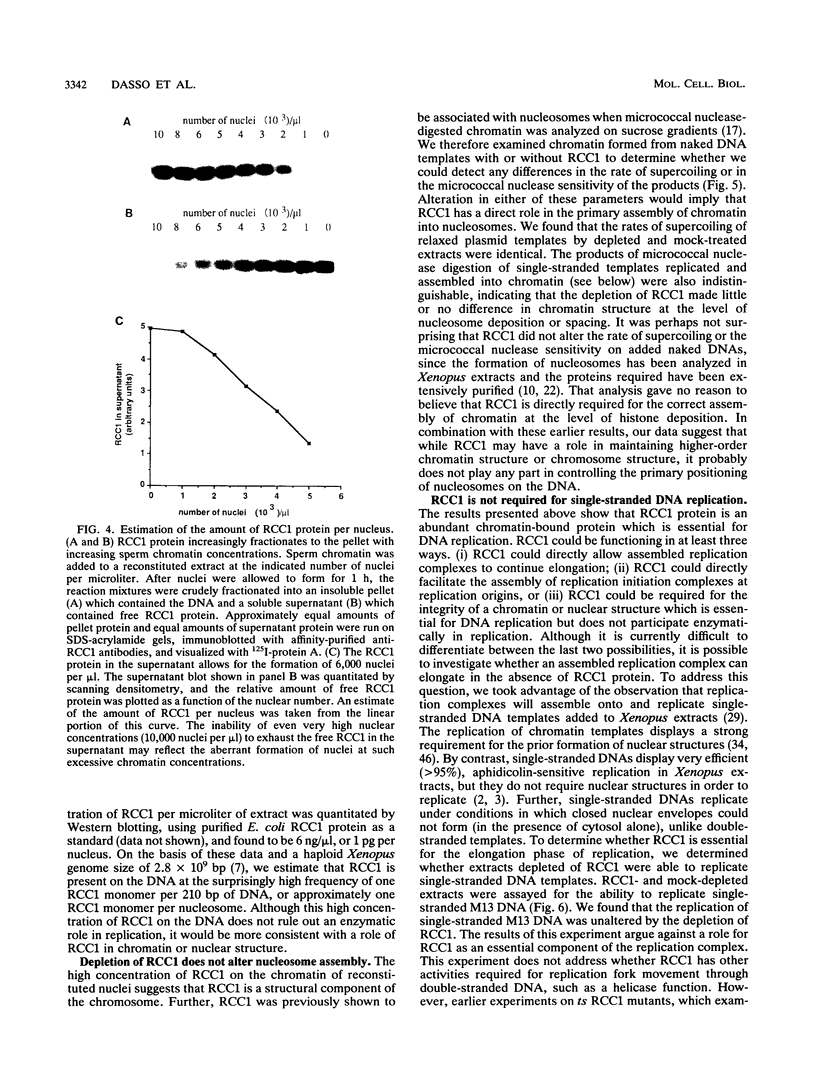
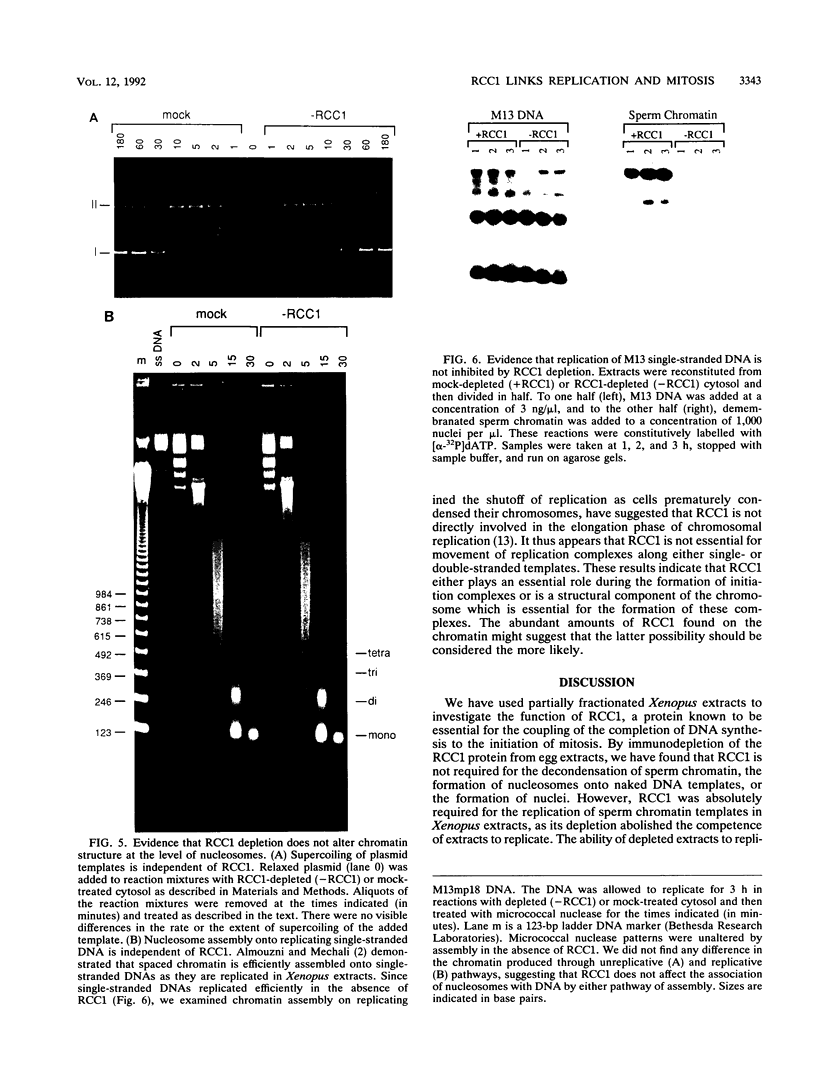
Abstract
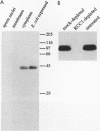
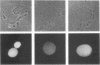
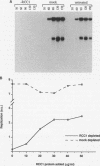
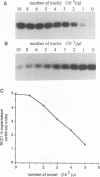
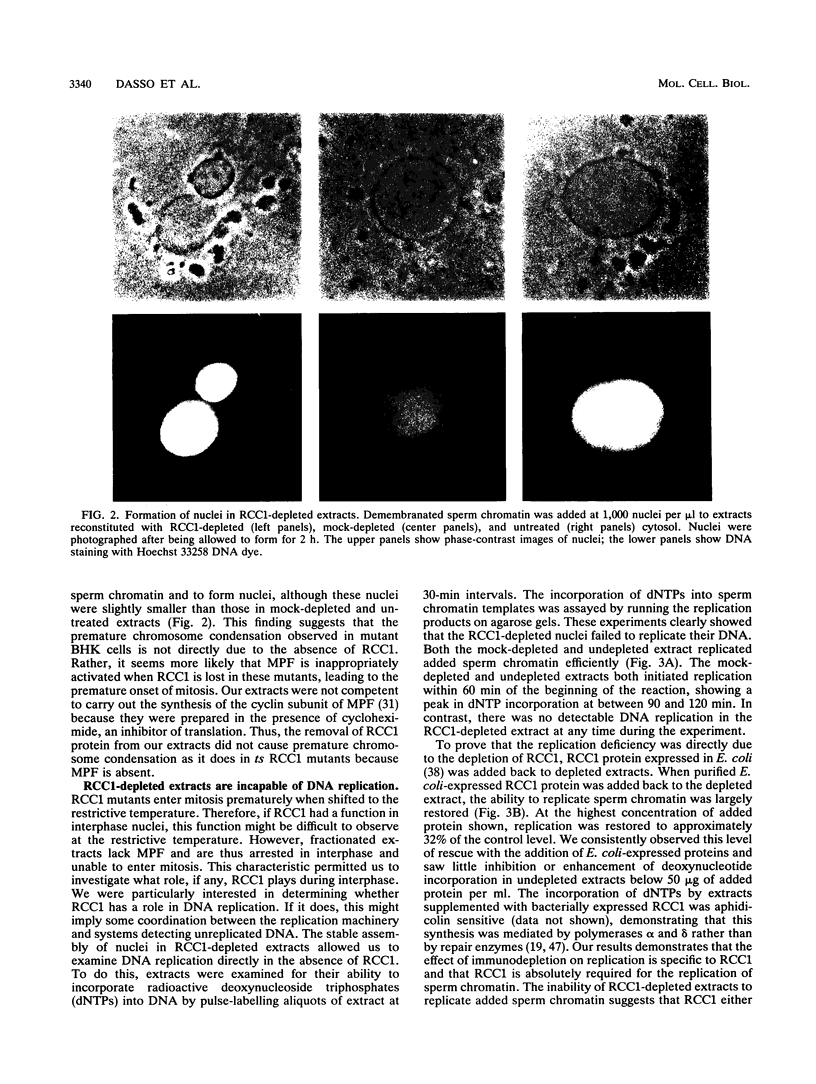
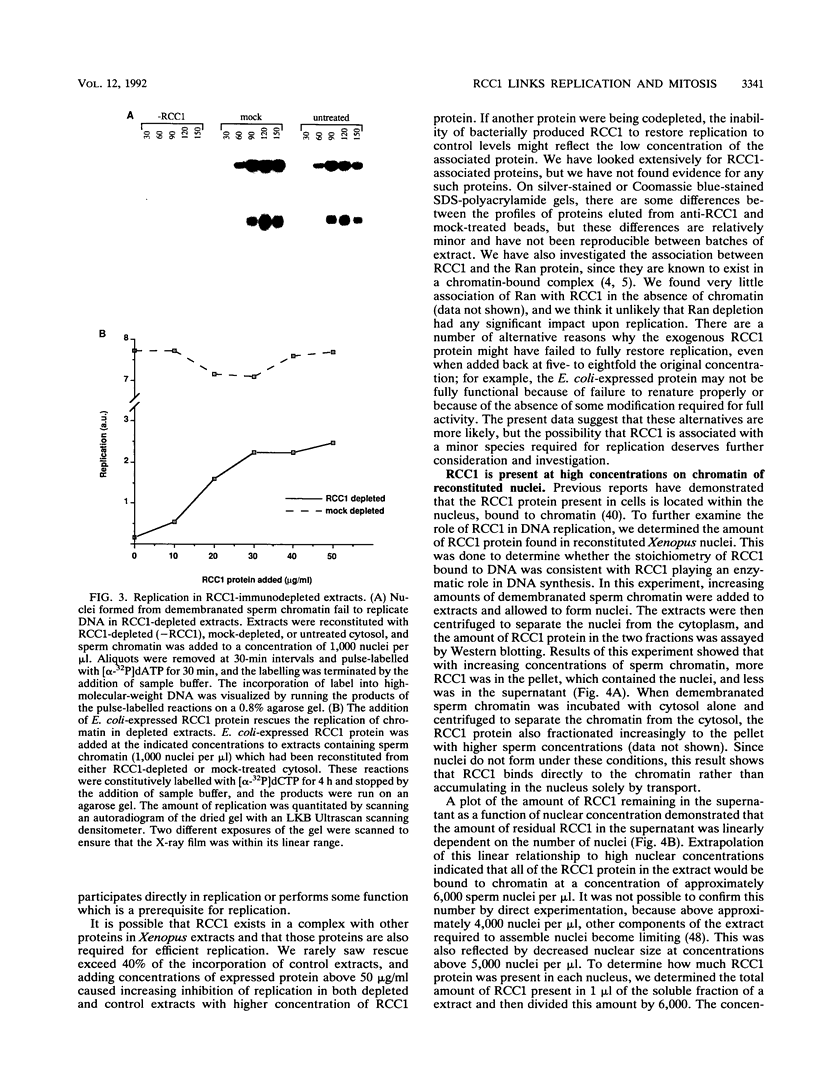
Temperature-sensitive mutants in the RCC1 gene of BHK cells fail to maintain a correct temporal order of the cell cycle and will prematurely condense their chromosomes and enter mitosis at the restrictive temperature without having completed S phase. We have used Xenopus egg extracts to investigate the role that RCC1 plays in interphase nuclear functions and how this role might contribute to the known phenotype of temperature-sensitive RCC1 mutants. By immunodepleting RCC1 protein from egg extracts, we find that it is required for neither chromatin decondensation nor nuclear formation but that it is absolutely required for the replication of added sperm chromatin DNA. Our results further suggest that RCC1 does not participate enzymatically in replication but may be part of a structural complex which is required for the formation or maintenance of the replication machinery. By disrupting the replication complex, the loss of RCC1 might lead directly to disruption of the regulatory system which prevents the initiation of mitosis before the completion of DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Clark M. W., Vijayraghavan U., Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol Gen Genet. 1990 Oct;224(1):72–80. doi: 10.1007/BF00259453. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Méchali M. Assembly of spaced chromatin promoted by DNA synthesis in extracts from Xenopus eggs. EMBO J. 1988 Mar;7(3):665–672. doi: 10.1002/j.1460-2075.1988.tb02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouzni G., Méchali M., Wolffe A. P. Competition between transcription complex assembly and chromatin assembly on replicating DNA. EMBO J. 1990 Feb;9(2):573–582. doi: 10.1002/j.1460-2075.1990.tb08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991 Nov 7;354(6348):80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Callan H. G. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):19–41. doi: 10.1098/rspb.1972.0039. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Kirschner M. W. Regulation of MPF activity in vitro. Cell. 1988 Apr 22;53(2):185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- Dasso M., Newport J. W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990 Jun 1;61(5):811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dilworth S. M., Black S. J., Laskey R. A. Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987 Dec 24;51(6):1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Mitosis-inducing factors are present in a latent form during interphase in the Xenopus embryo. J Cell Biol. 1988 Jun;106(6):2047–2056. doi: 10.1083/jcb.106.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilen E., Hand R., Basilico C. Decreased initiation of DNA synthesis in a temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Nov;105(2):259–266. doi: 10.1002/jcp.1041050209. [DOI] [PubMed] [Google Scholar]
- Engle D. B., Osmani S. A., Osmani A. H., Rosborough S., Xin X. N., Morris N. R. A negative regulator of mitosis in Aspergillus is a putative membrane-spanning protein. J Biol Chem. 1990 Sep 25;265(27):16132–16137. [PubMed] [Google Scholar]
- Enoch T., Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990 Feb 23;60(4):665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Forbes D. J. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990 Jan 12;60(1):17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Frasch M. The maternally expressed Drosophila gene encoding the chromatin-binding protein BJ1 is a homolog of the vertebrate gene Regulator of Chromatin Condensation, RCC1. EMBO J. 1991 May;10(5):1225–1236. doi: 10.1002/j.1460-2075.1991.tb08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Goscin L. P., Byrnes J. J. DNA polymerase delta: one polypeptide, two activities. Biochemistry. 1982 May 11;21(10):2513–2518. doi: 10.1021/bi00539a034. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Seiter A., Zentgraf H. Nucleosome assembly in vitro: separate histone transfer and synergistic interaction of native histone complexes purified from nuclei of Xenopus laevis oocytes. EMBO J. 1990 Apr;9(4):1309–1318. doi: 10.1002/j.1460-2075.1990.tb08240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Smythe C., Newport J. W. In vitro cell cycle arrest induced by using artificial DNA templates. Mol Cell Biol. 1992 Jul;12(7):3216–3223. doi: 10.1128/mcb.12.7.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991 Mar 8;64(5):903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984 Apr;98(4):1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Beach D. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell. 1991 Jul 26;66(2):347–360. doi: 10.1016/0092-8674(91)90624-8. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Wilson K. L., Dunphy W. G. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987 Jan 30;48(2):205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Newport J., Spann T. Disassembly of the nucleus in mitotic extracts: membrane vesicularization, lamin disassembly, and chromosome condensation are independent processes. Cell. 1987 Jan 30;48(2):219–230. doi: 10.1016/0092-8674(87)90425-9. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Eilen E., Basilico C. Premature of chromosome condensation in a ts DNA- mutant of BHK cells. Cell. 1978 Oct;15(2):475–483. doi: 10.1016/0092-8674(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Ishida R., Ajiro K., Yamamoto S., Takahashi T. The synthesis of protein(s) for chromosome condensation may be regulated by a post-transcriptional mechanism. J Cell Physiol. 1981 Nov;109(2):299–308. doi: 10.1002/jcp.1041090213. [DOI] [PubMed] [Google Scholar]
- Nishimoto T. The 'BN2' gene, a regulator for the onset of chromosome condensation. Bioessays. 1988 Oct;9(4):121–124. doi: 10.1002/bies.950090405. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Kobayashi H., Ohtsubo M., Nishimoto T. Cloning of Xenopus RCC1 cDNA, a homolog of the human RCC1 gene: complementation of tsBN2 mutation and identification of the product. J Biochem. 1990 Feb;107(2):228–235. doi: 10.1093/oxfordjournals.jbchem.a123031. [DOI] [PubMed] [Google Scholar]
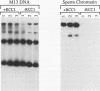
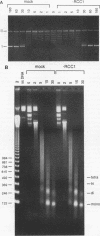
- Nishitani H., Ohtsubo M., Yamashita K., Iida H., Pines J., Yasudo H., Shibata Y., Hunter T., Nishimoto T. Loss of RCC1, a nuclear DNA-binding protein, uncouples the completion of DNA replication from the activation of cdc2 protein kinase and mitosis. EMBO J. 1991 Jun;10(6):1555–1564. doi: 10.1002/j.1460-2075.1991.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M., Okazaki H., Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989 Oct;109(4 Pt 1):1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M., Yoshida T., Seino H., Nishitani H., Clark K. L., Sprague G. F., Jr, Frasch M., Nishimoto T. Mutation of the hamster cell cycle gene RCC1 is complemented by the homologous genes of Drosophila and S.cerevisiae. EMBO J. 1991 May;10(5):1265–1273. doi: 10.1002/j.1460-2075.1991.tb08068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Engle D. B., Doonan J. H., Morris N. R. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988 Jan 29;52(2):241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Pu R. T., Morris N. R. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988 Apr 22;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- Poccia D. Remodeling of nucleoproteins during gametogenesis, fertilization, and early development. Int Rev Cytol. 1986;105:1–65. doi: 10.1016/s0074-7696(08)61061-x. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Pardee A. B. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986 Jun 6;232(4755):1264–1266. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- Sheehan M. A., Mills A. D., Sleeman A. M., Laskey R. A., Blow J. J. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988 Jan;106(1):1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Wilson K. L., Newport J. A trypsin-sensitive receptor on membrane vesicles is required for nuclear envelope formation in vitro. J Cell Biol. 1988 Jul;107(1):57–68. doi: 10.1083/jcb.107.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]