Abstract
Primary cardiac sarcomas are rare tumours carrying poor prognosis. Postradiation sarcoma has been reported in patients with breast, cervical and head and neck cancers. We report a case of a 56-year-old woman with stage IIA breast cancer diagnosed in 1997, submitted to mastectomy, adjuvant chemotherapy, radiotherapy and hormonotherapy. Pulmonary metastasis were detected in 2008 and treated with chemotherapy and hormonotherapy, being in complete remission since August 2009. She was admitted in December 2009 with a 3-week history of fever, dyspnoea, polyarthralgias and leg oedema. An echocardiography showed a mass in the left atrium. She was submitted to a surgical tumour resection and the histology revealed a sarcoma of intermediate degree of differentiation. Chemoradiation therapy was started and she remains alive after 3 years, without tumour regrowth or metastasis. This case is a therapeutic challenge, because the previous therapies for breast cancer hampered the options for extra chemoradiation therapy.
Background
Primary cardiac sarcomas are rare tumours carrying poor prognosis.1–4 To our knowledge, only about 300 cases have been reported until date.5 The majority (75%) of cardiac masses is benign, presenting as myxomas, and approximately three-fourth of the malignant are sarcomas.2 3 5–9 A diagnosis of sarcoma of the heart cannot be established with confidence preoperatively because signs, symptoms and images are non-specific.5 7 9 The authors describe a patient surviving at 36 months of follow-up after partial surgical resection, chemotherapy and radiotherapy (RT) for cardiac sarcoma, without tumour progression or metastasis, reviewing the literature for this unusual neoplasm.
Case presentation
A 56-year-old woman was admitted in December 2009 to the emergency department of our tertiary referral hospital with a 3-week history of fever, progressive dyspnoea, arthralgias of elbows, fists and ankles and leg oedema.
She was a diabetic and had been previously diagnosed stage IIA invasive ductal left breast cancer (T1N1M0Gx, oestrogen-receptor-positive in 40%) in 1997. Past cancer therapies included a modified radical mastectomy, adjuvant chemotherapy with fluorouracil, epirubicin and cyclophosphamide, thoracic wall RT with 50 Gy, and hormonotherapy (HT) with tamoxifen for 5 years.
In 2008, she was diagnosed with pulmonary metastasis of her previous breast cancer and was treated with docetaxel and capecitabine, followed by HT with letrozole. She was in complete clinical remission in August 2009. By that time a thoracic CT showed clear lungs and no cardiac abnormalities.
Upon examination, the temperature was found to be 38°C, arterial pressure was 116/55 mm Hg and rest heart rate was 110 beats/min. Besides the trace leg oedema, the physical examination was normal.
Investigations
Laboratory data revealed a normocytic normochromic anaemia (haemoglobin of 10.4 g/dl) and marked elevation of C reactive protein (142 mg/dl). The platelet and leucocytes counts, urea, creatinine, sodium, potassium, tests of coagulation and arterial blood gases were normal. Blood cultures were sterile.
An enlargement of cardiac silhouette was visible on chest x-ray, corresponding to an area of left atrium (figure 1). Subsequent transthoracic and transoesophagic echocardiography (TEE) showed a heterogeneous mass in the lateral wall of the left atrium, 36–47 mm in diameter, with projection to the mitral valve, which caused an inflow obstruction to the left ventricle, but no systolic dysfunction or pericardial effusion was detected (figure 2). High-resolution CT confirmed the echocardiography findings (figure 3).
Figure 1.
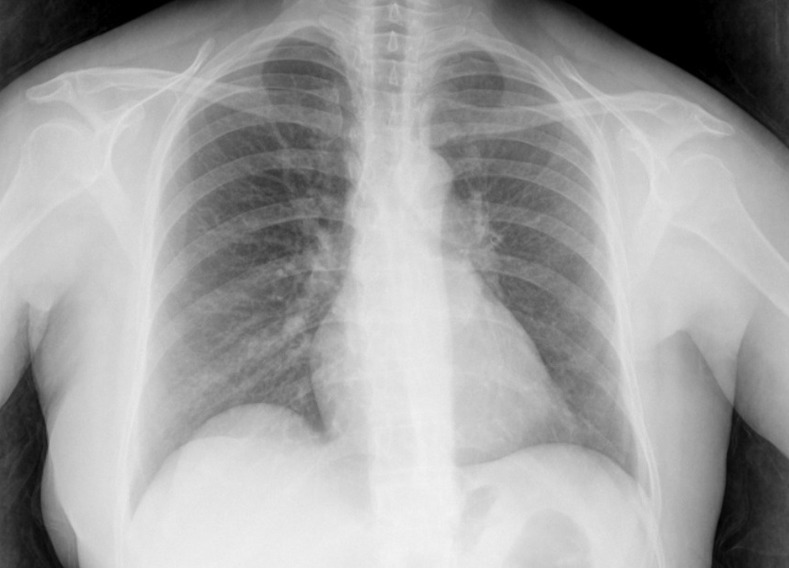
In chest x-ray, an enlargement of cardiac silhouette corresponding to left atrium area is visible.
Figure 2.
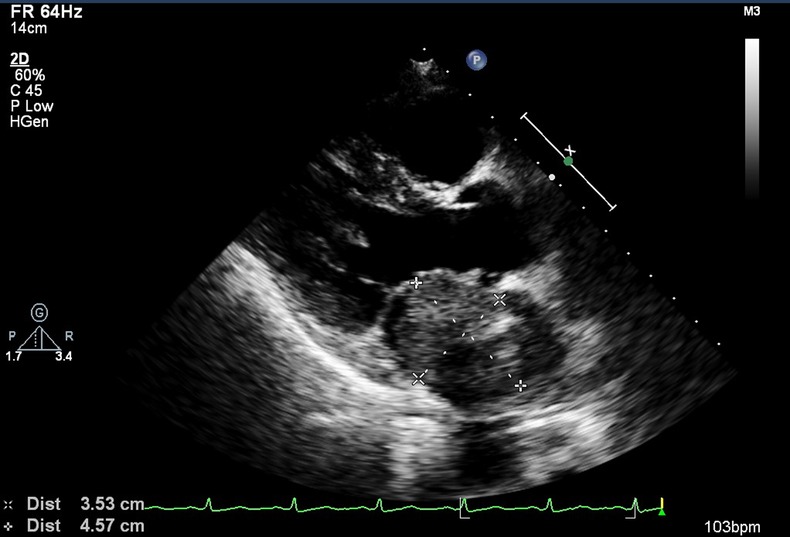
Transoesophagic echocardiography image showing a heterogeneous mass in the lateral wall of the left atrium, with 36×47 mm.
Figure 3.
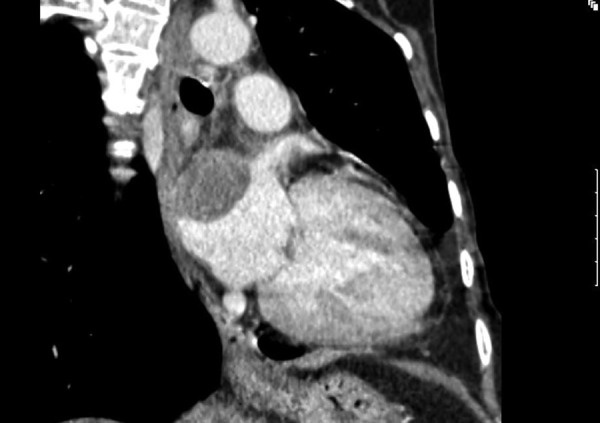
High-resolution CT image showing a left atrium mass.
Treatment
The patient was immediately transferred to another hospital to perform an emergency cardiothoracic surgery. During surgery a left atrial mass was reported, invading the left atrium wall and extending to the pulmonary veins, which limited the surgical procedure to a partial resection. The histological examination revealed a sarcoma of intermediate degree of differentiation (degree 2 from Federation Nationale des Centres de Lutte Contre le Cancer system).
A single episode of atrial fibrillation, reverted to sinus rhythm with amiodarone, was the only postoperative complication. The patient was then treated with six cycles of liposomal adriamycin and ifosfamide and heart RT (50 Gy in 25 fractions).
Outcome and follow-up
At present, 3 years later, the patient remains alive, without sarcoma growth or evidence of breast cancer.
Discussion
The majority of primary cardiac tumours are benign, most atrial myxomas, and only a very small percentage are malignant (0.001–0.028% of autopsies).6 10
Angiosarcoma is the most common primary cardiac malignant tumour, followed by others like leiomyosarcoma, rhabdomyosarcoma, fibrous histiocytoma, myxoid and undifferentiated sarcomas.1–3 6–8 10 11
The age of diagnosis is under 65 years, as in this patient, more frequently in the fourth decade.1 2 3 8 10
The clinical presentation depends on the tumour size and its anatomic location. Tumour type, growth rate, friability and invasiveness are also relevant factors that determine clinical features.3 5 10 Large tumours may be clinically silent and small tumours, in critical locations, may give rise to devastating consequences. Dyspnoea is the commonest symptom, with other common symptoms being chest pain, congestive heart failure, cough, palpitations, syncope, haemoptysis, fever, myalgias and embolic events.2 6 9 11
The differential diagnosis is very broad and must include thrombus, valvular vegetations, intracardiac metastasis, infectious and non-bacterial thrombotic or marantic endocarditis.
Diagnostic approaches include echocardiography, in particular TEE, which proved to be an effective but invasive tool. Other imaging modalities such as high-resolution CT and/or MRI can also be used, with the latter being complementary to echocardiography, providing detailed information about the extent of the tumour, the presence of haemorrhage or necrosis, vascularity and invasion of adjacent structures.9 11
Owing to the low frequency of malignant cardiac tumours there is no staging classification, such as TNM classification.12
Postradiation sarcoma has been reported in patients with breast, cervical and head and neck cancers.13 Criteria for postradiation sarcoma defined by Cahan et al and later revised by Murray et al include a history of exposure to radiation, no prior sarcoma and histological features distinct from previously diagnosed malignancy. Furthermore, radiation doses in the range of 25–75 Gy have been associated with the development of sarcomas.13–15 This patient was diagnosed with a sarcoma 12 years after mastectomy and chemoradiation therapy, filling the described criteria.
Surgical resection is not always an effective treatment for cardiac tumours because of the large mass of cardiac tissue involved, but only patients who undergo complete excision have the possibility of cure.3 6 This makes it highly important to find new non-invasive diagnostic tools for these tumours. TEE is useful in determining tumour size, anatomical localisation and valvular abnormalities and has a diagnostic sensitivity of 97% for non-myxomatous cardiac tumours. A definitive diagnosis depends on histopathological findings. Transvenous endomyocardial biopsy may be the best option and has several advantages over surgery, like significantly less risk of morbidity and mortality.16
The median survival for most sarcomas is about 6–24 months and 2-year survival rates range from 14% to 26%.1 2 10
With partial or no resection the median survival is 6–10 months versus 17–24 months after complete resection.10 There are reports of 23 months of survival after partial resection and chemotherapy, 5.5 years after chemotherapy and RT, 7-year survival with combined surgical, chemotherapy and RT, and 112 months after resection of tumour followed by heart transplantation.3 4 17 18 However, in case of patients with undifferentiated sarcoma the mean survival has been reported to be about 3 months.3 The prognosis of the patient that we report remains dismal because of the local extension, that impossibilities complete resection and the poor degree of differentiation of the sarcoma at presentation.
Left-sided tumours, a low mitotic index and the absence of metastasis were associated with a more favourable outcome.9
The roles of RT and chemotherapy in malignant cardiac tumour are still controversial. Owing to the rarity of the disease, there are no prospective studies to determine the efficacy of chemotherapy and RT in addition to surgery.1 2 The combination of several chemotherapeutic agents appears to be more effective than single-agent therapy, with ifosfamide, doxorubicin, cyclophosphamide and paclitaxel all being used with evidence of response.
Some authors defend that the value of RT in the treatment of heart tumours is limited by the potential late toxicities of RT on cardiac tissue and surrounding structures, like radiation cardiomyopathy and chronic pericarditis and by the evidence that adjuvant RT does not confer a statistically significant survival advantage.6 10 However low-dose RT might have a role as an adjuvant to complete surgery for cardiac sarcomas.6
An alternative treatment option reported with favourable results, particularly in localised cardiac sarcomas, is a complete cardiac excision with orthotopic heart transplantation, that enable improved local control.6 However, data on prolonged survival are still lacking, with a small number of case studies showing that cardiac transplantation leads to limited improvement in survival, with patients succumbing to either metastatic disease or transplant-related complications within 2–4 years.19 20
Targeted therapies based on cytogenetic analysis of the tumour cells may be a hope in the future, like the antiangiogenesis therapies in angiosarcomas and other therapies that promote induction of apoptosis.9
We report a patient with a metastasised breast cancer and a left atrial sarcoma of intermediate degree of differentiation, 12 years postadjuvant-breast radiation who, despite the poor prognosis associated with these diseases, is still alive and in clinical remission, 36 months after partial surgical resection, chemotherapy with adriamycin and ifosfamide and heart RT.
Learning points.
Primary cardiac sarcomas are rare tumours carrying poor prognosis.
Use of radiotherapy (RT) and chemotherapy is still controversial and only patients who undergo complete excision have a prognostic advantage.
A patient with a metastasised breast cancer and a left atrial sarcoma, 12 years postadjuvant-breast radiation is still alive and in clinical remission, 36 months after partial surgical resection, chemotherapy and heart RT.
The aetiological role of radiation in the sarcoma of this patient is controversial, but there are criteria for postradiation sarcoma and she meets it.
This case has turned a therapeutic challenge, because the previous therapies for breast cancer hampered the options for extra chemotherapy and RT.
Footnotes
Contributors: JR and SN performed cardiac sarcoma management, analyzed and interpreted the patient data and were major contributors in writing the manuscript. IM and FM performed sarcoma management and were contributors in writing the manuscript. All authors read and approved the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bakaeen FG, Jaroszewski DE, Rice DC, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg 2009;2013:1454–60 [DOI] [PubMed] [Google Scholar]
- 2.Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer 2008;2013:2440–6 [DOI] [PubMed] [Google Scholar]
- 3.Zhang PJ, Brooks JS, Goldblum JR, et al. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Hum Pathol 2008;2013:1385–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Movsas B, Teruya-Feldstein J, Smith J, et al. Primary cardiac sarcoma: a novel treatment approach. Chest 1998;2013:648–52 [DOI] [PubMed] [Google Scholar]
- 5.Bhandari V, Sakhi P, Munjal K, et al. Primary right atrial sarcoma. J Cancer Res Ther 2010;2013:347–9 [DOI] [PubMed] [Google Scholar]
- 6.Butany J, Nair V, Naseemuddin A, et al. Cardiac tumors: diagnosis and management. Lancet Oncol 2005;2013:219–28 [DOI] [PubMed] [Google Scholar]
- 7.Maraj S, Pressman GS, Figueredo VM. Primary cardiac tumors. Int J Cardiol 2009;2013:152–6 [DOI] [PubMed] [Google Scholar]
- 8.Gupta A. Primary cardiac sarcomas. Expert Rev Cardiovasc Ther 2008;2013:1295–7 [DOI] [PubMed] [Google Scholar]
- 9.Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumors of the heart: a review of tumor type, diagnosis and diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;2013:748–56 [DOI] [PubMed] [Google Scholar]
- 10.Hamidi M, Moody J, Weigel T, et al. Primary cardiac sarcomas. Ann Thorac Surg 2010;2013:176–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Bathina J, Qureshi S, et al. Cardiac tumors in a tertiary care cancer hospital: clinical features, echocardiographic findings, treatment and outcomes. Heart Int 2012;2013:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcovan D, Stoica L, Tinică G, et al. A primary epithelioid cardiac sarcoma—case report and review of the literature. Jurnalul de Chirurgie Iaşi 2010;2013:59–63 [Google Scholar]
- 13.Miandoab S, Gangadharam S, Sugarbaker D. Cardiac sarcoma 14 years after pleural mesothelioma. N Engl J Med 2005;2013:1929–30 [DOI] [PubMed] [Google Scholar]
- 14.Cahan WG, Woodard HQ, Higginbotham NL, et al. Sarcoma arising in irradiated bone. Cancer 1948;2013:3–29 [DOI] [PubMed] [Google Scholar]
- 15.Murray EM, Werner D, Greeff EA, et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys 1999;2013:951–61 [DOI] [PubMed] [Google Scholar]
- 16.Hosokawa Y, Kodani E, Kusama Y, et al. Cardiac angiosarcoma diagnosed by transvenous endomyocardial biopsy with the aid of transesophageal echocardiography and intra-procedural consultation. Int Heart J 2010;2013:367–9 [DOI] [PubMed] [Google Scholar]
- 17.Antunes M, Vanderdonck K, Andrade C, et al. Primary cardiac leiomyosarcomas. Ann Thorac Surg 1991;2013:999–1001 [DOI] [PubMed] [Google Scholar]
- 18.Pessotto R, Silvestre G, Luciani GB, et al. Primary cardiac leiomyosarcoma: seven-year survival with combined surgical and adjuvant therapy. Int J Cardiol 1997;2013:91–4 [DOI] [PubMed] [Google Scholar]
- 19.Bachet J, Banfi C, Martinelli L, et al. Heart transplantation and primary cardiac tumors. Ann Thorac Surg 1995;2013:262–3 [DOI] [PubMed] [Google Scholar]
- 20.Talbot SM, Taub RN, Keohan M, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg 2002;2013:1145–8 [DOI] [PubMed] [Google Scholar]


