Abstract
An 85-year-old man was admitted to the medical intensive care unit with a 10-day history of severe breathlessness, fever and cough. The patient was known to have chronic obstructive pulmonary disease and had been receiving corticosteroids in the preceding 18 months. He had been treated for tuberculosis 2.5 years previously. On examination he was febrile, tachycardic with a respiratory rate of 46/min. Auscultation revealed bilateral crepitation's and wheeze. Chest radiograph revealed patchy infiltrates on right lung. The patient developed respiratory depression and was mechanically ventilated. His sputum and endotracheal aspirates revealed Nocardia brasiliensis on culture which was found to be co-trimoxazole resistant. Once this became known imipenem was substituted for co-trimoxazole but unfortunately condition of the patient did not improve and he died following a cardiac arrest.
Background
Nocardiosis is usually an opportunistic infection and most commonly presents as pulmonary disease. Most common condition predisposing the patient to nocardiosis is underlying chronic lung disease, often in association with long-term corticosteroid therapy. Pulmonary infections are commonly caused by Nocardia asteroides while cutaneous or subcutaneous infections by Nocardia brasiliensis.1 Rarely N brasiliensis may also produce pulmonary infection. Though sulfonamides have remained the treatment of choice, some patients may not respond to sulfonamide therapy because of infection with sulfonamide-resistant Nocardia strains. The present report describes such a case and highlights the possibility of emergence of co-trimoxazole resistant N brasiliensis in India and treatment failures with empirical co-trimoxazole therapy.
Case presentation
An 85-year-old man was admitted to the medical intensive care unit with a 10-day history of severe breathlessness, fever and cough. The patient was known to have chronic obstructive pulmonary disease (COPD) and had been receiving corticosteroids in the preceding 18 months. He had been treated for tuberculosis 2.5 years previously. Patient was non-diabetic and non-hypertensive. No family history of similar cases was present.
On clinical examination his temperature was 38°C, pulse rate was 120/min, blood pressure was 120/86 mm Hg and respiratory rate was 46/min. Auscultation revealed bilateral crepitation's and wheeze. He also had CO2 retention and hyponatraemia. No abnormality was detected on examination of abdomen and central nervous system (CNS).
Investigations
Chest radiograph revealed patchy infiltrates on right lung. The whole of the left lung showed consolidated. Blood, sputum and endotracheal (ET) aspirates were sent for bacterial and fungal culture. Laboratory investigations revealed total leucocyte count of 19 000/mm3 and differential count of 90% neutrophils and 10% lymphocytes. The sputum and ET aspirate showed predominant branching, beaded, Gram-positive filaments 1 µm wide and up to 50 µm long resembling Nocardia spp. The bacterium was also found to be weakly acid fast. Blood cultures were negative after 7 days' aerobic incubation. Specimens were cultured on 5% sheep blood agar, MacConkey agar and Sabouraud dextrose agar. After 72 h of incubation yellow pigmented dry colonies with rough surface were noted on 5% sheep blood agar. Gram and ZN staining of these colonies revealed bacteria resembling Nocardia spp. The bacterium was identified as N brasiliensis by standard biochemical tests. Antibiotic susceptibility testing by Kirby-Bauer disc diffusion method2 indicated that the isolate was susceptible to aminoglycosides (gentamicin, tobramycin and amikacin) but resistant to erythromycin and ciprofloxacin. This pattern is typical of N brasiliensis. It was also susceptible to imipenem. Unlike other Nocardia spp. the isolate was found to be resistant to co-trimoxazole (figures 1 and 2 ).
Figure 1.
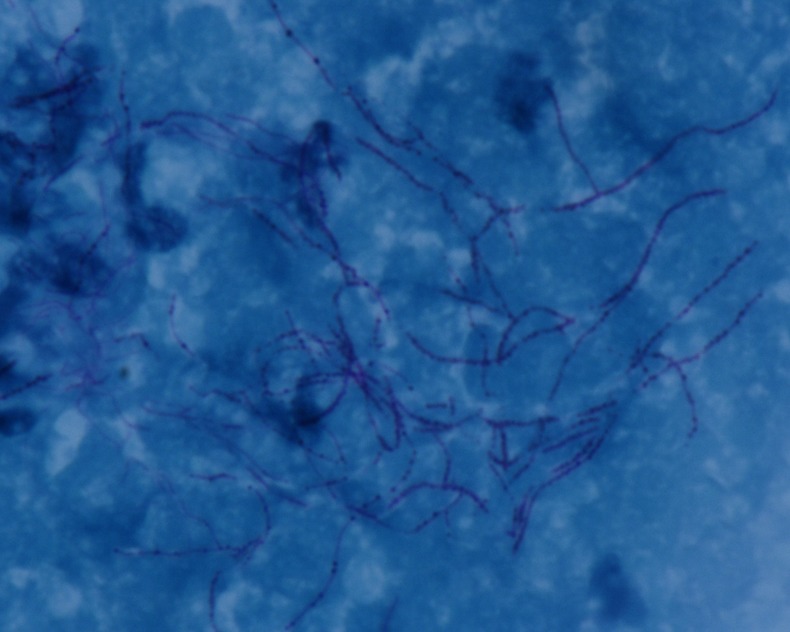
Photomicrograph of endotracheal aspirate showing acid fast, filamentous, branching bacteria (×1000).
Figure 2.

Colonies of Nocardia brasiliensis on blood agar.
Differential diagnosis
Community-acquired bacterial pneumonia due to Streptococcus pneumoniae, Haemophilus influenzae, Legionella pneumophila, Chlamydophila pneumoniae and Staphylococcus aureus
Viral pneumonia
Fungal pneumonia
Pulmonary tuberculosis
Treatment
Empirical treatment with ceftriaxone (1 g intravenous twice daily) and levofloxacin (750 mg intravenous once daily), was started. Because the patient had severe respiratory distress, he was intubated and mechanically ventilated. As soon as the organism was identified by microscopy the physician in charge was informed and the patient was soon started on co-trimoxazole. When antimicrobial susceptibility test results were available imipenem was substituted for co-trimoxazole.
Outcome and follow-up
Unfortunately the condition of the patient did not improve and he died after 2 days of starting imipenem, due to cardiac arrest.
Discussion
Nocardia spp. are acid-fast, filamentous aerobic actinomycetes causing superficial and deep-seated infections, usually in the immunocompromised host. COPD, especially when associated with steroid therapy, is significantly associated with pulmonary nocardiosis. Pulmonary nocardiosis is usually acquired by direct inhalation from soil, and person-to-person transmission is unusual.1 Patients usually present with the symptoms of chronic cough, chest pain, dyspnoea and haemoptysis. Thus, clinically the entity is a close mimic of pulmonary tuberculosis.3 Chronic pulmonary infection is the most serious form of nocardiosis. The disease may disseminate by bacteraemic spread to other organs, with approximately 45% cases of disseminated infection involving the CNS. Infection is facilitated by factors such as microorganism's ability to live inside macrophages by inhibiting phagosome–lysosome fusion and production of enzymes like superoxide dismutase and catalase.4 In a report from Spain, COPD was incriminated as the major predisposing factor followed by HIV infection and polymyalgia rheumatic.5 In this study, lobar consolidation was the predominant x-ray finding (60%) in the patients. The species isolated were N asteroides (80% cases) followed by Nocardia farcinica and Nocardia otitidiscaviarum. N brasiliensis primarily cause cutaneous infection and is the second most common isolated aerobic actinomycete.6 Very few studies of pulmonary nocardiosis in COPD patients have been published from India. In an Indian study by Chawla et al,7 N otitidiscaviarum, N asteroides and N brasiliensis were the most common species encountered in pulmonary nocardiosis. Our study shows that N brasiliensis can cause infection restricted mainly to the lungs in patients with chronic lung disease and this species should be looked for in any microbiological investigation of COPD. Early and correct microbiological diagnosis can help in decreasing disease-related morbidity and mortality.
Co-trimoxazole has been the drug of choice for nocardiosis but now resistance to this drug is being reported. Recently Uhde et al reported that of 765 isolates of Nocardia submitted to the Centers for Disease Control and Prevention in Atlanta, Georgia, from 1995 to 2004, 61% were resistant to sulfamethoxazole and 42% were resistant to co-trimoxazole. In a 2011 study of 186 Nocardia species isolated from patients in Spain, Larruskain et al described 16.1% resistance to co-trimoxazole. Another 2011 report from Canada described 43% of 157 Nocardia isolates recovered from Quebec from 1988 to 2008 as resistant to co-trimoxazole. Brown-Elliott et al8 reviewed 552 recent susceptibilities of clinical isolates of Nocardia from six major laboratories in the USA, and only 2% of the isolates were found to have resistant minimal inhibitory concentrations of co-trimoxazole.
Despite these reports of in vitro sulfonamide resistance, there have been only rare recent clinical reports describing treatment failure of Nocardia with co-trimoxazole.5 Our patient succumbed to this infection because it was caused by a co-trimoxazole resistant N brasiliensis which could not be diagnosed in time as susceptibility testing of Nocardia requires longer incubation. Only one case report from India has shown presence of co-trimoxazole resistant N brasiliensis.9 Local as well as Indian data of co-trimoxazole resistant Nocardia is severely lacking as most of the laboratories does not perform isolation, identification and susceptibility testing of Nocardia. Our report highlights the possibility of emergence of co-trimoxazole resistant N brasiliensis in India and treatment failures with empirical co-trimoxazole therapy. This possibility must prompt clinicians to use combined treatment or drugs with proven clinical response.
In patients with pulmonary nocardiosis and dissemination to other organs combined treatment is recommended including a sulfonamide and a bactericidal primary agent, or a combination of imipenem and amikacin. Experimental studies have shown in vitro synergy with imipenem-cefotaxime, cefotaxime-amikacin and imipenem-amikacin with good clinical results. When the CNS is involved, treatment with cefotaxime or ceftriaxone is also recommended. Linezolid has shown excellent in vitro activity against all of the clinically relevant species of Nocardia; moreover, the mechanism of action is unique, so that cross-resistance with other antimicrobials is unlikely. Unfortunately, the high cost and serious toxicities associated with prolonged use of the drug, such as bone marrow suppression, lactic acidosis, peripheral neuropathy and optic neuritis, appear to limit its use and relegate it to salvage therapy, alone or in combination with other antimicrobials.10
Immunocompetent patients with pulmonary nocardiosis or disseminated nocardiosis outside the CNS should be treated for 6–12 months and the duration of treatment must be at least 1 year for patients with CNS involvement. In the case of immunosuppressed patients, treatment should continue for 1 year and, if possible, the dose of the immunodepressant drug should be reduced.10
Learning points.
Possibility of nocardiosis must be considered in patients of chronic obstructive pulmonary disease with long-term steroids.
Our report highlights the possibility of emergence of co-trimoxazole resistant Nocardia brasiliensis in India and treatment failures with empirical co-trimoxazole therapy.
This possibility must prompt clinicians to use combined treatment or drugs with proven clinical response.
Footnotes
Contributors: The patient was clinically evaluated and treated by HD. Laboratory investigations were done by PG, VK and DK. All of them contributed in writing this article.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol 2003;2013:4497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambaye A, Kohner PC, Wollan PC, et al. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol 1997;2013:847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subhash HS, Christopher DJ, Roy A, et al. Pulmonary nocardiosis in human immunodeficiency virus infection: a tuberculosis mimic. J Postgrad Med 2001;2013:30–2 [PubMed] [Google Scholar]
- 4.Winn W, Allen S, Janda W, et al. eds. Koneman's color atlas and textbook of diagnostic microbiology. 6th edn Philadelphia: Lippincott Williams and Wilkins, 2006;861–3 [Google Scholar]
- 5.Mari B, Monton C, Mariscal D, et al. Pulmonary nocardiosis: clinical experience in ten cases. Respiration 2001;2013:382–8 [DOI] [PubMed] [Google Scholar]
- 6.Neil MM, Brown JM. The medically important aerobic actinomycetes, epidemiology and microbiology. Clin Microbiol Rev 1994;2013:357–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla K, Mukhopadhyay C, Pawanur P, et al. Pulmonary nocardiosis from a tertiary care hospital in Southern India. Trop Doct 2009;2013:163–5 [DOI] [PubMed] [Google Scholar]
- 8.Brown-Elliott BA, Biehle J, Conville PS, et al. Sulfonamide resistance in isolates of Nocardia spp. from a U.S. multicenter survey. J Clin Microbiol 2012;2013:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhwa V, Rai S, Kharbanda P, et al. A fatal pulmonary infection by Nocardia brasiliensis. Indian J Med Microbiol 2006;2013:63–4 [DOI] [PubMed] [Google Scholar]
- 10.Greenfield RA. Nocardiosis. http://emedicine.medscape.com/article/224123 (accessed 10 Feb 2012).


