Abstract
Drug-resistant Candida infection is a major health concern among immunocompromised patients. Antimicrobial photodynamic inactivation (PDI) was introduced as an alternative treatment for local infections. Although Candida (C.) has demonstrated susceptibility to PDI, high doses of photosensitizer (PS) and light energy are required, which may be harmful to eukaryotic human cells. This study explores the capacity of chitosan, a polycationic biopolymer, to increase the efficacy of PDI against C. albicans, as well as fluconazole-resistant clinical isolates in planktonic or biofilm states. Chitosan was shown to effectively augment the effect of PDI mediated by toluidine blue O (TBO) against C. albicans that were incubated with chitosan for 30 min following PDI. Chitosan at concentrations as low as 0.25% eradicated C. albicans; however, without PDI treatment, chitosan alone did not demonstrate significant antimicrobial activity within the 30 min of incubation. These results suggest that chitosan only augmented the fungicidal effect after the cells had been damaged by PDI. Increasing the dosage of chitosan or prolonging the incubation time allowed a reduction in the PDI condition required to completely eradicate C. albicans. These results clearly indicate that combining chitosan with PDI is a promising antimicrobial approach to treat infectious diseases.
Keywords: chitosan, Candida, antimicrobial, photodynamic inactivation
1. Introduction
Chitosan is a natural linear polycationic biopolymer comprising N-acetyl-D-glucosamine and β-1,4-linked D-glucosamine. In dilute acid solutions, the positive charge of chitosan interferes with the negatively charged residue of macromolecules on the surface of cells [1], presumably by competing with Ca2+ for electronegative sites on the membrane without conferring dimensional stability or rendering the membrane leaky [1]. In general, the bacteriostatic/bactericidal activity of chitosan is related to the protonated positive charge number of chitosan and the number of negative charges on the surface of the microbe [1,2]. The antimicrobial activity of chitosan was found against a wide variety of fungi, yeasts and bacteria [3]. Because of its safety and excellent biocompatibility [4], chitosan has been used in food preservation [3], medical and pharmaceutical applications for wound healing [5], drug delivery [6] and tissue engineering [7].
Candida is an opportunistic pathogen in humans. In recent years, fungal infection has become a leading cause of nosocomial infections [8,9], responsible for a range of diseases from superficial mucosal to systemic disorders. Candida infection is also encountered in immunocompromised patients, such as those with HIV [10] and severe burns [11]. In addition, implants and prostheses often harbor biofilms of C. albicans. Although antibiotics against fungal infection are available, resistant strains are frequently reported. Therefore, there is a compelling need for alternative antimicrobials effective in the treatment of multi-drug resistant fungal infections. Recently, the use of chitosan to control postharvest and phytopathogenic fungal disease has attracted much attention, due to imminent problems associated with chemical agents. The fungicidal activity of chitosan varies considerably according to type of chitosan, the host targeted and the environment in which it is applied [3]. N-carboxybutyl chitosan was shown to disturb the membrane functions of C. albicans[1,2]. In addition, plain 2% chitosan gel demonstrated the least inhibition of C. albicans[12]. However, the antimicrobial studies of chitosan against pathogenic Candidas are still limited.
Antimicrobial photodynamic inactivation (PDI) has been developed as an alternative therapeutic tool against bacterial infection, garnering considerable interest in the management of drug-resistant bacterial strains [13,14]. PDI as a bactericide employs a combination of nontoxic photosensitizer (PS) and visible light to generate cytotoxic species. Following light irradiation, the activated PS reacts with molecules in its direct environment, either through electron transfer producing free radicals (type I reaction) or through energy transfer, generating highly reactive singlet oxygen in the presence of oxygen (type II reaction). Unlike other therapies, PDI provides the advantage of dual selectivity; the PS is targeted to the infected area, and the light can be accurately directed to the affected tissue [13]. Furthermore, PDI causes direct damage to the cell wall and membranes, because of the direct binding of PS to these structures. Thus, targeted cells have little chance of developing resistance or increasing metabolic detoxification or export of the drug [15]. Evidence indicates that repeated PDI does not induce resistance in the bacteria against PDI treatment [16,17].
Eukaryotic species, such as C. albicans, are less susceptible to killing with PDI than prokaryotic bacteria [18]. Candida species are approximately 25~50-times larger than bacterial test species and, therefore, contain a larger number of targets per cell [18]. This may explain why C. albicans was shown to be susceptible to toluidine blue O (TBO) and methylene blue (MB) mediated PDI at higher doses of photosensitizers [19]. It has been demonstrated that PDI with MB or TBO, under conditions conducive to the effective killing of typical skin microbes, causes neither cytotoxicity [18,20,21] nor DNA damage to keratinocytes in vitro[22]. However, both humans and Candida are eukaryotic, and higher doses of photosensitizers or light irradiation might still be harmful to human cells.
In a previous study, we showed that chitosan can potentiate the efficacy of PDI against both Gram-(+) and Gram-(−) prokaryotic bacteria in planktonic cells and biofilms [23]. In the present study, the utility of chitosan in PDI against eukaryotic C. albicans was evaluated. Chitosan was shown to potentiate the efficacy of PDI in planktonic cells and biofilms of C. albicans. Following the administration of PDI, the addition of chitosan greatly augmented the killing of C. albicans and drug-resistant clinical isolates. Because the safety of chitosan as a biomaterial is well known, the combination of chitosan and PDI for the treatment of fungal infections shows considerable promise.
2. Results and Discussion
2.1. TBO Binding Assay and Survival Fraction as Related to Incubation Time and Dose
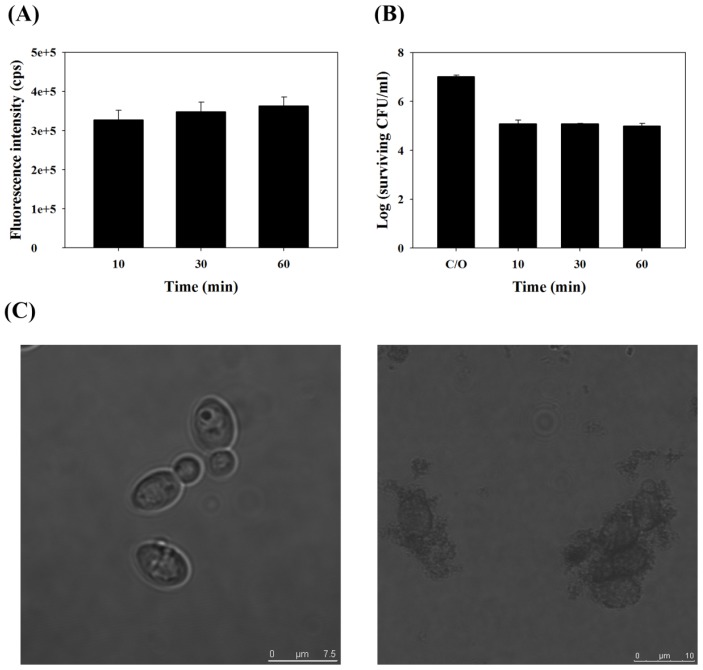
To examine the binding of TBO to C. albicans and the survival fraction as it relates to incubation time, we measured the intensity of fluorescence after incubating 1 × 107 CFU/mL C. albicans with 0.2 mM TBO for 10, 30 or 60 min. As shown in Figure 1A, the binding of TBO to C. albicans was significant and remained steady after incubation for 10 min. Following light irradiation (50 J/cm2), the surviving fraction decreased after 10 min of incubation with TBO and remained steady at 30 and 60 min (Figure 1B). Neither PS binding nor PDI increased significantly with an increase in incubation time, indicating that TBO binding and its subsequent PDI effect occurred very quickly, if not instantaneously. For the convenience of the following experiments, 30 min was selected as the standard incubation time.
Figure 1.
Toluidine blue O (TBO) binding (A) and survival fraction (B) of C. albicans were incubated with 0.2 mM TBO for different time and then measured with a spectrophotometer for the TBO binding (A) or subjected to 50 J/cm2 of the red light illumination. Each value is the mean from three independent experiments ± standard deviation. Neither fluorescence nor photodynamic inactivation (PDI) significantly increased as incubation time increased; (C) Morphology of C. albicans before (left panel) and after (right panel) PDI, as observed by confocal microscope. C. albicans was incubated with TBO (0.2 mM, 30 min), followed by red light illumination (50 J/cm2).
Analysis of the morphology of C. albicans through a confocal microscope was also carried out before and after PDI. Prior to illumination, the cell wall of C. albicans was intact and regular in shape (left panel, Figure 1C). However, the cell walls were fragmented and irregular in shape after PDI (right panel, Figure 1C). The cell walls of a number of C. albicans appeared swollen or disintegrated.
2.2. Chitosan Augments TBO or Ce6 Mediated PDI against Planktonic C. albicans
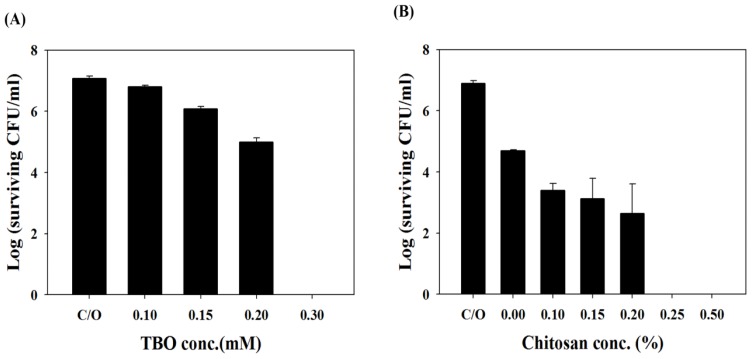
The impact of TBO concentration on PDI against C. albicans was found to be dose-dependent (Figure 2A). PDI is only capable of causing approximately 1- and 2-log10 reductions in viable cell count using 0.15 mM and 0.2 mM TBO, respectively. PDI with TBO at 0.3 mM completely eradicated C. albicans.
Figure 2.
(A) Cell survival fraction of planktonic C. albicans after being incubated with different concentrations of TBO for 30 min and subjected to 50 J/cm2 of the red light illumination; (B) Cell survival fraction of planktonic C. albicans after PDI (0.2 mM TBO and 50 J/cm2) followed by treatment with various concentrations of chitosan for 30 min and then plate counted. Each value is the mean from three independent experiments ± SD.
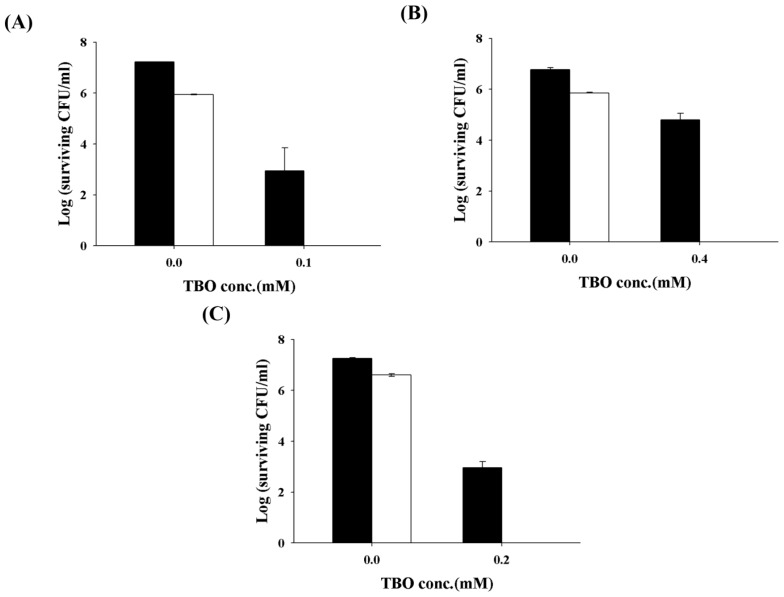
Antimicrobial PDI has provided new hope for controlling microbial infections. The main advantages of PDI are the ability to eradicate bacteria almost instantly, while avoiding damage to adjacent host tissue. A number of PSs have demonstrated the efficacy of PDI against microbial pathogens; however, many commonly used PSs, capable of significant phototoxicity against Gram-(+) bacteria, are ineffective against Gram-(−) bacteria, due to the properties of their outer membrane. In a previous study, we showed that encapsulating PSs in liposomes or micelles can improve their photochemical efficiency and subsequently enhance the efficacy of PDI in killing Gram-(+) bacteria [24]. In a recent study [23], we further determined that one of the most commonly studied biomaterials, chitosan, can potentiate the efficacy of PDI against Gram-(+) or Gram-(−) bacteria. As the cell wall compositions of C. albicans are quite different from bacteria, we wonder whether chitosan can exert the augmented effect in PDI against Candida. Therefore, various concentrations of chitosan were added following PDI with 0.2 mM TBO. As shown in Figure 2B, without chitosan incubation, PDI only devitalized C. albicans on a scale of 2-log10. However, the fungicidal effect was augmented with chitosan in a dose-dependent manner and resulted in complete killing after incubation for 30 min in 0.25% chitosan following PDI. The augmented effects of chitosan in PDI were also found in three clinical isolates of fluconazole-resistant C. albicans (Figure 3). The potentiated effects of chitosan in PDI are valuable, particularly when the photosensitizer is expensive or toxic to normal human tissue in clinical practice.
Figure 3.
Effect of chitosan on TBO-mediated PDI against fluconazole-resistant C. albicans strain 2008 no30 (A), 2008 no22 (B) and 2008 no19 (C). TBO at 0.1 mM, 0.4 mM and 0.2 mM were incubated with strain 2008 no30, no22 and no19 for 30 min, respectively. After light illumination, cells were incubated with 0.25% chitosan for 30 min and then plate counted. Closed column: PDI only. Open column: PDI plus chitosan. Each value is the mean from three independent experiments ± SD.
2.3. Chitosan Augments TBO Mediated PDI against the Biofilm of C. albicans
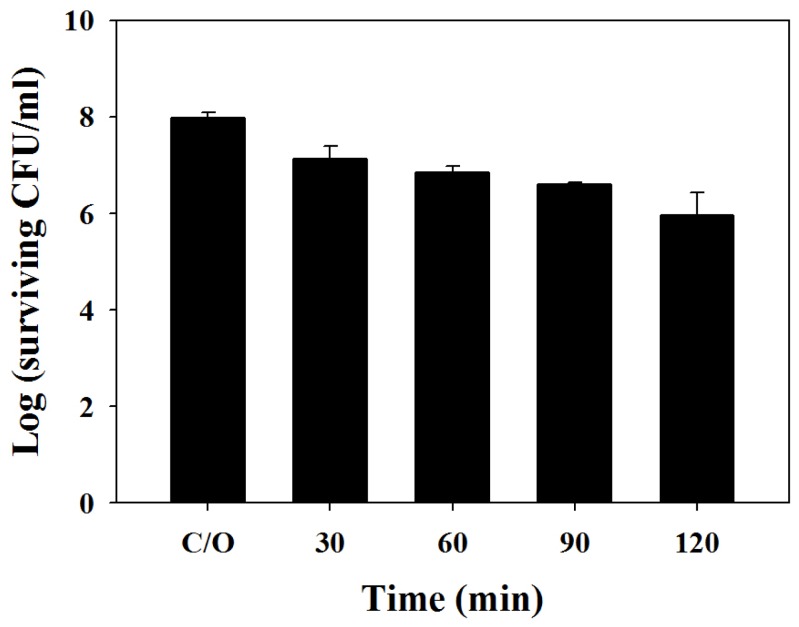
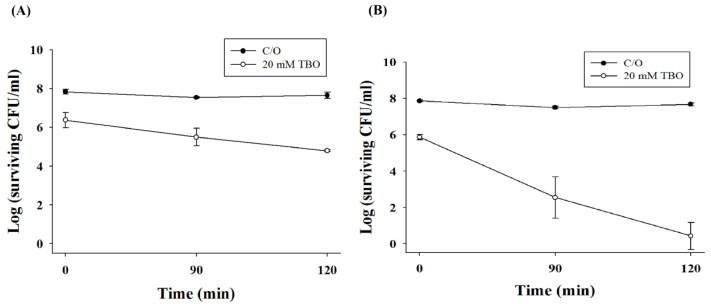
We further examined the potentiating effect of chitosan in PDI against the biofilms of C. albicans. Under a light energy dose of 100 J/cm2, 1- and 2-log10 reductions in viable counts were found in biofilm cells incubated with 20 mM TBO for 1 h or 2 h, respectively (Figure 4). However, the killing of biofilm cells of C. albicans increased following incubation with chitosan (0.5%, w/v) for 90 or 120 min (Figure 5). Complete killing of C. albicans in the biofilm was observed after the incubation of chitosan for 2 h following PDI with a TBO incubation time of 2 h (Figure 5B), suggesting that the augmentation of chitosan in PDI against biofilm cells is related to the level of damage. It has been reported that the biofilm of C. albicans is resistant to most antifungal drugs [25,26]. This explains why the required TBO concentration in the biofilm study is 100-times higher than that of planktonic cells. Taken together, these results clearly indicate that chitosan can potentiate the efficacy of PDI against planktonic, as well as biofilm cells.
Figure 4.
Cell survival fraction of C. albicans biofilm after TBO-mediated PDI. Biofilm cells were treated with 20 mM TBO for different periods of time, followed by light exposure at 100 J/cm2. Each value is the mean from three independent experiments ± SD.
Figure 5.
Effect of TBO-mediated PDI followed by chitosan treatment on biofilm of C. albicans. Biofilm cells were treated with 20 mM TBO for 1 h (A) or 2 h (B), followed by light exposure at 100 J/cm2. After PDI, biofilm cells were further treated with 0.5% chitosan for 90 and 120 min and then subjected to a plate count. Each point is the mean from three independent experiments ± SD.
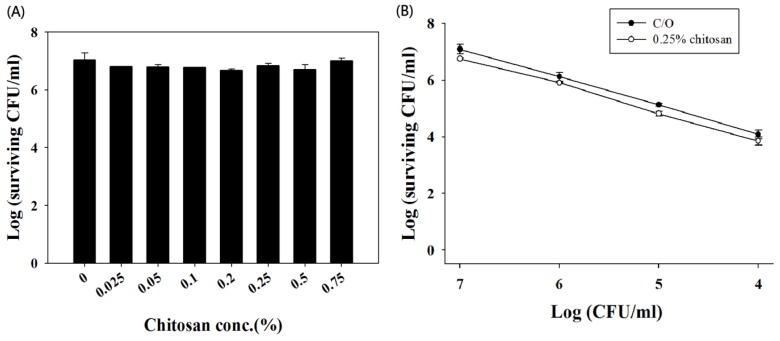
Although the antimicrobial activity of chitosan alone has been demonstrated, a complete lack of activity against microbes was observed after 18–48 h of incubation [3]. However, without PDI treatment, we found that various concentrations of chitosan alone did not demonstrate significant toxicity against C. albicans after 30-min incubation (Figure 6A). It has been reported that the concentration of chitosan required to exert antimicrobial activity varies according to the species and concentration of the microorganism. In this regard, it is possible that the potentiating effect of chitosan might be due to the reduced number of surviving C. albicans cells after PDI. To examine this further, various cell densities of C. albicans were incubated with 0.25% (w/v) chitosan for 30 min. However, no antimicrobial activity against C. albicans was observed (Figure 6B). These results indicate that the potentiating effect of chitosan worked after the C. albicans cells were damaged by PDI and was not associated with a decreased number of surviving cells.
Figure 6.
Chitosan alone did not demonstrate antimicrobial activity. (A) Chitosan of various concentrations did not influence the viability of C. albicans cells. Cells at a concentration of 1 × 107 CFU/mL were not affected after incubation with chitosan as high as 0.75% for 30 min; (B) Chitosan of 0.25% did not influence the viability of C. albicans at various cell densities. Data are the means of three independent experiments and the bars are the SDs.
Many studies have reported on the mechanisms underlying the antimicrobial activity of chitosan [27]. Park et al. reported that low molecular weight water-soluble (LMWS) chitosan exhibits antifungal activity, but no cytotoxicity against mammalian cells [28]. Using confocal microscopy, they showed that LMWS-chitosan is located in the plasma membrane and demonstrates membrane disrupting activity. In our analysis, using an electron microscope, we also observed that chitosan binds to the cell wall with or without PDI (data not shown). However, we observed no cell killing without PDI damage to the cells.
For clinical applications, an ideal antimicrobial PDI should exhibit extensive killing of the pathogen population with minimal damage to host tissues in the area of infection. Although PSs are capable of exerting phototoxic effects on microbes, damage to neighboring human cells is still possible if the PS dose is too high. It would be ideal if a lower safe dose of PS could function efficiently as a microbial killing agent. The potentiating effect of chitosan in TBO-mediated PDI against C. albicans either in planktonic cells or biofilms was demonstrated in this study, indicating that when PDI is combined with chitosan, a lower dose of PS and light may function effectively as a microbial killing agent. Chitosan is abundant in nature, and most studies have shown it to be non-toxic to mammalian cells. The combined use of chitosan with PDI offers a new direction for the eradication of all microbes, while preventing mutagenesis among survivors.
3. Experimental Section
3.1. Materials
Low-molecular-weight chitosan (MW ~20 kDa) with a degree of deacetylation (DDA) of ~90% was purchased from Shin Era Technology (Taipei, Taiwan). Toluidine blue O (TBO) and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Candida Strains and Growth Conditions
Three strains of C. albicans, namely, a wild-type strain SC5314 (ATCC MYA-2876D, kindly provided by L.Z. Den, Department of Medical Technology, National Taiwan University, Taipei, Taiwan) and three fluconazole-resistant clinical strains (2008 no. 19, 22 and 30) from the infection control lab at the National Taiwan University Hospital, Taipei, Taiwan, were used. C. albicans strains were grown aerobically overnight (12 h) in 50 mL yeast peptone dextrose (YPD) broth at 37 °C. Cells were then harvested following centrifugation at 5000× g for 5 min, washed three times with phosphate-buffered saline (PBS; pH 7.4) and suspended in PBS to produce a cell suspension containing 107 CFU/mL.
3.3. Biofilm Preparation
Biofilms of C. albicans were developed according to a published protocol [29]. In brief, washed C. albicans cells were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 1% glucose and adjusted to a cell density of approximately 1 × 107 cells/mL. Then, 100 μL of cell suspension was pipetted into each well of a 96-well polystyrene flat plate and shaken at 75 rpm for 1.5 h at 37 °C to permit the cells to adhere to the surface of the wells. Cell suspensions were then aspirated and each well was washed with 100 μL PBS to remove loosely adherent cells. A 200 μL RPMI 1640 medium supplemented with 1% glucose was pipetted into the washed wells. The plates were then incubated at 37 °C and shaken at 75 rpm for 48 h.
3.4. TBO Incubation
A cell suspension of C. albicans containing approximately 1 × 107 CFU/mL was mixed with equal volumes of TBO of various concentrations, cultured at 25 °C for 30 min in the dark and then centrifuged at 12,000× g for 1 min. Cells were then suspended in PBS. To perform a binding assay, the sample was lysed with lysis buffer (0.1 N NaOH-1% SDS) and held in the dark for 24 h at room temperature before being measured using a spectrophotometer (FluoroMax®-4, Horiba Jobin Yvon, NJ, USA) at an excitation wavelength of 624 nm. Fluorescence emission was measured at wavelengths ranging from 580 to 700 nm.
3.5. PDI in Planktonic Cells of C. albicans
In a typical experiment, 0.1 mL of C. albicans cell suspension containing approximately 107 CFU/mL was transferred into a well. Then, 0.1 mL of PBS solution (pH 7.4) containing TBO was added to the solution. Samples were incubated in the dark for 30 min at 25 °C and shaken at 100 rpm, unless otherwise specified. Samples were then centrifuged (12,000 × g, 1 min), washed once with PBS and resuspended using 200 uL PBS. The home-made light source used for TBO irradiation consisted of a high-power LED array with the wavelength centered at 630 ± 5 nm, delivered at an irradiance of 30 mW/cm2 and 50 J/cm2. Irradiated and non-irradiated samples were serially diluted 10-fold with PBS, and the colonies that formed on YPD agar plates after 18 h of incubation at 37 °C were counted. Analysis of morphology using a confocal microscope was carried out after Candida was first co-incubated with TBO (0.2 mM) and then treated with PDI using 50 J/cm2 (630 ± 5 nm, 30 mW) of red light.
3.6. TBO Mediated PDI in Biofilm Cells
Disks with biofilms were placed in a sterile 48-well microtiter plate and treated with 0, 20 and 40 mM of TBO in the dark for 30 min. Disks were then moved to a new plate containing PBS and irradiated using an LED array (630 ± 5mm, 30 mW) at 50 J/cm2. Following irradiation, the disk with biofilms were then placed into test tubes containing 10 mL sterile PBS and vigorously vortexed to remove the biofilm from the disks. The resulting microbial suspensions were suitably diluted and plated on tryptic soy agar. Colonies that formed after 18 h of incubation at 37 °C were counted.
3.7. Effect of Chitosan on PDI
A stock solution of chitosan (1% w/v) was prepared in 1% acetic acid and used within 1 month. Various concentrations of chitosan were prepared by taking aliquots from the chitosan stock and diluting them with culture medium. Chitosan was added to the cells after performing PDI. Following incubation for various durations, cells were washed out using fresh culture medium. In the presence or absence of chitosan, irradiated and non-irradiated Candida cells were serially diluted 10-fold with PBS, and the colonies that formed after 18 h of incubation at 37 °C were counted.
3.8. C. albicans Cell Survival Assay
Colony-forming units of Candida suspensions were counted using the following protocol: aliquots (10 μL) of appropriate dilutions (from 10−1 to 10−5) were plated on YPD agar plates and incubated at 37 °C in the dark for 18 h. The surviving fraction was calculated as NPDI/N0, where NPDI is the cell count (CFU/mL) after antimicrobial photodynamic therapy and N0 is the cell count (CFU/mL) in the initial sample. The toxicity of substrates in the dark, defined as the intrinsic toxicity of the compounds in the absence of light, was monitored by evaluating the surviving fraction of non-illuminated C. albicans samples and calculated as NDARK/N0, where NDARK is the cell count (CFU/mL) of the non-illuminated samples. All results were expressed as the mean ± SD. Differences between two means were assessed for significance using the two-tailed Student’s t-test, and p < 0.05 was considered significant.
4. Conclusions
The combination of PDI and chitosan was shown to potentiate the PDI efficacy in planktonic cells and biofilms of C. albicans. Due to the characteristics of biocompatibility and saltiness, chitosan is quite promising in augmenting the PDI efficacy for eradicating C. albicans infection.
Acknowledgments
Financial support for this study was provided by grants from the National Science Council of Taiwan (NSC 98-2320-B-002-016-MY3 and NSC 98-2320-B-038-012-MY3) and Department of Health (DOH100-TD-PB-111-TM006). The authors would also want to thank Sami M. Nazzal for his critical reading of this manuscript and Kuo-Liong Chien for statistical consultation.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Helander I.M., Nurmiaho-Lassila E.L., Ahvenainen R., Rhoades J., Roller S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001;71:235–244. doi: 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 2.Muzzarelli R., Tarsi R., Filippini O., Giovanetti E., Biagini G., Varaldo P.E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990;34:2019–2023. doi: 10.1128/aac.34.10.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabea E.I., Badawy M.E., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 4.Alonso M.J., Sanchez A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2010;55:1451–1463. doi: 10.1211/0022357022476. [DOI] [PubMed] [Google Scholar]
- 5.Ueno H., Yamada H., Tanaka I., Kaba N., Matsuura M., Okumura M., Kadosawa T., Fujinaga T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–1414. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 6.Fontana C.R., dos Santos D.S.J., Bosco J.M., Spolidorio D.M., Marcantonio R.A.C. Evaluation of chitosan gel as antibiotic and photosensitizer delivery. Drug Deliv. 2008;15:417–422. doi: 10.1080/10717540802007433. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.H., Li D.P., Wang W.J., Feng Q.L., Cui F.Z., Xu Y.X., Song X.H., van der Werf M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials. 2003;24:3213–3220. doi: 10.1016/s0142-9612(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 8.Hung C.C., Chen Y.C., Chang S.C., Luh K.T., Hsieh W.C. Nosocomial candidemia in a university hospital in Taiwan. J. Formos. Med. Assoc. 1996;95:19–28. [PubMed] [Google Scholar]
- 9.Liu C.Y., Liao C.H., Chen Y.C., Chang S.C. Changing epidemiology of nosocomial bloodstream infections in 11 teaching hospitals in Taiwan between 1993 and 2006. J. Microbiol. Immunol. Infect. 2010;43:416–429. doi: 10.1016/S1684-1182(10)60065-5. [DOI] [PubMed] [Google Scholar]
- 10.Phelan J.A., Saltzman B.R., Friedland G.H., Klein R.S. Oral findings in patients with acquired immunodeficiency syndrome. Oral Surg. Oral Med. Oral Pathol. 1987;64:50–56. doi: 10.1016/0030-4220(87)90116-2. [DOI] [PubMed] [Google Scholar]
- 11.Ballard J., Edelman L., Saffle J., Sheridan R., Kagan R., Bracco D., Cancio L., Cairns B., Baker R., Fillari P., et al. Positive fungal cultures in burn patients: A multicenter review. J. Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 12.Ballal N.V., Kundabala M., Bhat K.S., Acharya S., Ballal M., Kumar R., Prakash P.Y. Susceptibility of Candida albicans and Enterococcus faecalis to Chitosan, Chlorhexidine gluconate and their combination in vitro. Aust. Endod. J. 2009;35:29–33. doi: 10.1111/j.1747-4477.2008.00126.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamblin M.R., Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jori G., Fabris C., Soncin M., Ferro S., Coppellotti O., Dei D., Fantetti L., Chiti G., Roncucci G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 15.Winckler K.D. Special section: Focus on anti-microbial photodynamic therapy (PDT) J. Photochem. Photobiol. B. 2007;86:43–44. doi: 10.1016/j.jphotobiol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright M., Crossley K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeter Biodeg. 2004;53:119–126. [Google Scholar]
- 17.Francesco G., Manuele M., Annalisa C., Debora A., Lia F., Gabrio R. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeina B., Greenman J., Purcell W.M., Das B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001;144:274–278. doi: 10.1046/j.1365-2133.2001.04013.x. [DOI] [PubMed] [Google Scholar]
- 19.Souza R.C., Junqueira J.C., Rossoni R.D., Pereira C.A., Munin E., Jorge A.O. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med. Sci. 2010;25:385–389. doi: 10.1007/s10103-009-0706-z. [DOI] [PubMed] [Google Scholar]
- 20.Zeina B., Greenman J., Corry D., Purcell W.M. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br. J. Dermatol. 2002;146:568–573. doi: 10.1046/j.1365-2133.2002.04623.x. [DOI] [PubMed] [Google Scholar]
- 21.Soukos N.S., Wilson M., Burns T., Speight P.M. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg. Med. 1996;18:253–259. doi: 10.1002/(SICI)1096-9101(1996)18:3<253::AID-LSM6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Zeina B., Greenman J., Corry D., Purcell W.M. Antimicrobial photodynamic therapy: Assessment of genotoxic effects on keratinocytes in vitro. Br. J. Dermatol. 2003;148:229–232. doi: 10.1046/j.1365-2133.2003.05091.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai T., Chien H.F., Wang T.H., Huang C.T., Ker Y.B., Chen C.T. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2011;55:1883–1890. doi: 10.1128/AAC.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai T., Yang Y.T., Wang T.H., Chien H.F., Chen C.T. Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg. Med. 2009;41:316–322. doi: 10.1002/lsm.20754. [DOI] [PubMed] [Google Scholar]
- 25.Nett J.E., Guite K.M., Ringeisen A., Holoyda K.A., Andes D.R. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 2008;52:3411–3413. doi: 10.1128/AAC.01656-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra J., Kuhn D.M., Mukherjee P.K., Hoyer L.L., McCormick T., Ghannoum M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganan M., Carrascosa A.V., Martinez-Rodriguez A.J. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganisms and its mechanism of action. J. Food Prot. 2009;72:1735–1738. doi: 10.4315/0362-028x-72.8.1735. [DOI] [PubMed] [Google Scholar]
- 28.Park Y., Kim M.H., Park S.C., Cheong H., Jang M.K., Nah J.W., Hahm K.S. Investigation of the antifungal activity and mechanism of action of LMWS-Chitosan. J. Microbiol. Biotechnol. 2008;18:1729–1734. [PubMed] [Google Scholar]
- 29.Jin Y., Yip H.K., Samaranayake Y.H., Yau J.Y., Samaranayake L.P. Biofilm-forming a ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003;41:2961–2967. doi: 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]