Abstract
Background:
Ameloblastoma is characterized as a slow growing, non-metastatic and a locally invasive tumor with a high risk of recurrence. Immunohistochemical evaluation of ameloblastomas using epithelial and connective tissue specific markers help in studying the histogenesis and assessing the biological behavior. The aim of the study was to study the expression patterns of cytokeratin, vimentin, smooth muscle actin (SMA), S100 and CD34 in ameloblastomas.
Materials and Methods:
The material for the study consisted of 24 cases of ameloblastomas. The excised specimens were grossed and bits were taken from different areas of the specimen. Based on the histopathology, the cases were classified into different types and stained for immunohistochemistry.
Results:
The cases showed strong positivity to cytokeratin, vimentin, moderate positivity for SMA and S100. Five cases were also moderately positive for CD34 in blood vessels.
Conclusion:
The results and hypothesis achieved from the study, proved to be consistent, not only augmenting the already existing hypothesis but also imparting new concepts of hypothesis.
Keywords: Ameloblastoma, CD34, cytokeratin, S100, smooth muscle actin, vimentin
INTRODUCTION
Odontogenic tumors are tumors that are thought to arise from tooth forming tissues.[1] Ameloblastoma is an odontogenic tumor which has commanded considerable attention in the medical and dental literature, since the beginning of 19th century. Ameloblastoma is characterized as a slow growing, non-metastatic and a locally invasive tumor with a high risk of recurrence. Ameloblastomas can be classified in many ways namely: according to the location as intraosseous and peripheral types, according to histopathology broadly classified into solid, unicystic and desmoplastic variants. The histological sub variants can be further classified as follicular and plexiform types which in turn may show acanthomatous, basal cell, granular cell changes etc.
Several types of odontogenic lesions, particularly odontogenic keratocyst and solid ameloblastoma, although defined as benign, demonstrate locally aggressive behavior and a potential lethal nature.[1–3]
Cytokeratin is an intermediate filament, which is a major structural protein of epithelial cells. They are multigene family of proteins that differ in their distribution in different types of epithelia. While virtually all epithelial cells possess the potential to elaborate keratins, odontogenic epithelium in its fully differentiated state is not a keratinized tissue. In odontogenic neoplasms, the patterns of cytokeratin may get altered and has been extensively studied.[4] Vimentin is a part of the intermediate filament family and expressed in fibroblasts and endothelial cells. Unlike other intermediate filament proteins, vimentin is expressed along with desmin during the early stages of cellular development. It is speculated that vimentins are involved in intracellular transport of proteins between the nucleus and plasma membrane.[5] The pattern of cytokeratin and vimentin expression in odontogenic tumor may help in studying the histogenesis and in predicting their biologic behavior. Myofibroblasts play a very important role in wound healing and remodeling. It is now well accepted that myofibroblasts play a very important role in tumor invasion and angiogenesis. In wound healing and tumorogenesis, the trans-differentiation of fibroblasts to myofibroblasts marks the stromal changes. These stromal changes may contribute to tumor invasion. Immunohistochemical assessment of the frequency of stromal myofibroblasts in different histological types of ameloblastomas will help in predicting their aggressive biological behavior.[6] Intra-tumoral blood vessels are known to play an important role in cancer growth by supplying oxygen and nutrients, and also associated with metastasis. Immunohistochemical staining for CD34 is a sensitive marker of vascular endothelium used to evaluate microvessel density in numerous tumors like ameloblastoma.[7] Oral tissues have not been extensively studied for S100 protein. The immunolocalization of this protein is used to demonstrate evidence of neuroectodermal histogenesis.[8]
A number of clinico-pathologic studies on ameloblastomas have been published in English literature. Similar studies on ameloblastomas in India have not yet been sufficiently published in comparison with that of English and other language literature. Therefore, in this study an attempt has been made to evaluate the histogenesis and biological behavior of ameloblastomas using cytokeratin, vimentin, S100, SMA and CD34.
MATERIALS AND METHODS
The material for the study consisted of 24 cases of ameloblastomas. The paraffin embedded blocks were retrieved from the Department of Oral and Maxillofacial Pathology and Microbiology, Saveetha University, Chennai.
The blocks were sectioned and stained with hematoxylin and eosin. Based on the histopathology, the cases were classified into different types and selected according to the individual markers studied. Different histological types of ameloblastomas studied include, follicular ameloblastoma, plexiform ameloblastoma, desmoplastic ameloblastoma and unicystic ameloblastoma. Positive controls for various markers were included. Twenty-four tissue blocks were considered for immunohistochemical staining for cytokeratin, vimentin and 20 tissue blocks for smooth muscle actin, CD34 and 10 tissue blocks for S100.
Immunohistochemistry
Immunohistochemical analysis was performed on 3 micron paraffin embedded tissue sections on gelatin coated glass slides. After heat drying, sections were deparaffinized in xylene and subsequently rehydrated in gradients of ethanol. Antigen retrieval to unmask antigenic sites was done using 10 mM citric acid solution (pH 6) followed by a washing step with Tris buffered saline (TBS pH 7.6). Further incubations with pre-diluted ready to use primary mouse monoclonal anti-cytokeratin antibody (AE1/AE3) (Biogenex, San Ramon), monoclonal mouse anti-vimentin antibody (V9) (Biogenex, San Ramon), monoclonal mouse anti-smooth muscle actin antibody (IA4) (Biogenex, San Ramon), monoclonal mouse anti CD34 antibody (Q Bend/10) (Biogenex, San Ramon), monoclonal mouse anti S100 antibody (N 1573) (DAKO Carpenteria, CA, USA) was performed at 37° C for 30 min. Sections were washed again and incubated with biotinylated secondary antibody (Biogenex, San Ramon) for 30 min followed by the streptavidin-biotin-peroxidase (Biogenex, San Ramon) for 30 min at room temperature. Colored reactions were developed by incubating with 3'3'-diaminobenzidine (0.05 diaminobenzidine in 0.05 M Tris buffer, pH 7.6, and 0.01 H2O2) and subsequently counterstained with Harris hematoxylin and mounted with DPX (Dysterene Plasticizer Xylene). Positive and negative controls were included in all reactions. Presence of brown colored reaction localized to the nucleus or cytoplasm was considered as positive reaction. The intensity of the immunostaining was classified as negative, weak or strong from three fields in a blinded analysis performed by two independent pathologists.
For cytokeratin, S100, vimentin, and smooth muscle actin, the intensity of staining and staining location were considered. A scale of 0 to + + was used. The location of staining was assessed at both tissue and cellular level. Concerning the tissue level location, staining of lining cells, stellate reticulum and connective tissue were assessed. Further the areas were graded based on the staining intensity as, 0 - absence of staining, + - weak positive staining, + + - strong positive staining. At cellular level, staining of cell membrane, cytoplasm and nucleus were assessed and graded as follows, M - membranous positivity, C - cytoplasmic positivity, N - nuclear positivity and B - both. For CD34, the numbers of vessels were assessed in 3 random locations, at 10× and 40× magnification. The microvessel density (MVD) was calculated as the mean of 3 recorded values.
Statistical analysis
All the parameters were tabulated and assessed for statistical significance using SPSS statistical package. Differences in the distribution of cytokeratin, vimentin, smooth muscle actin, CD34 and S100 expression among different types of ameloblastomas were compared using the Pearsons Chi-square test. Association between the variables for CD34 microvessel density was calculated using Kendal's Tau correlation. Significance was set at P <0.05. Kappa statistics for interobserver variability was not performed considering the sample size and the number of markers.
RESULTS
The study group consisted of 24 cases and the results obtained for various markers are as follows,
Cytokeratin
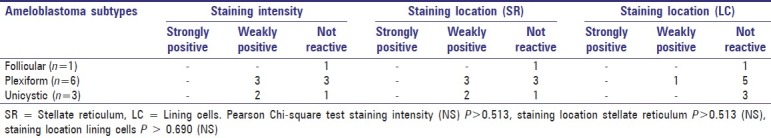
Twenty-four cases of ameloblastomas were stained for cytokeratin with strong positivity in 9 cases, weak positivity in 11 cases and not reactive in 4 cases. Maximum number of positivity was observed in plexiform ameloblastomas and was statistically significant (P<0.049). The intensity of staining based on histological subtypes is depicted in Table 1.
Table 1.
Cytokeratin staining intensity and staining location in stellate reticulum and lining cells in different types of ameloblastomas
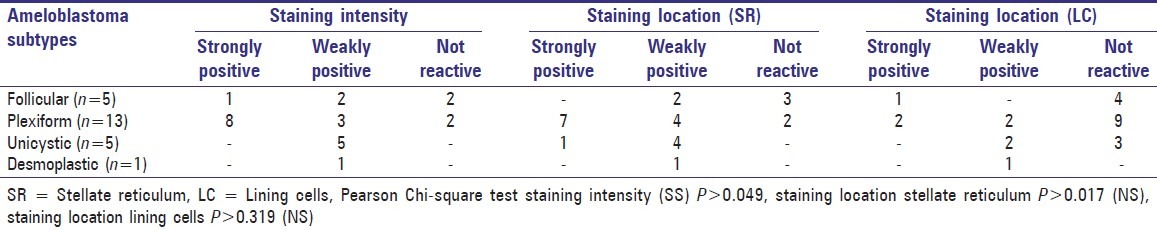
The pattern of cytokeratin expression was seen localized to both the stellate reticulum and ameloblastomatous lining cells [Figure 1]. Cytokeratin expression in stellate reticulum was strongly positive in 8 cases, weakly positive in 11 cases and negative in 5 cases, whereas only 3 cases exhibited strong positivity in lining cells of the tumor islands. Majority of the cases (n = 16) were negative for cytokeratin in the lining cells.
Figure 1.
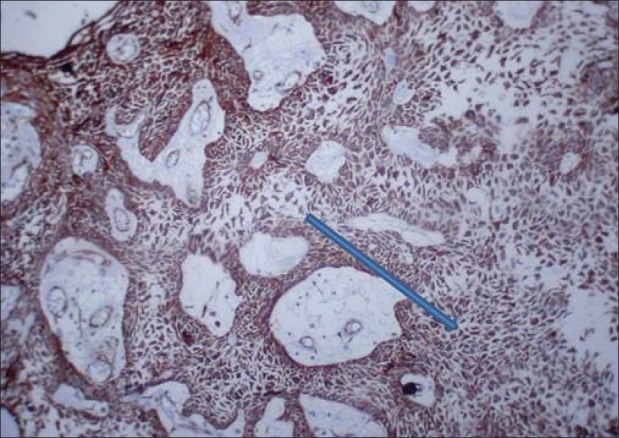
Plexiform strands showing strong positivity for cytokeratin ×10
Vimentin
Twenty-four cases of ameloblastomas were assessed for vimentin expression.
Concerning the staining intensity, 16 cases were strongly positive, 6 cases weakly positive and 2 cases were negative. None of the cases demonstrated positivity in the ameloblastomatous epithelium, except one case which showed a weak expression pattern in the stellate reticulum region.
Vimentin expression was seen localized to the connective tissue region and the expression pattern was assessed separately for connective tissue near the periphery and near the tumor islands. Connective tissue at the periphery was strongly positive in 15 cases, weakly positive in 6 cases and negative in 3 cases. In contrast, 13 cases demonstrated weak positive staining for the connective tissue near the tumor islands and the rest were negative [Figure 2] [Table 2]. A stronger expression pattern of vimentin was seen for the mature connective tissue (n = 13) when compared to the immature connective tissue (n = 1) [Figure 3].
Figure 2.
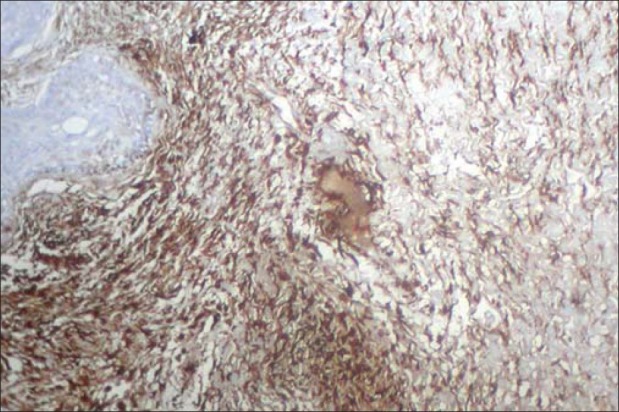
Mature connective tissue near the islands showing strong positivity for vimentin ×10
Table 2.
Vimentin staining intensity and staining location in stellate reticulum areas and connective tissue stroma
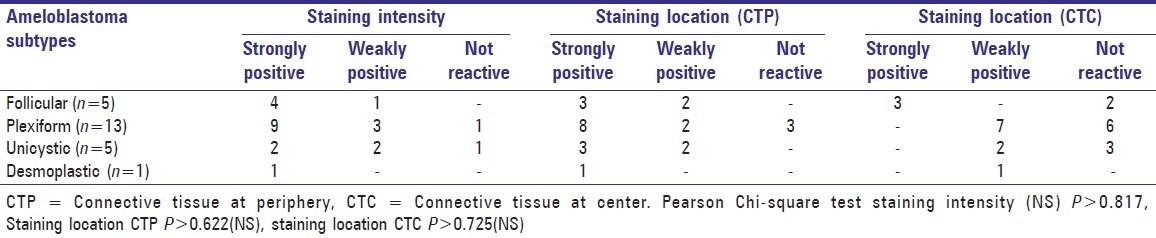
Figure 3.
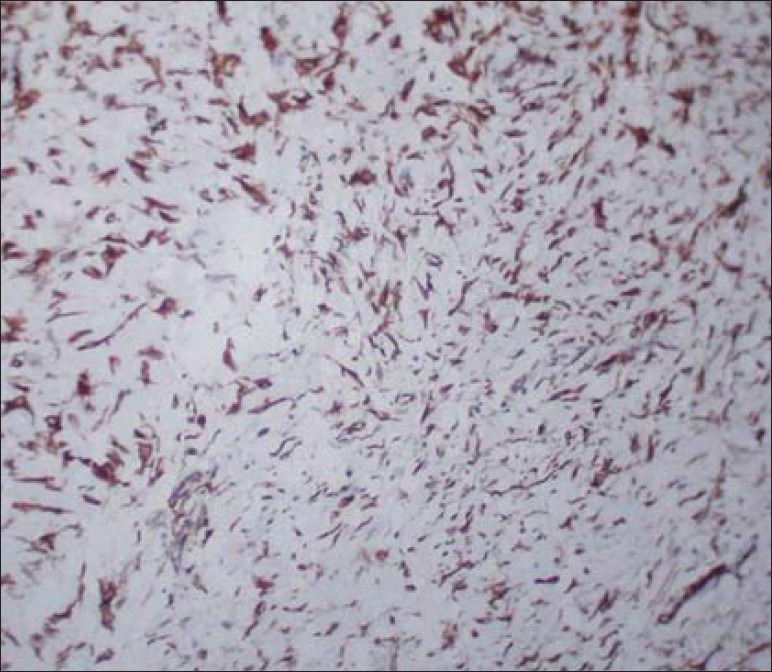
Immature connective tissue showing strong positivity for vimentin ×10
Smooth muscle actin
Twenty cases (n = 20) of ameloblastomas were assessed for the pattern of expression of smooth muscle actin (SMA).
SMA expression was strongly positive in 3 cases, weakly positive 11 cases and negative in 6 cases [Table 3]. SMA expression pattern was assessed for both cells around the blood vessels, tumor front and was strongly positive in 2 cases, respectively [Figure 4]. Weak positive staining was seen around the blood vessels in 12 cases and the rest were negative. When compared to SMA expression around the blood vessels only 7 cases exhibited weak positivity at the tumor front and the remaining 11 cases were negative. The SMA expression pattern at the tumor front was found to be statistically significant (P < 0.032).
Table 3.
Smooth muscle actin staining intensity and staining location around blood vessels and tumor front
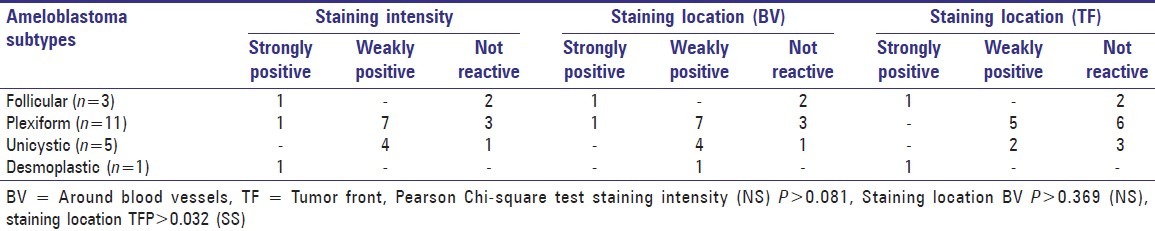
Figure 4.
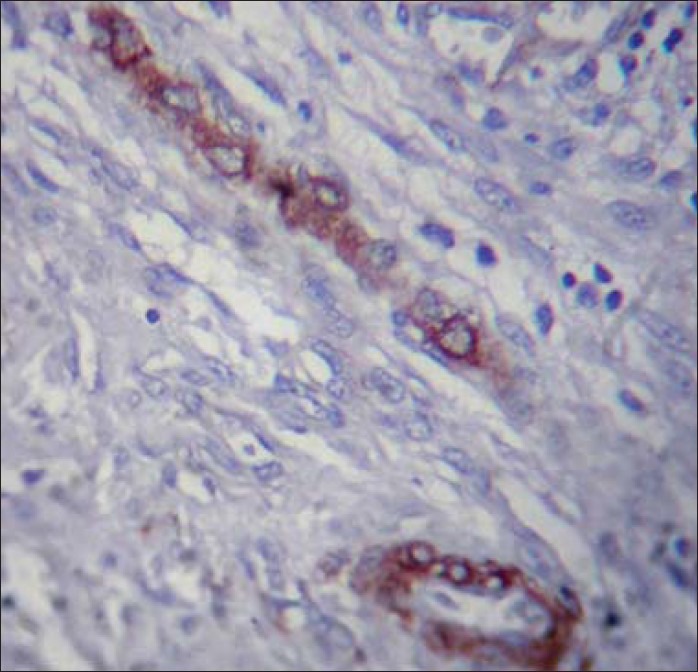
Myofibroblasts showing smooth muscle actin positivity predominantly around the blood vessels and few within the connective tissue ×40
CD34
Twenty cases of ameloblastomas were assessed for CD34 positive vessels microvessel density (MVD) at 10× and 40× magnification.
Microvessel density at ×10
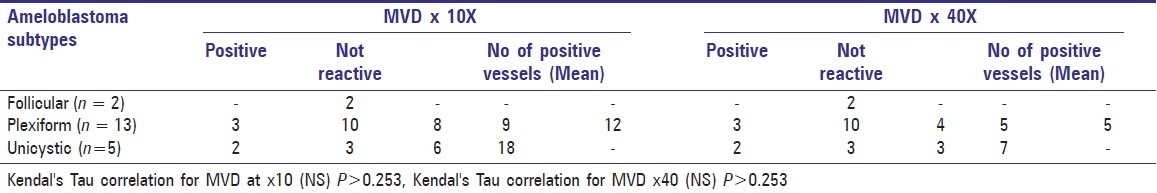
Totally out of 20 cases, 5 cases exhibited CD34 positive vessels at 10× magnification. Remaining cases were all negative for CD34 positive vessels. Of the total 2 cases of follicular ameloblastomas none exhibited CD34 positive vessels. Of the total of 13 cases of plexiform ameloblastomas, 3 cases revealed the presence of CD34 positive vessels and their mean values are 8, 9, and 12 respectively. Of the total of 5 cases of unicystic ameloblastomas, CD34 positive vessels were present in 2 cases and their mean values are 6 and 18, respectively [Table 4].
Table 4.
CD34 Microvessel density (MVD) positive blood vessels at 10× and 40× magnification in different ameloblastoma types
Microvessel density at ×40
Totally out of 20 cases, 5 cases exhibited CD34 positive vessels at 40× magnification. Remaining cases were all negative for CD34 positive vessels. Of the total 2 cases of follicular ameloblastomas none exhibited CD34 positive vessels. Of the total of 13 cases of plexiform ameloblastomas, 3 cases revealed the presence of CD34 positive vessels and their mean values are 4, 5, and 5 respectively [Figure 5]. Of the total of 5 cases of unicystic ameloblastomas, CD34 positive vessels were present in 2 cases and their mean values are 3 and 7, respectively.
Figure 5.
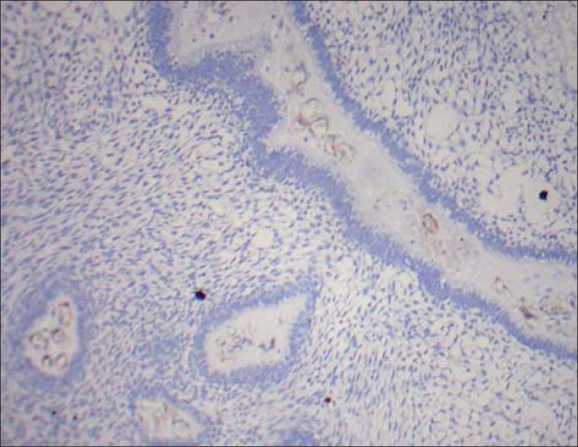
Proliferative plexiform type showing CD34 positive blood vessels at ×10 magnification
S100
Ten cases (n = 10) were considered for S100 staining and 5 cases demonstrated weak positive staining and was seen localized to stellate reticulum [Figure 6] except for one case where the staining was localized to lining cells. The rest of the cases were found to be negative [Table 5].
Figure 6.
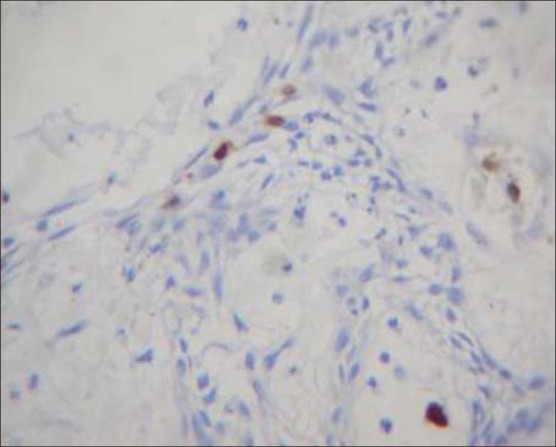
S100 weak positive staining seen localized to stellate reticulum at ×10 magnification
Table 5.
S100 staining intensity and staining location in stellate reticulum and lining cells
DISCUSSION
Odontogenic tumors, similar to odontogenic cysts, arise from the dental apparatus and, in addition, mimic various stages of tooth formation. In spite of being the commonest odontogenic tumor, paucity still exists in understanding the molecular mechanisms associated with the pathogenesis and biological behavior of this neoplasm.
The present study consisted of 5 cases of follicular ameloblastomas, 13 cases of plexiform ameloblastomas, 5 cases of unicystic ameloblastomas and 1 case of desmoplastic ameloblastoma, and the above typing was based on the H and E sections.
Cytokeratins are subdivided into type I and II with molecular weight ranging from 40-67 Kda. In our study, we used AE1/AE3, the cocktail keratin which consists of antibodies to CK 1, 5, 8, 10, 14 and 19.
According to Fukumashi et al.[9] CK8 and 19 are normally found in the odontogenic epithelium and reacted positively to the constituting cells in all types of ameloblastomas. Similarly all our 24 cases studied reacted positively for cytokeratins due to the presence of CK8 and 19 in our cocktail antibody. This finding reiterates that all types of ameloblastomas including unicystic are derived from the odontogenic epithelium. Majority of the cases in our study were plexiform ameloblastomas. Considering the intense positivity of cytokeratin in plexiform ameloblastomas, it can be argued that, considering the fact that plexiform is the most primitive form, the cytokeratin expression could indicate recapitulation of the odontogenic epithelium to the original oral epithelium. Thus because of this functional discrepancy, the ameloblastomas are not able to induce hard tissue formation.[10] However, it is assumed that since we used a cocktail cytokeratin and since the individual component cytokeratins have been extensively studied, it is anticipated to be expressed in a similar pattern.
Regarding the cytokeratin positivity in desmoplastic ameloblastomas, it is consistent with the review by Siar, et al.[11] There was a weak, focal and inconsistent staining in ameloblastomatous islands. With regard to the staining pattern of the lining cells in ameloblastomas, only 2 of 24 cases had weak focal staining. This positivity could be attributed to the expression of CK14 in the lining cells, as seen by Crivelini et al.[12]
In unicystic ameloblastomas, a weak staining pattern of cytokeratin in the lining cells and stellate reticulum is seen in all 5 cases, reemphasizing the fact that it is a true neoplasm and a unique subtype of ameloblastoma. Moreover, all the cases showed weak staining in both lining and stellate reticulum, which indicates a shift in oral cytokeratin profile to odontogenic cytokeratin profile.
Vimentin supports the cell membrane and also plays an important role in intracellular transport of proteins between the nucleus and plasma membrane. In our study, intense staining for vimentin was observed in the connective tissue stroma of 15 cases at the periphery and weak staining in 6 cases at tumor islands. The intense staining at the periphery of the tumor suggests normal pattern of connective tissue of that anatomical site. The general lack of much connective tissue stroma around the epithelial component of plexiform ameloblastomas likely affects the vimentin results in those lesions, as most of our cases were of plexiform ameloblastomas. Interestingly, the vimentin expression in unicystic ameloblastomas was mostly confined to the subepithelial region of the lining odontogenic epithelium. This may be due to the odontogenic inductive effect exerted on the connective tissue by the proliferating odontogenic epithelium. This again reiterates the fact which we discussed in cytokeratins that, unicystic ameloblastomas represents a more mature type. The one case of desmoplastic ameloblastoma in our study was intensely positive for vimentin. This is in conjunction with Siar, et al.,[11] who suggested that connective tissue component may play a role in expansion. Hence we hypothesize that, based on our vimentin expression pattern; a balanced induction is required from both the epithelium and connective tissue for the hard tissue formation. The degree of maturation of the connective tissue relates to the inductive potential of the mesenchyme to the epithelium and vice versa. Thus, more the connective tissue matures, less the propensity to form hard tissue. According to literature, the proliferating cell rests of Serres try to lyse the mature connective tissue so as to revert it to immature ectomesenchyme for initiating an inductive effect.[13] In our study, the presence of mature connective tissue in the periphery and a less mature connective tissue subjacent to the tumor as shown by vimentin expression is thus well established.
Angiogenesis, the growth of new blood vessels from pre-existing ones, is one of the essential phenotypes of tumor formation.[14] Characterizing the tumor microvasculature on the basis of tissue samples provides important prognostic information in malignancy. CD34 antigen is a heavily glycosylated type I transmembrane protein with a molecular weight ranging from 110-120 Kda. Normally CD34 is expressed on early lymphopoietic progenitor cells, small vessel endothelial cells, embryonic fibroblasts and some fetal and adult nerve tissue cells. Estimation of microvessel density using CD34 has been extensively studied by various researchers in various carcinomas including oral squamous cell carcinomas. Recently, Nagatsuka, et al.,[15] assessed microvessel density using CD34 in oral squamous cell carcinomas and found increased microvessel density in tumor areas. They suggested that CD34 is highly expressed on highly proliferating blood vessels and endothelial cells and associated with metastasis and cancer cell infiltration. In relation to odontogenic tumors, there are few studies on microvessel density and ameloblastomas, in which Hiroyuki, et al.[16] have found increased microvessel density in ameloblastomas when compared to normal tooth germs. Our study consisted of 20 cases and CD34 positive microvessels were found only in five cases. Microvessel density (MVD) was assessed by counting CD34 positive vessels both at 10× and 40× magnification using Weidener's criteria. There was intense staining of CD34 in two cases which was histopathologically diagnosed as a proliferative plexiform ameloblastoma. Though H and E staining lacks evidence of malignancy, there was an increased proliferation and budding of basal cells. Hence we preferred to use the term, proliferative plexiform ameloblastoma as suggested by Harvey Kessler.[17] This terminology was preferred to reemphasize the fact that, though benign, the conventional ameloblastoma can have an aggressive behavior as that in malignancy. This was proved by our study results where increased microvessel density was observed in two aggressive cases of ameloblastomas with increased proliferative activity. It can be suggested that though the epithelium did not show any features of typical cytological atypia, CD34 microvessel density can be a marker of aggressive behaviour almost equal to malignancy. Our staining pattern on CD34 positive microvessel density suggests that, though ameloblastomas are benign infiltrative tumors, increased microvessel density in certain types can be suspected for invasive behavior as in malignancy.
Myofibroblasts are modulated fibroblast that expresses alpha-smooth muscle actin, which is key contractile protein, found in smooth muscle cells. The presence of smooth muscle actin positive myofibroblasts in odontogenic lesions has not been thoroughly investigated. In our results, 3 cases showed intense staining both around blood vessels and connective tissue stroma, 11 cases showed weak staining particularly around the blood vessels. The smooth muscle actin positivity around the blood vessels was justified by Tomasek, et al.,[18] by explaining the presence of smooth muscle actin positive proto myofibroblasts. The proto myofibroblasts are the immature myofibroblasts that do not express alpha smooth muscle actin. It is suggested that smooth muscle actin positive proto myofibroblasts might be recruited from the fibroblasts surrounding the blood vessels, pericytes or smooth muscle cells of the vasculature. The proto myofibroblasts at the later stages, depending on the nature of the tumor, may get transformed into mature myofibroblasts expressing alpha smooth muscle actin. This can be one of the stromal factors which may have a role in limiting the invasiveness of ameloblastomas. Also, 3 cases in our study including one case of desmoplastic ameloblastoma showed intense staining in the connective tissue stroma, apart from the peri-vascular positivity. This suggests that the myofibroblasts which is present in the stroma other than the blood vessels, could be a mature myofibroblast and their presence may facilitate tumor progression, which can be a attributed to the invasiveness of desmoplastic ameloblastoma as proposed by Hinz, et al.[19] Moreover in our study, most of the cases showed smooth muscle actin positivity at the tumor front of the lesion, (P < 0.0321 SS) which clearly indicates the pivotal role of smooth muscle actin positive myofibroblasts in tumor progression. This is in concurrence with the study by Marilena, et al.,[20] in which smooth muscle actin positive myofibroblasts were seen close to the ameloblastomatous islands.
S100 is a 20-30 Kda calcium binding protein, was thought to be of neural crest origin, but now known to have a wider distribution. The main function of S100 is probably attributable to calcium binding properties and plays a role in assembly and disassembly of microtubules and cilia. Our study consisted of 10 cases and 5 were found to be weakly positive. Though this positivity can be attributed to neural crest origin, but with accordance to Murase, et al.,[21] S100 positive cells could indicate Langerhans cells, which are usually accompanied with a high degree of inflammatory cell infiltration in the lesions. Despite the fact that Langerhans cells have an antigen presenting role in association with inflammatory infiltrate, our cases demonstrated minimal inflammatory cell infiltrate. The presence of focal S100 positivity within the ameloblastic strands could be because of the presence of Langerhans cells. This further supports our hypothesis that ameloblastomas indicate the recapitulation of odontogenic epithelium to the original oral epithelium. Sensory nerve associated factors like Trk A and S100 are expressed in normal basal keratinocytes and also by the presence or absence of epithelial cell rests of Malassez as suggested by Yammamoto, et al.,[22] Pincelli, et al.,[23] Richard et al.[24] This augments our hypothesis that an immunohistochemistry (IHC) marker like S100 expressed in the epithelium can also be expressed in ameloblastomas supporting the histogenic concept of derivation from the oral epithelium.
CONCLUSION
To summarize the present study re-emphasize the histogenic concepts of ameloblastomas that they are derived from the oral and odontogenic epithelium. The increased expression of smooth muscle actin and CD34 in ameloblastomas mark its aggressiveness and thereby predicting their biological behavior. The results and hypothesis achieved from the study, proved to be consistent, not only augmenting the already existing hypothesis and also imparting new concepts of hypothesis. The current immunohistochemical markers could be evolved further for a better understanding of the biological behavior of ameloblastomas, which can lead to better management and treatment. Further studies with more number of cases could further throw light on the biology of ameloblastomas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Barnes L, Eveson JW, Reichart PA, Sidransky B, editors. Lyon: IARC; 2005. World Health Organization classification of tumors: Pathology and genetics of tumors of the head and neck. [Google Scholar]
- 2.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 3.Brannon RB. The odontogenic keratocyst: A clinico-pathologic study of 312 cases. Part II: Histologic features. Oral Surg Oral Med Oral Pathol. 1977;43:233–55. doi: 10.1016/0030-4220(77)90161-x. [DOI] [PubMed] [Google Scholar]
- 4.Moll R. Molecular diversity of cytokeratins: Significance for cell and tumor differentiation. Acta Histochem Suppl. 1991;41:117–27. [PubMed] [Google Scholar]
- 5.Webb PP, Moxham BJ, Ralphs JR, Benjamin M. Cytoskeleton of the mesenchymal cells of the rat dental papilla and dental pulp. Connect Tissue Res. 1995;32:71–6. doi: 10.3109/03008209509013708. [DOI] [PubMed] [Google Scholar]
- 6.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: A key player in the control of tumor cell behaviour. Int J Dev Biol. 2004;48:509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 7.Kumamoto H, Ohki K, Ooya K. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in ameloblastomas. J Oral Pathol Med. 2002;31:28–34. doi: 10.1046/j.0904-2512.2001.10061.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap C, Williams C, Barker B, Hof R. An investigation of S100 protein in embryonic dental papillae of rats. Oral Surg Oral Med Oral Pathol. 1984;58:575–8. doi: 10.1016/0030-4220(84)90082-3. [DOI] [PubMed] [Google Scholar]
- 9.Fukumashi K, Enokiya Y, Inoue T. Cytokeratins expression of constituting cells in ameloblastomas. Bull Tokyo Dent Coll. 2002;43:13–21. doi: 10.2209/tdcpublication.43.13. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo A, Ueno S. Immunohistochemical demonstration of keratin in ameloblastoma as an indication of tumor differentiation. J Oral Maxillofac Surg. 1991;49:282–8. doi: 10.1016/0278-2391(91)90221-7. [DOI] [PubMed] [Google Scholar]
- 11.Siar CH, Ng KH. Patterns of expression of intermediate filaments and S100 protein in desmoplastic ameloblastoma. J Nihon Univ Sch Dent. 1993;35:104–8. doi: 10.2334/josnusd1959.35.104. [DOI] [PubMed] [Google Scholar]
- 12.Crivelini MM, de Araujo VC, de Sousa So, Araujo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003;9:1–6. doi: 10.1034/j.1601-0825.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 13.Geraldo MC. Ameloblastoma: A behavioral and histological paradox. Braz Dent J. 1990;1:5–15. [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 15.Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, Sugahara T, et al. Various immunostaining patterns of CD31, CD34 and Endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:70–6. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 16.Hiroyuki K, Kousuke O, Kiyoshi O. Association between vascular endothelial growth factor (VEGF) expression and tumour angiogenesis in ameloblastomas. J Oral Pathol Med. 2002;31:28–34. doi: 10.1046/j.0904-2512.2001.10061.x. [DOI] [PubMed] [Google Scholar]
- 17.Kessler HP. Intraosseous ameloblastomas. Oral Maxillofac Surg Clin North Am. 2004;16:309–22. doi: 10.1016/j.coms.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 19.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblast. Curr Opin Biotechnol. 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in stroma of odontogenic cysts and tumors can contribute to variations in the biological behaviour of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Murase N, Tatemoto Y, Iwai Y, Okada Y, Mori M. Langerhans cells in odontogenic tumors and cysts as detected by S-100 protein immunohistochemistry. Basic Appl Histochem. 1990;34:135–41. [PubMed] [Google Scholar]
- 22.Yamashiro T, Fujiyama K, Fukunaya T, Wang Y, Takano Epithelial rests of malassez express immunoreactivity of TrK A and its distribution is regulated by sensory nerve innervations. J Histochem Cytochem. 2000;48:979–84. doi: 10.1177/002215540004800711. [DOI] [PubMed] [Google Scholar]
- 23.Pincelli C, Marconi A. Autocrine nerve growth factor in human keratinocytes. J Dermatol Sci. 2000;22:71–9. doi: 10.1016/s0923-1811(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 24.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 Proteins in the Epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]


