Abstract
Background:
Presence of cervical metastasis is one of the factors influencing the outcome of patients with carcinoma of the head and neck, its early detection is potentially very important. Triplex ultrasonography technology have definitive role in detecting clinically undetectable involvement of lymph nodes (LNs). The purpose of this study was to evaluate, whether triplex ultrasonography characterization of cervical LNs could, with an acceptable degree of certainty, differentiate malignant from benign/reactive LNs, in order to prevent invasive diagnostic procedures.
Materials and Methods:
A total of 50 patients with oral cancer, were subjected to ultrasonographic investigation of the neck for grayscale, color flow imaging, and pulsed Doppler. All the parameters were compared with histopathologic examination. Correlation was then made between ultrasound and Doppler investigation and histopathology results to evaluate the sensitivity specificity, positive predictive value (PPV) and negative predictive value (NPV) of color Doppler ultrasonography in detecting metastatic neck nodes.
Results:
Study results showed that malignant LNs, especially metastatic nodes, are accompanied with significantly high resistive index (RI) and pulsatility index (PI) values, rounded shape, size, loss of central hilar echogenicity and peripheral vascularity. Among these sonographic findings, nodal shape (longitudinal nodal diameter to transverse diameter ratio or L/T ratio), RI and PI values were more accurate for differentiating benign from malignant LNs.
Conclusion:
In this study, triplex sonographic findings had relatively high accuracy in differentiating benign from malignant cervical LNs. Ultrasound hence can be recommended for initial non-invasive evaluation of the neck in patients with oral cancers with or without palpable cervical lymph nodes.
Keywords: Cervical lymph nodes, color mapping, spectral doppler, triplex ultrasound
INTRODUCTION
Oral cancer is a major cause of morbidity worldwide; 90% of oral malignancies are squamous cell carcinomas.[1] Oral cancer is the sixth most frequent neoplasm in the world, with an increasing incidence in developing countries.[2] Early detection would drastically bring down the morbidity and mortality associated with advanced stages of oral cancer.
Most lymph nodes in the human body are in the cervicofacial area so invasive squamous cell carcinoma of the upper aero-digestive tract has a strong potential for metastatic spread to the cervical lymph nodes. The involvement of the lymph nodes with metastatic deposits is always associated with a poor prognosis, approximately 50% poorer than for patients with equivalent tumors with no nodal involvement. Hence, the neck status is the single most important indicator of prognosis in head and neck cancers and early detection of LNs involvement has great therapeutic and prognostic implications.[3]
An increase in nodal size was found to be an effective imaging criterion for the detection of metastatic cervical nodes with CT and MR imaging.[4] However, size determination alone is not effective enough for detecting metastatic nodes. Therefore, several studies have attempted to improve diagnostic accuracy by assessing the internal architecture of the node and using other tissue-specific imaging techniques such as sonographically guided fine-needle aspiration biopsy and Fluorodeoxyglucose positron emission tomography. Recently, Curtin, et al.,[5] showed that combined information on the size and internal architecture of the node facilitated the detection of nodes that were metastatic from squamous cell carcinoma of the head and neck, confirming, at least in part, the significance of the assessment of the internal architecture of a node for the detection of metastatic nodes.
Triplex sonographic evaluation of enlarged nodes is also based on assessment of the internal architecture as well as size determination of the node, and abnormalities in the node may be reflected by increased parenchymal echogenicity or loss of hilar echogenicity in malignant disease.[6] In addition, the recent development of Doppler sonography technology has shed light on the diagnostic significance of changes in nodal blood flow in differentiating metastatic from non-metastatic nodes. Studies have shown that sonography used for staging head and neck tumors is more sensitive than clinical examination and even CT scan for detection of cervical LNs involvement.[6]
The purpose of this study is to demonstrate triplex ultrasonographic characterization of cervical lymph nodes could, with acceptable degree of certainty, differentiate malignant from benign lymph nodes, in order to prevent invasive diagnostic procedures.
MATERIALS AND METHODS
A total of 50 patients, who were referred to the Department of Cranio Maxillofacial Plastic and Reconstructive Surgery at College of Dental Sciences, Davangere, Karnataka from August 2008 to August 2010, of both sexes, with carcinoma of different regions of the oral cavity, confirmed with incision biopsy and had clinically palpable cervical lymph nodes (suspected of metastasis), were included in this study.
Written and verbal consent were obtained by the subjects prior to the start of study in accordance with the declaration of Helsinki (World Medical Association). All cervical regions of these patients were scanned by three experienced radiologists separately, with PHILIPS Envisor-C (Shenzhen Lontek Electronic Technology Co., Limited, China). Broad band (3-12MHz), grayscale at 3-12 MHz and power Doppler at 5 MHz along with linear array transducer of 7-12 MHz frequencies and all the findings were properly registered. All patients were in supine position and their neck hyper extended. The radiologists were unaware of pathology results during examination. As lymph nodes are encountered during the examination, their site is noted as per the classification given by American Joint Committee on Cancer.
Ultrasonographic, high resolution pulsed and color Doppler ultrasonography findings were documented under following parameters, and these parameters are defined as follows:
Shape: LNs were divided into two groups round or oblong.
Size: LNs were divided into two groups size <0.8 and >0.8 cm.
L/T ratio (roundness index): LNs were divided in two groups, L/T ≤ 2 and L/T > 2.
Echotexture: The LNs were divided in two groups, those with homogenous and those with heterogenous echotexture.
Margin: Based on their margins LNs were divided in two groups, loss of capsular intactness and capsule intact lymph nodes.
Central Hilar Echogenicity: Absence or presence of hilar echogenicity
Vascular pattern: The LNs vascular pattern were assessed by color mapping and classified as central, peripheral and mixed types.
Arterial resistive index (RI): This parameter was defined as the mean RI of two to three arterial vessels within LNs.
Pulsatility index (PI): For a metastatic node was defined as the mean PI of two to three arterial vessels within LNs.
The lymph nodes obtained surgically were subjected to histopathological examination done in the Department of Oral Pathology, College of Dental Sciences, Davangere for presence of metastasis and compared with the ultrasonographic and Doppler findings to cross check for true positive and true negative results. Lymphomatous and metastatic LNs were classified as malignant and reactive LNs as benign cervical nodes.
Data collected were computerized and analyzed using the Statistical Package for Social Sciences (SPSS) version 17.0. Differences in means of Resistivity index and Pulsatility index were statistically evaluated by means of Student's t-test. The diagnostic value of the ultrasonographic findings and central color flow (flow pattern) was statistically evaluated by means of Chi-square test. P value of 0.05 or less was considered as statistically significant. Diagnostic validity and predictive value of high resolution pulsed and color Doppler ultrasonography was done by assessing the sensitivity, specificity, positive prediction value and negative prediction value compared to histopathologic results.
RESULTS
A total of 50 cases of squamous cell carcinoma of the oral cavity were included in this study, according to the inclusion and exclusion criteria. In them, 119 lymph nodes were detected by ultrasonography and color Doppler investigation of the patients. Of the 119 nodes, 48 (40.33%) of the nodes were metastatic on histopathologic examination and 71 (59.7%) were benign [Table 1].
Table 1.
Depicts distribution of parameters used in the study as compared to histopathological results
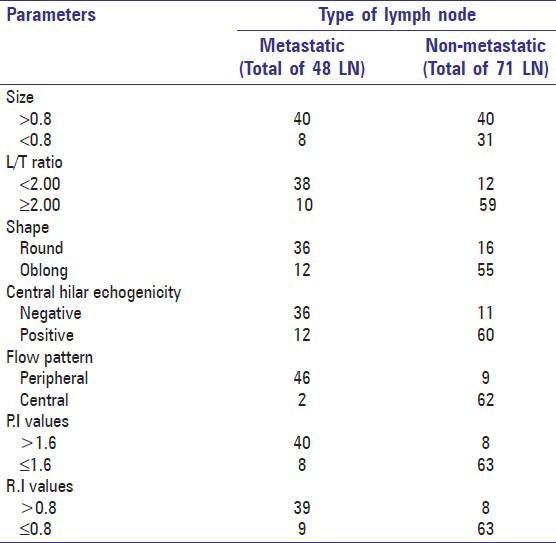
Results of triplex ultrasonographic assessment of LNs are shown in Table 1. For the 48 metastatic nodes, the observed results were as follows. Thirty-eight ((79.1%) had an L/T ratio <2.00 and 10 (20.9%) had a ratio >2.00. Statistically significant difference between L/T ratio of malignant and benign LNs was observed. L/T ratio ≤2 had statistically significant association with malignancy (P value < 0.00). Forty (83.4%) of the metastatic nodes were >0.8 cm in size and 8 (16.6%) were <0.8 cm in size. Nodal size >0.8 had statistically significant association with malignancy (P value < 0.02) [Table 1].
Thirty-six (75%) of the lymph nodes were round in shape and 12 (25%) had oblong shape [Figure 1]. Round shape had significant relation with malignancy with a P value of 0.000. Thirty-six (75%) showed negative or absence of hilar echoes and 12 (25%) showed positive or presence of hilar echogenicity. There was statistically significant difference in hilar echogenicity between metastatic and non-metastatic lymph nodes with a P value of 0.00 [Table 1].
Figure 1.
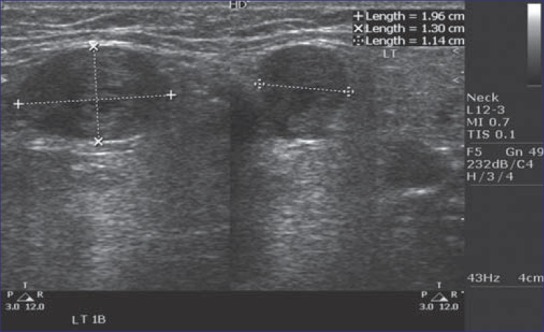
Triplex ultrasound scan showing long axis transverse axis of lymph nodes
There was no difference in capsular intactness and echogenic texture among metastatic and non-metastatic lymph nodes.
Some degree of vascularity was detected in all LNs by color Doppler assessment. Forty-six (95.8%) of the metastatic nodes had a peripheral parenchymal pattern of blood flow and 2 (4.2%) had a hilar (central) pattern of blood flow as compared to non-metastatic nodes in which 9 (12.6%) showed peripheral parenchymal pattern of blood flow and 62 (87.32%) showed a hilar pattern of blood flow [Figures 2 and 3]. Peripheral vascular pattern had statistically significant association with malignant LNs (P value <0.000) [Table 1].
Figure 2.
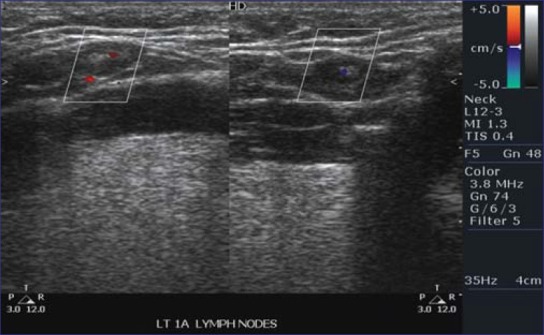
Triplex ultrasound scan showing central vasculature
Figure 3.
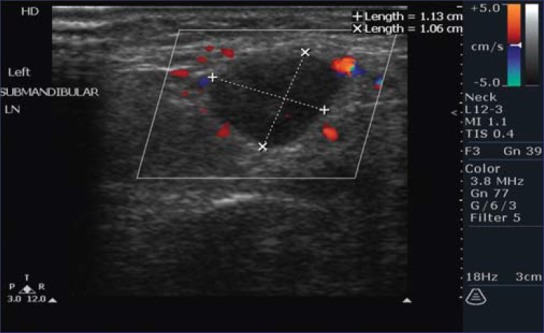
Triplex ultrasound scan showing peripheral vasculature
In 48 metastatic nodes, 40 nodes (83.3%) had a PI value >1.5 and in 8 (16.7%) of the nodes, PI value <1.5 was observed. Similarly, RI value of >0.8 was observed in 39 (81.25%) metastatic nodes and a RI value <0.8 was seen in 9 (18.75%) metastatic nodes [Figure 4]. Statistically significant difference between mean arterial RI and PI value of malignant and benign LNs was observed with t values of 11.91 and 11.84 respectively (P values <0.05) [Table 1].
Figure 4.
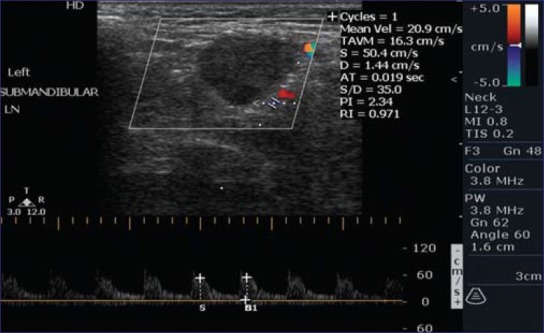
Triplex ultrasound showing R.I and P.I
DISCUSSION
The role of ultrasonography in evaluating superficial lymph nodes is well documented in literature.[6,7] In the present study, the results depicts that sonography can accurately differentiate metastatic from non-metastatic nodes in the neck. Furthermore, we found that the better performance of sonography for depicting metastatic nodes appears to be because of its greater ability to delineate changes in the internal architecture of the node.[7]
The present study showed metastatic nodes were larger in size (>0.8 cm) compared with non-metastatic nodes. Our result is consistent with the study done by Giovagnorio, et al.,[8] in Italy and Mazaher, et al.,[9] in Iran. More recent studies concluded that the size criteria is inaccurate to predict metastasis as the phenomenon of micrometastasis with extracapsular spread has been demonstrated in nodes as small as 2 mm in diameter and inflammation may also cause nodal enlargement. However in patients with proven head and neck cancers, increasing size of lymph nodes on serial ultrasound examinations is highly suspicious of metastases.[10,11]
In our study, the majority of LNs with a ratio of L/T > 2 were benign and the majority of those having a ratio of L/T ≤ 2 were malignant, with a specificity of 83.09%, sensitivity of 79.16%, positive predictive value of 76% and negative predictive value of 85.5 %, L/T ratio was more useful as a screening tool as it was more sensitive in ruling out whether a node was metastatic [Table 2]. Ultrasound showed the tendency for benign nodes to be oval (L/T > 2) and malignant nodes to be round (L/T < 2). Our findings are consistent with those of other authors.[6,12–15]
Table 2.
Depicts sensitivity, specificity, positive predictive value and negative predictive value of parameters compared to histopathological results
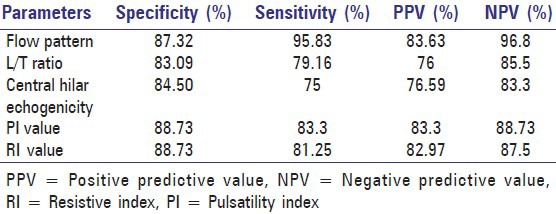
The sonographic criteria presence or absence of hilar echoes was used in this study. Results of the study showed difference in hilar echogenicity between metastatic and non-metastatic lymph nodes with a specificity of 84.5%, sensitivity 75%, positive predictive value 76.59% and negative predictive value 83.3 % [Table 2]. Our study is consistent with the findings of studies done by Vassallo, et al. and Rubaltelli, et al.,[7,13] Our study showed that echotexture and capsular intactness are not valuable criteria for differentiating benign from malignant LNs which is contrasting to the results of other studies.[6,16]
It is well known that normal and reactive lymph nodes have intense vascularization with rich cortical capillary circulation, and this explains the appearance of the intense homogeneous enhancement that we observed in 87.32% of 71 non-metastatic lymph nodes. Lymph node metastases are generally less vascularized than healthy lymph node parenchyma and therefore behave as areas of perfusion defects; moreover, the presence of completely avascular necroses in metastatic lymph nodes is frequent. Indeed, of the 48 metastatic lymph nodes examined in our survey, 44 showed well-defined areas of perfusion defects, and 4 showed scarce or absent perfusion due to widespread invasion of confluent areas of necrosis and neoplastic infiltration.
In our series, the mean arterial resistive index RI (>0.8) and Pulsatility index (P.I) were significantly higher in malignant LNs (>1.5) than in benign LNs. It has been suggested that the high vascular resistance is generally due to the compression of intranodal vessels by the tumor cells. Our result showed that analysis of nodal vascular pattern on color Doppler sonography is valuable in differentiating benign from malignant nodes and it is also highly sensitive (sensitivity of RI and PI was 86.3%) in detecting borderline size or with shape mimicking a benign node [Table 2]. The findings of this study compare favorably with those of other researchers.[15,17–19]
CONCLUSION
In conclusion using all the ultrasound potentials, which are grayscale, color flow imaging, power Doppler and pulse Doppler, lymphadenopathy in patients with oral cancer can be satisfactorily divided into benign and malignant cases. It can provide the macroscopic appearance of the node in question non-invasively and aids in better treatment plan.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Shah JP. 1st ed. Canada: BC Decker; 2001. Cancer of the Head and Neck; p. 100. [Google Scholar]
- 2.Watkinson J, Wilson J, Gaze MN. 4th ed. London: Hodder Arnold Publishers; 2000. Head and neck surgery; p. 10. [Google Scholar]
- 3.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–9. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 4.Sumi M, Ohm M, Nakamura T. Comparision of Sonography and CT for Differentiating Benign from Malignant cervical lymph nodes in patients with squamous cell carcinoma of the Head and Neck. AJR Am J Roentgenol. 2001;176:1019–24. doi: 10.2214/ajr.176.4.1761019. [DOI] [PubMed] [Google Scholar]
- 5.Curtin HD, Ishwaran H, Mancuso AA, Dalley RW, Caudry DJ, McNeil BJ. Comparison of CT and MR imaging in staging of neck metastases. Radiology. 1998;207:123–30. doi: 10.1148/radiology.207.1.9530307. [DOI] [PubMed] [Google Scholar]
- 6.Sharifkashani SM, Sharifan H. Triplex ultrasonographic assessment of cervical lymph nodes. Acta Medica Iranica. 2004;42:441–4. [Google Scholar]
- 7.Rubatelli L, Khadivi Y, Tregnaghi A, Stramare R, Ferro F, Borsato S, et al. Evaluation of Lymph Node Perfusion Using Continuous Mode Harmonic Ultrasonography with a Second-Generation Contrast Agent. J Ultrasound Med. 2004;23:829–36. doi: 10.7863/jum.2004.23.6.829. [DOI] [PubMed] [Google Scholar]
- 8.Giovagnorio F. Color Doppler sonography in the evaluation of superficial lymphomatous lymph nodes. J Ultrasound Med. 2002;21:403–8. doi: 10.7863/jum.2002.21.4.403. [DOI] [PubMed] [Google Scholar]
- 9.Mazaher, Sharifkashani Sh, Sharifan H. Triplex ultrasonographic assessment of cervical lymph nodes. Acta Medica Iranica. 2004;42:441–4. [Google Scholar]
- 10.Som P. Lymph nodes of the neck. Radiology. 1987;165:593–600. doi: 10.1148/radiology.165.3.3317494. [DOI] [PubMed] [Google Scholar]
- 11.Sinnatamby CS, Last RJ. 6th ed. New York: Elsevier/Churchill Livingstone; 2006. Anatomy regional and applied; pp. 376–8. [Google Scholar]
- 12.Solbiati L, Cioffi V, Ballarati E. Ultrasonography of the Neck. Radiolo Clin North Am. 1992;30:941–53. [PubMed] [Google Scholar]
- 13.Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of Benign from Malignant Superficial Lymphadenopathy: The Role of High-resolution US. Radiology. 1992;183:215–20. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 14.Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of Cervical Lymphadenopathy: Usefulness of Color Doppler Sonography. AJR Am J Roentgenol. 1997;168:1311–6. doi: 10.2214/ajr.168.5.9129432. [DOI] [PubMed] [Google Scholar]
- 15.Dragoni F, Cartoni C, Pescarmona E, Chiarotti F, Puopolo M, Orsi E. The role of high resolution pulsed and color doppler ultrasound in the differential diagnosis of benign and malignant lymphadenopathy. Cancer. 1999;85:2485–90. doi: 10.1002/(sici)1097-0142(19990601)85:11<2485::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Yusa H, Yoshida H, Ueno E. Ultrasonographic criteria for Diagnosis of Cervical Lymph Node Metastasis of Squamous cell carcinoma in the Oral and Maxillofacial Region. J Oral Maxillofac Surg. 1999;57:41–8. doi: 10.1016/s0278-2391(99)90631-6. [DOI] [PubMed] [Google Scholar]
- 17.Chang DB, Yuan A, Yu CJ, Luh KT, Kuo SH, Yang PC. Differentiation of benign and malignant cervical lymph nodes with color doppler sonography. AJR Am J Roentgenol. 1994;162:965–8. doi: 10.2214/ajr.162.4.8141027. [DOI] [PubMed] [Google Scholar]
- 18.Choi MY, Lee JW, Jang KJ. Distinction between Benign and Malignant Causes of Cervical, Axillary and Inguinal Lymphadenopathy: Value of Doppler Spectral Waveform Analysis. AJR Am J Roentgenol. 1995;165:981–4. doi: 10.2214/ajr.165.4.7677005. [DOI] [PubMed] [Google Scholar]
- 19.Wu CH, Chang YL, Hsu WC, Ko JY, Sheen TS, Hsieh FJ. Usefulness of Doppler Spectral Analysis and Power Doppler Sonography in the differentiation of cervical Lymphadenopathies. AJR Am J Roentgenol. 1998;171:503–9. doi: 10.2214/ajr.171.2.9694484. [DOI] [PubMed] [Google Scholar]


