Abstract
Cryptococcus, an opportunistic yeast, known to afflict immune-compromised patients is often overlooked in immune-competent patients. This has led to increasing morbidity and mortality worldwide. We present a case of Cryptococcus causing sternal osteomyelitis in an immune-competent individual. Till date no case of Cryptococcus causing sternal osteomyelitis in an immune-competent patient has been reported in the English literature since 1946–2011. With the rising incidence of Cryptococcus infection it should be included among the list of infections causing osteomyelitis. Early detection and prompt treatment can decrease both morbidity and mortality.
Background
Cryptococcus was first isolated in the environment as yeast in peaches and subsequently in human as cryptococcal osteomyelitis of tibia in 1894–1895 by Busse and Bushke.1 Cryptococcosis, is an opportunistic fungal infection popularly known as the infectious disease of the immunocompromised. Its association with AIDS, lymphoma, sarcoidosis, steroid therapy, tuberculosis, organ transplantation and any other immune-compromised state is well known. It is very notorious for causing fatal central nervous system (CNS) infections like meningitis and pulmonary infections like pneumonia and ARDS. The less commonly involved organs are the skin, joints, eyes, urinary tract, prostate and medullary cavity of bones of which vertebra is the most common site.1 2
The yearly incidence of cryptococcosis is between 0.4 and 1.3 cases/100 000 people in the general population.3 The incidence of sternal osteomyelitis is 1–3% of all types of osteomyelitis.4 A review of the English literature showed only 56 cases of isolated cryptococcal osteomyelitis in immunocompetent patients. The most common organisms causing sternal osteomyelitis are Staphylococcus aureus, Pseudomonas aeruginosa and in some Mycobacterium.5 Till date there is no documented report of cryptococcal sternal osteomyelitis in immunocompetent hosts. Therefore, we present one such case.
Case presentation
A 41-year-old African-American woman with 2-month history of lower chest discomfort presented with acute onset lower chest pain and a bulge in the same area since 1 day. Two months ago, her primary care physician treated her as gastroesophageal reflux disease for chest discomfort with antacids. She had no history of radiation of the pain, nausea, vomiting, headache, fever, neck pain, night sweats, cough, shortness of breath, loss of weight, loss of appetite, similar episodes in past, history of tuberculosis or contacts and no history of travel, bird exposure or drug abuse. The pain aggravated with movement and was not relieved with narcotic pain medications, which were her home medications. Her vitals were stable except for mild tachycardia. Examination of her chest revealed a round-shaped, semisoft, non-fluctuant, 3×3×1 cm mass in the lower sternal area between the breasts above the epigastrium. The mass was non-tender, non-erythematous; not discharging and no punctum were visible. The surrounding area appeared normal. No breast mass or tenderness was present. No cervical or axillary lymph nodes were palpable. No neurodeficits were noted. Heart and lung examinations were normal too.
Investigations
Chest x-ray and ECG were normal. Urine and serum toxicological screen were negative. Thyroid function tests were normal. Her labs were normal except for erythrocyte sedimentation rate (ESR) of 30, C reactive protein (CRP) of 6.992 and alkaline phosphatase (ALP) of 148 IU/l. Chest CT scan with contrast showed osteolytic lesion involving the lower sternum eroding the anterior plate of sternum to involve the soft tissue anterior to sternum. The posterior plate of sternum and xiphoid process were intact. However, CT scan was inconclusive for osteomyelitis or neoplasm. Hence, infectious disease specialist and oncologist were consulted. The patient was empirically treated with intravenous vancomycin 1 g daily. HIV ELISA test was negative. A radioisotope bone scan was performed which showed increased tracer uptake in the lower sternal area only. A core biopsy of the sternal mass was sought, which showed budding yeasts. This was regarded as a contaminant initially. The multiple myeloma screen of serum and urine protein immunoelectrophoresis was negative. Quantitative immunoglobulin levels were also normal but immunofixation showed IgA monoclonal gammopathy. A bone survey was carried out which showed no metastasis. Mammogram was performed to rule out any breast lesion and it was negative. Two sets of blood cultures were negative.
Treatment
Consequently, within 5 days the mass became fluctuant and started to discharge pus, which on direct microscopy showed budding yeasts and Propionibacterium acne. Therefore, a decision to treat it as osteomyelitis was made with intravenous fluconazole 800 mg daily and intravenous clindamycin 600 mg every 8 h. Vancomycin was then discontinued. Despite antifungal treatment for 6 days, there was no symptomatic relief and worsening of the discharge from the lesion. Therefore, a decision for surgical intervention was made. Osteoectomy of the lower half of sternum and omental flap transposition from abdomen to mediastinum to cover the defect was performed. The patient responded to this procedure well with no issues. The sternum obtained from the surgery showed acute and chronic inflammation with giant cells and histiocytes containing Cryptococcus neoformans which was demonstrated using Gomori methamine stains (GMS; see figure 1) and Periodic acid schiff (PAS; see figure 2). Five days after the surgery, the culture from abscess drainage during surgery grew tan mucoid colonies on saborauds dextrose agar (SDA) at 37°C. The organism hydrolysed urea. In view of the neurotropic nature of this organism, a decision to perform lumbar puncture was made but the patient refused the procedure. Serum cryptococcal antigen test using latex agglutination technique was positive with titres of 1:32, but blood culture was negative.
Figure 1.
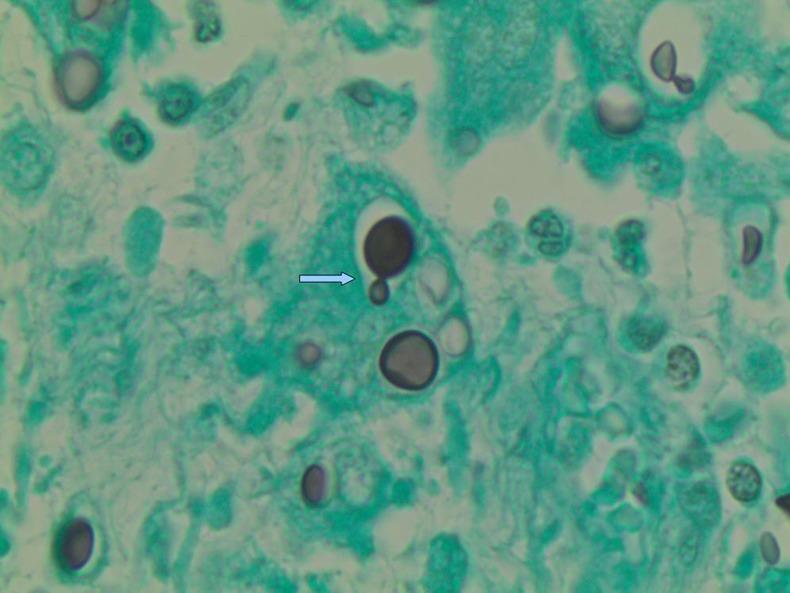
Cryptococcus neoformans using Gomorimethamine Silver stain at ×100 magnification. Arrow demonstrates ‘budding’ typical feature of Cryptococcus.
Figure 2.
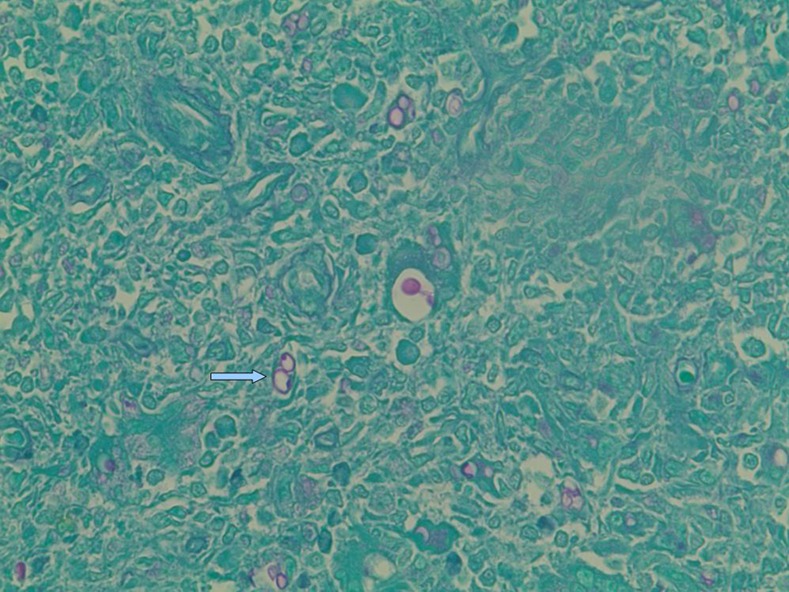
Cryptococcus neoformans using a Periodic acid schiff stain at ×40 magnification (arrow).
Based on the ease of administration and side effect profile of the available antifungals, fluconazole was started. However after the minimum inhibitory concentrations of different antifungals were available, intravenous liposomal amphotericin 375 mg daily and flucytosine 1 gm q6 h were added and fluconazole was discontinued. Nevertheless, in 5 days the patient gradually developed acute renal failure secondary to amphotericin and hence, she was switched back to intravenous fluconazole 200 mg daily.
Outcome and follow-up
Postsurgery she was symptomatically relieved and her ESR, CRP and ALP showed a significant drop. She was discharged home 10 days after surgery, on oral fluconazole 400 mg daily for a total of 12 weeks. After 1 year of this treatment, the patient was asymptomatic and has had no recurrences with cryptococcal infection.
Discussion
Cryptococcosis previously known as torulosis or European blastomycosis or Busse-Buschke disease is caused by an encapsulated budding yeast in both humans and animals. There are two most common pathogenic species namely, C neoformans and Cryptococcus gatti. In most cases, C neoformans is known to affect the immunocompromised host and C gatti, the immunocompetent host. Cryptococcus is distributed worldwide. C neoformans is found predominantly in the USA and exists in the soil contaminated by pigeon excreta and C gatti is found in the eucalyptus trees of the tropical and subtropical areas.6 It is transmitted to humans from the environment via inhalation. Animal to human or human-to-human transmission is not known to occur except for direct inoculation. Lung and CNS are the most common sites of infection.1 7
C neoformans is a spherical to oval, encapsulated, yeast-like organism, that is, 2–20 μm in diameter. Replication occurs via budding from a relatively narrow base. It is not a dimorphic fungus since both saprobic and parasitic forms are yeast. It can be usually detected by staining with India ink appearing as cells with halo due to lack of staining of the capsule. The capsule can also be stained with muciramine. The organism by itself poorly stains on H&E stain but is easily detected with PAS and GMS stains. Culture on SDA agar produces urease-positive mucoid colonies usually within 3–5 days. Species identification can be further established by carbohydrate assimilation test or phenoloxidase activity. Both of which are positive for C neoformans. C neoformans is detected in bodily fluids by latex agglutination or enzyme-linked assays. Both of these tests are sensitive and specific for the diagnosis. C neoformans uses creatine for its growth hence, explains its persistence in the pigeon excreta. Lungs and CNS are the primary sites of colonisation or infection.7
The host immune status is more crucial than the pathogens virulence in cryptococossis. In vivo and vitro studies have demonstrated the following three main lines of human defense against C neoformans; macrophages, inflammatory phagocytic cells, T-cell and B-cell responses. Macrophages containing the yeast cells generate cytokines and recruit various inflammatory cells like NK cells, monocytes and neutrophils from the bloodstream to the site of infection. The yeast-infested cells also act as antigen-presenting cells in inducing differentiation and proliferation of specific T and B lymphocytes. The complement system also plays a role in provision of opsonins and chemotactic factors. Thus, an effective host response requires an efficient interaction of cellular and humoral immune system. An imbalance or defect in any pathway leads to disseminated infection via the yeast infested macrophages to different sites via bloodstream or lymphatic.7
C neoformans evades host defenses by the following mechanisms; (1) polysaccharide capsule (glucuronoxylomannin, is the main component of capsule that inhibits cytokines induced by phagocytosis, leukocyte migration and recruitment of inflammatory cells. The capsule also physically blocks the opsonin effect. Thereby inhibits both humoral and cell-mediated immunity. The capsule can undergo phenotype switching to evade immune response), (2) melanin production (has antioxidant, thermotolerant and microbicidal properties. Melanin is postulated to be the reason for neurotropism of C neoformans), (3) enzymes (serine proteases aid in tissue invasion), (4) ability to grow at body temperature.7 8
The authors are intrigued with the clinical presentation of the patient and primary site of infection. Applying the above facts to our patient, even if an assumption is made that the lungs were the site of colonisation and that C neoformans might have migrated from the lungs to the bone via haematogeneous or lymphatic route, it is difficult to explain sternum as a site of infection. Since, both the blood supply and lymphatic drainage are poor in the sternum especially the lower part of the sternum. The serum cryptococcal antigen titres were elevated proving that the patient was colonised. Nonetheless, there was no evidence of breach in the host immunity (neither cellular nor humoral) since the patient was immunocompetent. In addition, the patient does not have any history of surgery or implantation in the past explaining the colonisation. One study performed in New York City showed majority of adults possess antibody to this organism and most children acquire antibodies to cryptococcal antigens before age of 10.9 Furthermore, there are no clear guidelines as to the duration of treatment in Cryptococcal osteomyelitis with antifungals in non-AIDS patients. Further studies are also required in the treatment approach of Cryptococcal osteomyelitis whether medical management alone is sufficient or it needs to be combined with surgery.
Learning points.
Isolated Cryptococcal osteomyelitis is an extremely rare but treatable condition.
Therefore, Cryptococcosis must be included in the list of infections causing osteolytic lesions on radiology.
There should be a low threshold for biopsy of bone10 or soft tissue mass in such cases to confirm diagnosis and early institution of therapy to ensure complete recovery.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Berdajs D. Delayed Primary Versus Late Secondary Wound Closure in Sternum Infections.University of Lausanne Hospitals. ClinicalTrials.gov Identifier NCT01473979. http://clinicaltrials.gov/ct2/show/NCT01473979 (accessed Feb 2012) [DOI] [PubMed]
- 2.Chen J, Varma A, Diaz Z, et al. Cryptococcus neoformans strains and infection in apparently immunocompetent patients. Emerg Infect Dis 2008;2013:755–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryptococcosis Statistics Centers for disease control and prevention. http://www.cdc.gov/fungal/cryptococcosis/statistics.html (accessed 5 Jan 2012).
- 4.Cunha B. Osteomyelitis in elderly patients. Aging and infectious diseases. Clin Infect Dis 2002;2013:287–93 [DOI] [PubMed] [Google Scholar]
- 5.Goldman DL, Khine H, Abadi J, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001;2013:E66. [DOI] [PubMed] [Google Scholar]
- 6.McAdam AJ, Sharpe AH. Infectious siseases. In: Kumar V, Abbas A, Fausto N, et al. eds Robins and Cotran pathologic basis of disease. 8th edn Philadelphia, PA: W.B. Saunders Company, 2009:384 [Google Scholar]
- 7.Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 1995;2013:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray PR. Pathogenesis of fungal diseases. In: Murray PR, Rosenthal KS, Pfaller MA, Medical microbiology. 6th edn Philadelphia, PA: C.V. Mosby, 2009:717 [Google Scholar]
- 9.Toone E, Jr, Kelly J. Joint and bone disease due to mycotic infection. Trans Am Clin Climatol Assoc 1956;2013:91–103 [PMC free article] [PubMed] [Google Scholar]
- 10.Vasa M, Ohikhuare C, Brickner L. Primary sternal tuberculosis osteomyelitis: a case report and discussion. Can J Infect Dis Med Microbiol 2009;2013:e181–4 [DOI] [PMC free article] [PubMed] [Google Scholar]


