Abstract
A 78-year-old man was diagnosed with bladder carcinoma in situ and was successfully treated with intravesical bacillus Calmette-Guérin (BCG) instillations. At 6 months after the last dose, he developed fever, weight loss and malaise. He had an extensive negative workup at an outside hospital and was treated empirically with ciprofloxacin for 2 weeks. The fever resolved but returned months later and he was readmitted with pancytopenia, elevated alkaline phosphatase and ground glass opacities on the chest CT. Bone marrow and liver biopsies showed non-caseating granulomas and were negative for acid-fast bacillus (AFB) and fungal stains. Mycobacterium tuberculosis complex PCR of the bone marrow was negative. Owing to the high clinical suspicion of disseminated BCG infection, the patient was treated empirically. After 9 weeks of incubation, the bone marrow AFB culture grew Mycobacterium bovis. Within 2 months of treatment his symptoms resolved and his laboratory results normalised.
Background
Intravesical bacillus Calmette-Guérin (BCG) instillations are commonly used to treat superficial bladder cancer. Minor side effects are common, but serious adverse events occur in less than 5% of patients, most of them localised.1 Less than 1% of patients develop serious complications of disseminated BCG infection.2 The pathogenesis of disseminated BCG infection has been postulated to be a hypersensitivity reaction or an ongoing infection.2 3 The majority of these events present with symptoms early after treatment and of those that have late presentation there are only rare reports of disseminated disease.3 4 We present a case of culture-proven disseminated BCG infection with liver, lung and bone marrow involvement presenting with fever of unknown origin and pancytopenia.
Case presentation
A 78-year-old man was transferred to our hospital for evaluation of fever of unknown origin. He presented with intermittent fever, anorexia, 20 pounds weight loss, malaise and cough with clear phlegm for the last 4 weeks. He had a similar episode 8 months ago. At that time, he had an extensive workup at a different hospital that did not reveal any possible aetiology of his symptoms. He was eventually treated empirically 2 months later with ciprofloxacin for 2 weeks and the fever resolved after the antibiotic therapy.
His medical history was significant for coronary artery bypass graft and carotid endarterectomy 24 years ago, and bladder carcinoma in situ diagnosed 1 year ago. He was treated with 15 weekly intravesical BCG instillations, without concurrent haematuria, cystitis or traumatic catheterisation and receiving the last dose 6 months before the first episode of fever. He was a non-smoker and had travelled extensively in the last 10 years to Panama, Ecuador, the Balkans and Russia, but not recently. He had a positive purified protein derivative (PPD) skin test 50 years ago and was treated with isoniazid for 1 year. He did not have any recent sick contacts or tuberculosis contacts. He was a retired professor and his family history was not significant.
On examination, he looked chronically ill and pale, his blood pressure was 144/70, pulse 94/min, temperature 96.2°F, respiratory rate 17/min and weight 165 lbs. He had no oral or skin lesions, and no lymphadenopathy. His cardiac sounds were regular without murmurs, his lungs were clear to auscultation, his abdomen was soft and non-tender without organomegaly, had no leg oedema and his neurological examination was normal.
Investigations
After the first episode of fever, investigations at another hospital showed pancytopenia and elevated alkaline phosphatase, normal serum and urine protein electrophoresis, normal thyroid stimulating hormone and immunoglobulin levels, negative quantiferon assay, negative blood and urine cultures, negative antibodies for tick borne diseases, Cytomegalovirus (CMV), Mycoplasma, Listeria, Brucella, HIV and hepatitis, and a negative rheumatological antibody panel.
After the second episode of fever, he had more investigations before being transferred to our hospital; an echo showed no vegetations and a white cell scan showed no abnormal areas of uptake. He still had pancytopenia and an elevated alkaline phosphatase and blood and urine cultures were negative. A bone marrow and liver biopsy were performed and revealed non-caseating granulomas with negative acid-fast bacillus (AFB) and fungal stains. Unfortunately, cultures were not sent from the biopsy specimens.
On admission to our hospital, the white cell count was 11 000/μl, haemoglobin was 9.9 mg/dl, platelets were 90 000/μl and alkaline phosphatase was 120 U/l. A chest CT showed ground glass opacities in both lung bases (figure 1). A thorough review of the history and previous investigations was performed, and owing to high clinical suspicion for disseminated BCG infection, a decision was made to repeat the bone marrow biopsy, sending this time the specimen for AFB and fungal cultures and Mycobacterium tuberculosis complex PCR. The bone marrow biopsy revealed non-caseating granulomas with negative AFB and fungal stains (figure 2). The PCR was negative but after 9 weeks of incubation the AFB culture grew Mycobacterium bovis, sensitive to isoniazid, rifampin and ethambutol, and resistant to pyrazinamide.
Figure 1.
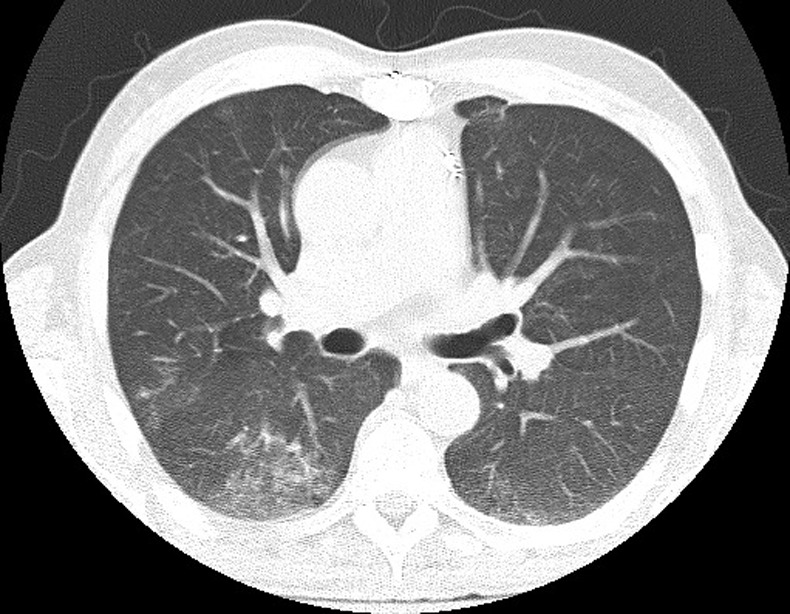
CT of the chest showing bibasilar ground-glass opacities.
Figure 2.
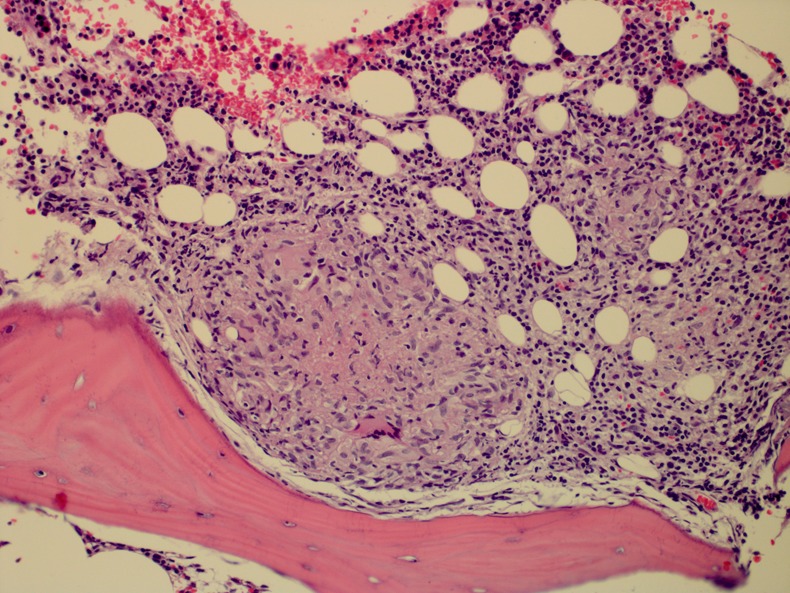
Non-necrotising granulomas in the bone marrow space.
Differential diagnosis
Possible causes of fever and non-caseating granulomas in the liver and bone marrow include sarcoidosis, tuberculosis, non-tuberculous mycobacteriae, fungal infections, CMV, Q fever and brucellosis. Malignancies like Hodgkin lymphoma, non-Hodgkin lymphoma and renal cell carcinoma have been described as causes of hepatic granulomas, and also exposure to drugs like allopurinol, sulfas, chlorpropamide and quinidine.
In this particular case, there was no exposure to such drugs neither evidence of malignancy on the bone marrow biopsy or CT scans. The patient was neither immunosuppressed nor had significant geographical exposure, so fungal infections, CMV and Q fever were very unlikely. The two main diagnostic possibilities were sarcoidosis and mycobacterial disease.
Treatment
Despite a negative PCR and pending AFB culture result, the patient was treated empirically for disseminated BCG infection with isoniazid, rifampin and ethambutol as the clinical suspicion was high. After 1 month, he developed transaminitis and his medications were stopped. They were gradually reintroduced with ciprofloxacin replacing isoniazid.
Outcome and follow-up
Within 2 months of treatment, his energy levels increased, the febrile episodes disappeared, the pancytopenia resolved, and the alkaline phosphatase level normalised. He was treated for a total of 12 months.
Discussion
Intravesical instillation of BCG remains the most effective therapy for in situ and superficially invasive transitional carcinoma of the bladder, achieving cure rates of 80%.5 Minor side effects are common, but serious adverse events occur in less than 5% of patients.1
Mild cystitis, haematuria and transient mild fever are common side effects that resolve spontaneously within 48 h and can be managed symptomatically.2 Severe local effects occur in less than 1% of patients and include granulomatous prostatitis, epididymo-orchitis, ureteral obstruction, bladder contracture and renal abscess. Transient high fever occurs in 3% of patients, and it can be difficult to tell which ones of these patients will develop disseminated disease.2
Less than 1% of patients develop disseminated BCG infection. Granulomatous hepatitis and pneumonitis occur in 0.7% of patients, full blown sepsis occurs in 0.4%, arthralgias in 0.5% and cytopenias in 0.1%.1 The organisms likely gain access to lymphatics and blood through disruption of the uroepithelial cells, which can occur during traumatic bladder catheterisation or concurrent cystitis, which are both known risk factors for systemic BCG absorption.1 Advanced age is also a risk factor.6
The interval between the administration of BCG and the onset of these complications is variable, ranging from hours to months, although cases have been described years after the last instillation.7–9 These complications can occur after a single dose of BCG.7
The diagnosis of disseminated BCG infection is very difficult as the symptoms are non-specific and the history of intravesical BCG instillations can be easily overlooked, especially if they were not recent. This can lead to a delayed diagnosis and extensive investigations, so having a high index of clinical suspicion is essential. Aetiological confirmation is also difficult, as usually the AFB stain on histopathology, mycobacterial cultures and PCR are negative.10 This finding has led many authors to postulate that the pathogenesis of this disease is hypersensitivity mediated rather than septic,3 11 although case reports have demonstrated viable organisms in a variety of tissues.12–15
Histopathology of liver, lung and bone marrow biopsies usually demonstrates non-necrotising granulomas,7 12 15 although necrotising granulomas have also been described to occur in the lungs.8 A long incubation period is required for M bovis to grow in mycobacterial cultures because of its very slow replication rate and fastidiousness.
At least 10 patients with bone marrow involvement have been described in the medical literature.8 15–21 In these patients the interval from the last treatment to the onset of symptoms ranged from several hours to 4 years. All of them had high fever, sweating and pancytopenia. Four of them had abnormal liver enzymes and jaundice, three of them had lung infiltrates, five of them had weight loss and two presented with sepsis. The bone marrow biopsy showed non-caseating granulomas in nine of them and caseating granulomas in one. Two patients developed bone marrow fibrosis, one of them progressive with a fatal outcome.18 The bone marrow culture was positive in four patients.
Disseminated BCG infection should be treated for at least 9–12 months.22 M bovis is inherently resistant to pyrazinamide, so treatment is with isoniazid, rifampin and ethambutol for 2 months and then isoniazid and rifampin for 7 months. Fluoroquinolones are very effective against M bovis, so they can be used as a second line agent when isoniazid and rifampin need to be avoided because of concern of liver toxicity.
Learning points.
Disseminated bacillus Calmette-Guérin (BCG) infection may have a delayed presentation as a fever of unknown origin and may be seen in multiple organ systems as seen in our case with bone marrow, liver and lung involvement.
A high suspicion for BCG infection is needed because the symptoms are non-specific and the exposure to BCG may be many months or years prior.
Acid-fast bacillus (AFB) smears and cultures are commonly negative but the cultures should be incubated for a longer period than usual as it is a notorious slow growing organism as evidenced by the growth of our culture at 9 weeks.
Both AFB cultures and Mycobacterium tuberculosis complex PCR should be sent from biopsies since the combined diagnostic yield may be increased.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol 1992;2013:596. [DOI] [PubMed] [Google Scholar]
- 2.Lamm DL. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis 2000;2013(Suppl 3):S86. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez OY, Musher DM, Brar I, et al. Spectrum of bacille Calmette-Guérin (BCG) infection after intravesical BCG immunotherapy. Clin Infect Dis 2003;2013:140. [DOI] [PubMed] [Google Scholar]
- 4.Marans HY, Bekirov HM. Granulomatous hepatitis following intravesical bacillus Calmette-Guerin therapy for bladder carcinoma. J Urol 1987;2013:111–12 [DOI] [PubMed] [Google Scholar]
- 5.Brake M, Loertzer H, Horsch R, et al. Long-term results of intravesical bacillus Calmette-Guérin therapy for stage T1 superficial bladder cancer. Urology 2000;2013:673. [DOI] [PubMed] [Google Scholar]
- 6.Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guérin therapy. Urol Oncol 2008;2013:137. [DOI] [PubMed] [Google Scholar]
- 7.Case records of the Massachusetts General Hospital Weekly clinicopathological exercises. Case 29–1998. A 57-year-old man with fever and jaundice after intravesical instillation of bacille Calmette-Guérin for bladder cancer. N Engl J Med 1998;2013:831. [DOI] [PubMed] [Google Scholar]
- 8.Nadasy KA, Patel RS, Emmett M, et al. Four cases of disseminated Mycobacterium bovis infection following intravesical BCG instillation for treatment of bladder carcinoma. South Med J 2008;2013:91–5 [DOI] [PubMed] [Google Scholar]
- 9.Mehta AR, Mehta PR, Mehta RL. A cough conundrum in a patient with a previous history of BCG immunotherapy for bladder cancer. BMJ Case Rep. Published: 24 Oct 2012. doi:10.1136/bcr-2012-007327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safdar N, Abad CL, Kaul DR, et al. Clinical problem-solving. An unintended consequence—a 79-year-old man with a 5-month history of fatigue and 20 lb (9 kg) weight loss presented to his local physician. N Engl J Med 2008;2013:1496–501 [DOI] [PubMed] [Google Scholar]
- 11.Jasmer RM, McCowin MJ, Webb WR. Miliary lung disease after intravesical bacillus Calmette-Guérin immunotherapy. Radiology 1996;2013:43–4 [DOI] [PubMed] [Google Scholar]
- 12.McParland C, Cotton DJ, Gowda KS, et al. Miliary Mycobacterium bovis induced by intravesical bacille Calmette-Guérin immunotherapy. Am Rev Respir Dis 1992;2013:1330–3 [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Múgica M, Gómez JM, Vázquez VB, et al. Pancreatic and psoas abscesses as a late complication of intravesical administration of bacillus Calmette-Guerin for bladder cancer: a case report and review of the literature. J Med Case Rep 2009;2013:7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellinger WC, Oldenburg WA, Alvarez S. Vascular and other serious infections with Mycobacterium bovis after bacillus of Calmette-Guérin therapy for bladder cancer. South Med J 1995;2013:1212. [DOI] [PubMed] [Google Scholar]
- 15.Viallard JF, Denis D, Texier-Maugein J, et al. Disseminated infection after bacille Calmette-Guérin instillation for treatment of bladder carcinoma. Clin Infect Dis 1999;2013:451. [DOI] [PubMed] [Google Scholar]
- 16.Korać M, Milosević B, Lavadinović L, et al. Disseminated BCG infection in patients with urinary bladder carcinoma. Med Pregl 2009;2013:592–5 [DOI] [PubMed] [Google Scholar]
- 17.Nemeth J, Stoiser B, Winkler HM, et al. Bone marrow infection with bacillus Calmette-Guérin (BCG) after intravesical immunotherapy. Wien Klin Wochenschr 2008;2013:121–3 [DOI] [PubMed] [Google Scholar]
- 18.Vaisban E, Melamed-Snapir Y, Braester A, et al. Bone marrow fibrosis and caseating granulomas associated with intravesicular BCG treatment. Eur J Intern Med 2005;2013:301–3 [DOI] [PubMed] [Google Scholar]
- 19.Elmer A, Bermes U, Drath L, et al. Sepsis and multiple organ failure after BCG-instillation for bladder cancer. Internist (Berl) 2004;2013:935–9 [DOI] [PubMed] [Google Scholar]
- 20.Andrès E, Kuhnert C, Perrin AE, et al. Sepsis syndrome and bone marrow granulomatosis after intravesical instillation of BCG. Presse Med 1999;2013:1753–4 [PubMed] [Google Scholar]
- 21.Dederke B, Riecken EO, Weinke T. A case of BCG sepsis with bone marrow and liver involvement after intravesical BCG instillation. Infection 1998;2013:54–7 [DOI] [PubMed] [Google Scholar]
- 22.LoBue PA, Moser KS. Treatment of Mycobacterium bovis infected tuberculosis patients: San Diego County, California, United States, 1994–2003. Int J Tuberc Lung Dis 2005;2013:333. [PubMed] [Google Scholar]


