PubMed] [Google Scholar]
- 14.Linn CE, Roelofs WL. Response specificity of male pink bollworm moths to different blends and dosages of sex pheromone. J Chem Ecol. 1985;11(11):1583–1590. doi: 10.1007/BF01012203. [DOI] [PubMed] [Google Scholar]
- 15.Schlyter F, et al. A model for peak and width of signaling windows: Ips duplicatus and Chilo partellus pheromone component proportions—does response have a wider window than production? J Chem Ecol. 2001;27(7):1481–1511. doi: 10.1023/a:1010377528683. [DOI] [PubMed] [Google Scholar]
- 16.Baker TC, Hansson BS, Löfstedt C, Löfqvist J. Adaptation of antennal neurons in moths is associated with cessation of pheromone-mediated upwind flight. Proc Natl Acad Sci USA. 1988;85(24):9826–9830. doi: 10.1073/pnas.85.24.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernays EA. Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu Rev Entomol. 2001;46:703–727. doi: 10.1146/annurev.ento.46.1.703. [DOI] [PubMed] [Google Scholar]
- 18.Showers WB, Reed GL, Robinson JF, Derozari MB. Flight and sexual activity of the European corn borer. Environ Entomol. 1976;5(6):1099–1104. [Google Scholar]
- 19.Malausa T, et al. Assortative mating in sympatric host races of the European corn borer. Science. 2005;308(5719):258–260. doi: 10.1126/science.1107577. [DOI] [PubMed] [Google Scholar]
- 20.Lassance JM, Löfstedt C. Concerted evolution of male and female display traits in the European corn borer, Ostrinia nubilalis. BMC Biol. 2009;7:10. doi: 10.1186/1741-7007-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groot AT, Classen A, Staudacher A, Schal C, Heckel DG. Phenotypic plasticity in sexual communication signal of a noctuid moth. J Evol Biol. 2010;23(12):2731–2738. doi: 10.1111/j.1420-9101.2010.02124.x. [DOI] [PubMed] [Google Scholar]
- 22.Frolov AN, et al. From Russia with lobe: Genetic differentiation in trilobed uncus Ostrinia spp. follows food plant, not hairy legs. Heredity (Edinb) 2012;108(2):147–156. doi: 10.1038/hdy.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabata J, Takanashi T, Ishikawa Y. Pheromone analysis of wild female moths with a PBAN C-terminal peptide injection for an estimation of assortative mating in adzuki bean borer, Ostrinia scapulalis. J Chem Ecol. 2003;29(12):2749–2759. doi: 10.1023/b:joec.0000008018.52213.65. [DOI] [PubMed] [Google Scholar]
- 24.Cardé RT, et al. European corn borer: Pheromone polymorphism or sibling species? Science. 1978;199(4328):555–556. doi: 10.1126/science.199.4328.555. [DOI] [PubMed] [Google Scholar]
- 25.Allison JD, Cardé RT. Male pheromone blend preference function measured in choice and no-choice wind tunnel trials with almond moths, Cadra cautella. Anim Behav. 2008;75(1):259–266. [Google Scholar]
- 26.Galizia CG, Rössler W. Parallel olfactory systems in insects: Anatomy and function. Annu Rev Entomol. 2010;55:399–420. doi: 10.1146/annurev-ento-112408-085442. [DOI] [PubMed] [Google Scholar]
- 27.Andersson MN, Schlyter F, Hill SR, Dekker T. 2012. What reaches the antenna? How to calibrate odor flux and ligand-receptor affinities. Chem Senses 37(5):403–420, 10.1093/chemse/bjs009.
- 28.Su CY, Martelli C, Emonet T, Carlson JR. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci USA. 2011;108(12):5075–5080. doi: 10.1073/pnas.1100369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66(2):287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silbering AF, Okada R, Ito K, Galizia CG. Olfactory information processing in the Drosophila antennal lobe: Anything goes? J Neurosci. 2008;28(49):13075–13087. doi: 10.1523/JNEUROSCI.2973-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witzgall P, Bengtsson M, Rauscher S, Liblikas I. Identification of further sex pheromone synergists in the codling moth, Cydia pomonella. Entomol Exp Appl. 2001;101(2):131–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.