Abstract
Skeletal muscle and bone form highly-integrated systems that undergo significant age-related changes, but the relationships between muscle mass and trabecular versus cortical bone or trabecular microarchitecture have not been systematically investigated. Thus, we examined the association between appendicular skeletal muscle mass relative to height2 (relative ASM) and bone parameters at several sites assessed by conventional as well as high-resolution peripheral QCT in a cohort of 272 women and 317 men aged 20 to 97 years. In women, relative ASM was associated with cortical thickness (CtTh) at the femoral neck, lumbar spine, radius and tibia (age-and physical activity adjusted r = 0.19 to 0.32; all p < 0.01). Relative ASM was also associated with trabecular volumetric bone mineral density (vBMD) at the femoral neck and spine (all p < 0.05), and trabecular bone volume to tissue volume (BV/TV), number (TbN), thickness (TbTh) and separation (TbSp) at the radius (all p ≤ 0.05). In all men, relative ASM was associated with CtTh at all sites (age- and physical activity adjusted r = 0.17 to 0.28; all p < 0.01). Associations between relative ASM and trabecular vBMD at the spine in men were lost after adjusting for age; however, relative ASM was associated with trabecular vBMD at the femoral neck and TbN and TbSp at the radius (all p < 0.01). We also investigated circulating factors associated with bone health that may be indicative of relative ASM and found that serum IGFBP-2 levels were the most robust negative predictors of relative ASM in both sexes. Collectively, these data add to the growing body of evidence supporting the highly-integrated nature of skeletal muscle and bone, and provide new insights into potential biomarkers that reflect the health of the musculoskeletal system.
Keywords: sarcopenia, osteoporosis, aging, sex steroids
INTRODUCTION
Skeletal muscle and bone share a common embryonic origin, form an integrated system that provides form and physical function, and exhibit remarkable changes across the lifespan. In older persons, excessive deterioration of skeletal muscle (sarcopenia) and bone (osteopenia) are prevalent, pose a significant risk for adverse health outcomes, and account for greater than $18.5 and $16.9 billion in annual healthcare costs, respectively (1–4). Developing a better understanding of the complex relationship between these fundamental components of the musculoskeletal system may reveal new strategies for early identification, prevention and treatment of sarcopenia and osteopenia, as well as their consequences.
Humans achieve peak skeletal muscle mass and bone density in young adulthood. After age ~45 years, skeletal muscle mass progressively declines in men and women, particularly, in the lower body (5). Alterations in skeletal muscle quantity and quality are highly associated with weakness and functional decline; these, in turn, are leading risk factors for falls (6), loss of independence (7), institutionalization (8), and even death (9–11). The loss of skeletal muscle, a recognized mechanical mediator of bone health, is also a plausible contributor to age-related changes in bone mass and strength. In fact, very recent data indicate that secreted factors may mediate cross-talk between bone cells (osteocytes) and muscle cells (12,13).
Multiple studies have demonstrated positive associations between skeletal muscle mass and bone mineral density (BMD) as assessed by dual-energy X-ray absorptiometry (DXA) at various skeletal sites (e.g., (14,15)), and the lower skeletal muscle mass in women has been implicated in their increased prevalence of osteoporosis (5). However, the advent of higher-resolution imaging technologies that perform measures of cortical and trabecular geometry and microstructure allows for much more detailed analyses of bone compartments and microstructure separately (16). To our knowledge, how these more discriminating measures of bone health relate to skeletal muscle mass in men and women across the lifespan has not been carefully examined.
Moreover, multiple biological factors have been causally implicated in the regulation of the musculoskeletal system. In particular, skeletal muscle and bone are highly responsive to estrogens and androgens. The age-associated decline in testosterone production has long been associated with sarcopenia (17), while the decrease in estradiol is linked to bone loss and osteoporotic fractures in men and women (18,19). Components of the insulin-like growth factor (IGF) system also play a role in muscle and bone health (20–23). IGF-I and –II mediate anabolic effects on constituent skeletal muscle and bone cells (24,25); and, recently, IGF binding protein-2 (IGFBP-2), an inhibitor of the trophic effects of IGFs, has emerged as a negative regulator of BMD in men and women (26,27). These factors, along with circulating concentrations of bone turnover markers, serve as reasonable biological indicators, or biomarkers, of bone health. Whether or not their concentrations are also associated with skeletal muscle mass has not been assessed.
Thus, in the present study we examined the relationship between skeletal muscle mass relative to body size and cortical and trabecular geometry and microstructure at multiple skeletal sites in a relatively large, population-based sample of men and women aged 20 to 97 years. We also assessed the relationship between relative skeletal muscle mass and biomarkers of bone health, sex steroids, and components of the IGF system.
MATERIALS AND METHODS
Study Subjects
Subjects were recruited from an age-stratified, random sample of Rochester, MN residents as described in detail previously (28). This population is highly characteristic of the United States White population, but Blacks and Asians are underrepresented (29). The sample spanned ages from 21 to 97 years and included 375 women and 325 men. Ninety-four women and 2 men were on some form of estrogen therapy (defined as oral or transdermal preparations with or without a progestin) and were excluded from the present study. Moreover, 4 women and 2 men were on bisphosphonates and their data were excluded from this study. An additional 5 women and 4 men did not have lean mass data from DXA and were also excluded from the analyses. All studies were approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from all subjects prior to evaluation. Data was collected between November 2000 and May 2006.
Study protocol
Subjects were evaluated at the outpatient Clinical Research Unit following an overnight fast. They consumed their habitual diet the day prior to study without any dietary restrictions. Following a blood draw, the subjects underwent the various imaging procedures described below.
Total body DXA and Determination of Lean Mass
A total body DXA was performed (Prodigy, GE Medical Systems, Madison, WI) using software version 6.10.029. The lean mass of the arms and legs (appendicular skeletal muscle mass, ASM) was normalized to body size by dividing it by height (m2), herein referred to as relative ASM, as previously described (30).
Lumbar Spine and Femoral Neck Quantitative Computed Tomography (QCT)
Single energy CT scans were made at the proximal femur and lumbar spine with a multi-detector Light Speed QX-I scanner (GE Medical Systems, Wakesha, WI) (28,31). Calibration standards scanned with the subject were used to convert CT numbers directly to equivalent volumetric BMD (vBMD) in mg/cm3 (32). To study age- and sex-specific structural changes in bone mineral distribution and structure, we developed software for the analysis of bone structure, geometry and volumetric density from the CT images, specific details of which have been previously published (31,33). These images were also used in finite element (FE) models to estimate the strength of the proximal femur, as described in detail previously (34).
Radius and tibia cortical parameters by pQCT
We used standard pQCT imaging to obtain cortical parameters at the distal radius and tibia. Details regarding the scanning protocol in this cohort using the Densiscan 1000 instrument (Scanco, Bassersdorf, Switzerland) have been described previously (16,28). While we used high-resolution pQCT (HRpQCT) to assess trabecular parameters (see below), the more proximal scanning sites at the distal radius and tibia by standard pQCT provided more robust measures and correlations of cortical bone parameters with relative ASM and are the data presented. In addition, in the prototype HRpQCT device used in the initial characterization of this cohort, scans of the tibia were not possible, and standard pQCT provided the cortical parameters at the tibia.
Radius trabecular parameters by HRpQCT
Details regarding the high resolution HRpQCT imaging used in this cohort have previously been reported (35) and are summarized briefly here. Due to the lack of availability of this new instrument initially, the HRpQCT measurements were done ~2 years after the other measurements. The HRpQCT scans were obtained in 233 (86%) of the 272 women used in the present study (105 premenopausal and 128 postmenopausal women) and in 281 (89%) of the 317 men. The non-dominant wrist (or in the case of a prior wrist fracture, the non-fractured wrist) was scanned using a prototype of the XtremeCT (Scanco Medical AG, Bassersdorf, Switzerland). The in vivo measurement protocol included the acquisition of a three-dimensional stack of 116 high-resolution QCT slices at the distal end of the radius, using an effective energy of 40 keV, slice thickness of 89 μm, field of view of 90 mm, image matrix of 1024 × 1024 pixels, and pixel size of 89 μm.
The processing and analysis of the images has also been extensively described and validated (36–39). Briefly, bone volume/total volume (BV/TV) is first derived from the trabecular vBMD. Recognizing that individual trabeculae will not be resolved at their correct thickness due to partial volume effects, a thickness-independent structure extraction is employed to assess trabecular microarchitecture. To this end, the 3D ridges (the center points of the trabeculae) are detected in the gray-level images (37) and trabecular number ( TbN, 1/mm) is then taken as the inverse of the mean spacing of the ridges (38). Trabecular thickness (TbTh, mm) is then derived as BV/TV ÷ TbN, and trabecular separation (TbSp, mm) is derived as (1-BV/TV) ÷ TbN, as is done in standard histomorphometry (40). The validity of this approach has been rigorously tested by comparing the HRpQCT methodology with 28 μm resolution μCT (39), with very high correlation (correlation coefficients of 0.96–0.99) between the μCT and HRpQCT measurements. The key point is that HRpQCT resolution has to be sufficient to adequately resolve the distance between the trabecular ridges (1/ TbN, or ~ 300–500 μm), not necessarily to resolve individual trabeculae (~100 μm or less).
Serum measurements
Serum osteocalcin (OC) was measured using a two site immunoradiometric assay (CIS-US, Bedford, MA; interassay CV, 8%). Serum amino-terminal propeptide of type I collagen (PINP) was measured by radioimmunoassay (DiaSorin, Stillwater, MN; interassay CV < 9%). Both serum cross-linked C-telopeptide of type I collagen (CTX) (Nordic Biosciences, Herlev, Denmark; interassay CV < 10%) and serum tartrate-resistant acid phosphatase isoform type 5b (TRAP 5b) were measured by ELISA (Immunodiagnostic Systems, Fountain Hills, AZ; interassay CV < 14%). Serum estradiol (E2), estrone (E1), and testosterone (T) were measured using LC-MS/MS (API 5000, Applied Biosystems-MDS Sciex, Foster City, CA), as previously described (41). Values as low as 1.25 pg/mL for E2 and E1 and 1 ng/dL for T were detectable by this method, with interassay CVs of 8%, 8%, and 6% for E2, E1, and T, respectively. The non-sex hormone binding globulin bound (SHBG), biologically-active (Bio) fraction of E2 and T was measured as previously described (42); interassay CVs for each were each < 12%. SHBG was measured by radioimmunoassay (RIA) (Wien Laboratories, Succasunna, NJ, USA; interassay CV, 7%). Total IGF-I and IGF-II were each measured by a two-site immunoradiometric assay (IRMA), after separation from their binding proteins with a simple organic solvent extraction (Diagnostic Systems Laboratories, Webster, TX, USA; interassay CVs, 6% for each. IGFBP-3 was also measured by a two-site IRMA (Diagnostic Systems Laboratories; interassay CV, 14%). IGFBP-2 was measured by a double antibody RIA (Diagnostic Systems Laboratories; interassay CV, 16%).
Self-reported Physical Activity
Habitual physical activity levels over the preceding 12 months were estimated by assessing the frequency (average times per week/year), duration (hours/day), and intensity (light, moderate, vigorous) of various daily activities, including recreation and sports, as well as stair climbing, walking and the number of sweat-producing activities per week as described previously (43). Caloric expenditure estimates (kcal) were then generated for individual subjects based on body weight, the intensity and duration of activity, and published metabolic equivalent task (MET) and used for analyses (44).
Statistical Analyses
Unadjusted, age-adjusted, and age- and physical activity-adjusted Pearson correlation coefficients were used to assess relationships between relative ASM and the various bone density variables, microstructural parameters, and biochemical markers. Linear regression models were used to test for interactions with gender. Sex steroids in women were log-transformed to approximate the normal distribution. A p-value < 0.05 was considered significant. All analyses were performed using SAS version 9 (SAS Institute, Cary, NC) and Splus (TIBCO Corporation, Palo Alto, CA).
RESULTS
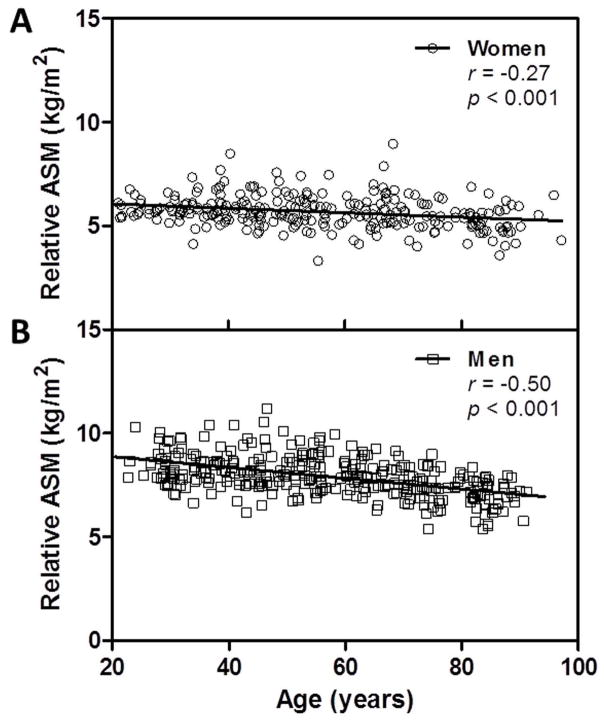
The study population for our analyses consisted of 272 women, of whom 122 were premenopausal and 150 were postmenopausal, and 317 men, of whom 124 were < 50 years of age and 193 were ≥ 50 years old. Subject characteristics are outlined in Table 1. Since the fundamental relationships between relative ASM and most of the variables did not differ by menopausal status in women or age < 50 years versus > 50 years in men, they are reported for all women and all men combined. Relative ASM was greater among the men than the women and was significantly inversely associated with advancing age in women and men (r = −0.27 and −0.50, respectively; both p < 0.001) (Figures 1A and 1B). Relative ASM was also significantly associated with estimates of physical activity after adjusting for age in women and men (r = 0.44 and 0.34, respectively; both p < 0.001).
Table 1.
Characteristics of study subjects (mean ± standard deviation (SD)).
| Women (n = 272) | Men (n = 317) | |
|---|---|---|
| Age (Years) | 55.7 ± 19.3 | 56.8 ± 18.5 |
| Height (m) | 1.63 ± 0.07 | 1.77 ± 0.07 |
| Weight (kg) | 73.6 ± 16.1 | 89.0 ± 16.0 |
| BMI (kg/m2) | 27.7 ± 5.6 | 28.4 ± 4.3 |
| PA (kcal/wk × 103) | 27.7 ± 9.2 | 36.2 ± 12.5 |
| Relative ASM (kg/m2) | 5.7 ± 0.8 | 7.9 ± 1.0 |
| Total Body BMC (kg) | 2.42 ± 0.40 | 3.24 ± 0.47 |
Abbreviations: BMI, body mass index; PA, physical activity; ASM, appendicular skeletal mass; BMC, bone mineral content
Figure 1.
Relation of relative appendicular skeletal muscle mass (ASM) to age in women (A) and men (B).
Relative ASM is associated with bone density and architecture of the central skeleton
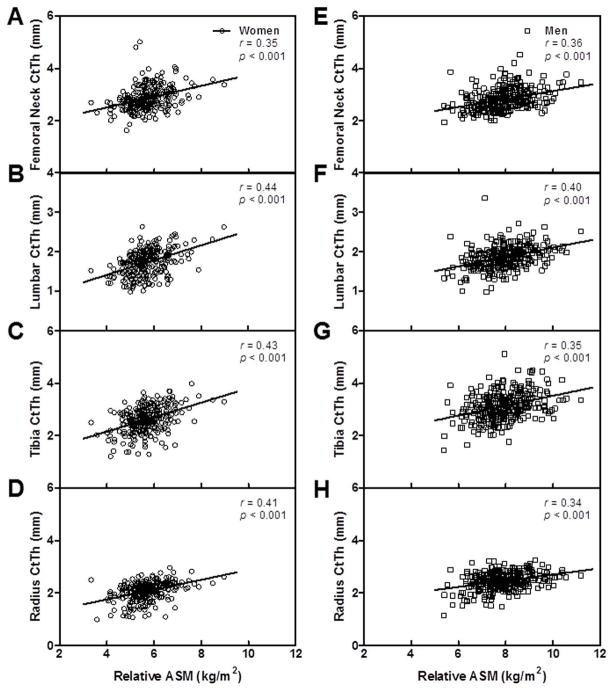
In women, relative ASM exhibited significant unadjusted and age-adjusted associations with femoral neck vBMD and both femoral neck and lumbar spine trabecular vBMD (Table 2). Cortical vBMD of the femoral neck and FE-derived proximal femur strength were also associated with relative ASM. Moreover, there were significant age-adjusted associations between relative ASM and bone area of the lumbar spine. A significant but less impressive association was found with bone area of the femoral neck. Relative ASM was also associated with endocortical area of lumbar spine, but not the femoral neck. Femoral neck CtTh (Figure 2A) and lumbar spine CtTh (Figure 2B) showed significant unadjusted and age-adjusted associations with relative ASM. As reported in Table 2, after further adjusting the above associations for age and physical activity levels, only the relationship between relative ASM and bone area of femoral neck and endocortical area of the lumbar spine were no longer significant.
Table 2.
Pearson correlation coefficients (rp-value unadjusted/rp-value age-adjusted/rp-value age- and physical activity adjusted) between relative ASM and bone parameters at the central skeletal sites in women and men. Statistics in bold are significant at p < 0.05.
| Skeletal Site/Parameter | Women | Men |
|---|---|---|
| Unadjusted/age-adjusted/age- and physical activity adjusted | Unadjusted/age-adjusted/age- and physical activity adjusted | |
| Femoral Neck (QCT) | ||
| Total vBMD | 0.36<0.001/0.28<0.001/0.190.002 | 0.36<0.001/0.130.022/0.110.055 |
| Trabecular vBMD | 0.37<0.001/0.30<0.001/0.190.003 | 0.43<0.001/0.170.003/0.150.010 |
| Cortical vBMD | 0.28<0.001/0.180.003/0.140.029 | 0.170.003/0.020.705/0.010.823 |
| Bone area | 0.050.388/0.120.049/0.030.689 | 0.100.086/0.22<0.001/0.170.003 |
| Endocortical area | −0.090.135/0.000.936/−0.050.433 | −0.010.803/0.130.027/0.100.096 |
| CtTh | 0.35<0.001/0.30<0.001/0.190.002 | 0.36<0.001/0.26<0.001/0.20<0.001 |
| Proximal femur strength | 0.46<0.001/0.43<0.001/0.30<0.001 | 0.48<0.001/0.30<0.001/0.24<0.001 |
| Lumbar Spine (QCT) | ||
| Trabecular vBMD | 0.36<0.001/0.26<0.001/0.200.001 | 0.37<0.001/0.060.264/0.060.275 |
| Bone area | 0.150.018/0.25<0.001/0.160.012 | 0.130.026/0.31<0.001/0.24<0.001 |
| Endocortical area | 0.040.550/0.180.004/0.100.097 | 0.060.311/0.27<0.001/0.20<0.001 |
| CtTh | 0.44<0.001/0.37<0.001/0.28<0.001 | 0.40<0.001/0.23<0.001/0.190.001 |
Figure 2.
Relation of relative appendicular skeletal muscle mass (ASM) to CtTh at the femoral neck, lumbar spine vertebrae, tibia and radius in women (A–D) and men (E–H).
In the men, total vBMD of the femoral neck and proximal femur strength were positively associated with relative ASM, both before and after adjusting for age (Table 2). Relative ASM was also associated with trabecular vBMD of the femoral neck, but not of the lumbar spine. While the age-adjusted relationships between relative ASM and femoral neck vBMD and trabecular vBMD were significant in women and men, additional analyses revealed a significant interaction with gender (p = 0.020 and 0.002, respectively) and a steeper slope in men compared to women. Bone area and endocortical area of the femoral neck exhibited positive associations with relative ASM in men following adjustment for age. Relative ASM was also associated with these parameters at the lumbar spine. Similar to women, relative ASM demonstrated a positive unadjusted and age-adjusted association with CtTh of the femoral neck and lumbar spine (Figures 2E and F); however, the analysis to assess the interaction with gender at the lumbar spine was significant (p = 0.032) and revealed a steeper slope in men compared to women. In the men, relative ASM was no longer associated with total vBMD or endocortical area at the femoral neck after further adjusting for both age and physical activity (Table 2).
Relative ASM is associated with cortical parameters of the peripheral skeleton
Relative ASM was not positively associated with cortical vBMD at the tibia after adjusting for age in the women (age-adjusted p = 0.054), but was at the radius. However, this association was no longer significant after adjusting for physical activity. Relative ASM showed significant unadjusted and age-adjusted associations with bone area but not endocortical area of the tibia and radius in the women, and the associations with bone area remained significant following further adjustment for physical activity (Table 3). As was observed in the central skeleton, relative ASM was strongly associated with CtTh at both the tibia and radius in the women (Figures 2C and D).
Table 3.
Pearson correlation coefficients (rp-value unadjusted/rp-value age-adjusted/rp-value age- and physical activity adjusted) between relative ASM and bone parameters at the peripheral skeletal sites in women and men. Statistics in bold are significant at p < 0.05.
| Skeletal Site/Parameter | Women | Men |
|---|---|---|
| Unadjusted/age-adjusted/age- and physical activity adjusted | Unadjusted/age-adjusted/age- and physical activity adjusted | |
| Tibia (pQCT) | ||
| Cortical vBMD | 0.28<0.001/0.120.054/0.100.119 | 0.26<0.001/0.040.480/0.050.370 |
| Bone area | 0.27<0.001/0.30<0.001/0.190.002 | 0.21<0.001/0.27<0.001/0.21<0.001 |
| Endocortical area | −0.020.685/0.060.337/−0.010.897 | −0.020.791/0.050.349/0.010.805 |
| CtTh | 0.43<0.001/0.35<0.001/0.32<0.001 | 0.35<0.001/0.30<0.001/0.28<0.001 |
| Radius (pQCT) | ||
| Cortical vBMD | 0.28<0.001/0.130.036/0.060.316 | 0.28<0.001/0.020.783/0.010.879 |
| Bone area | 0.25<0.001/0.27<0.001/0.200.002 | 0.180.001/0.29<0.001/0.23<0.001 |
| Endocortical area | −0.090.137/0.030.599/0.030.627 | −0.060.263/0.100.068/0.070.230 |
| CtTh | 0.41<0.001/0.32<0.001/0.23<0.001 | 0.34<0.001/0.21<0.001/0.170.002 |
| Radius (HRpQCT) | ||
| BV/TV | 0.37<0.001/0.32<0.001/0.24<0.001 | 0.170.005/−0.040.512/−0.050.370 |
| TbN | 0.49<0.001/0.45<0.001/0.34<0.001 | 0.190.001/0.25<0.001/0.190.001 |
| TbTh | 0.23<0.001/0.180.005/0.130.044 | 0.100.100/−0.160.006/−0.150.016 |
| TbSp | −0.45<0.001/−0.40<0.001/−0.30<0.001 | −0.22<0.001/−0.20<0.001/−0.140.016 |
Similar to women, relative ASM was associated with bone area but not endocortical area of the tibia and radius in the men prior to and following adjustment for age (Table 3). Relative ASM also showed a significant unadjusted and age-adjusted association with CtTh at the tibia and radius (Figures 2G and H). However, no significant age-adjusted relationships were observed between relative ASM and cortical vBMD at the tibia or radius in men. Of note, all significant age-adjusted relationships between relative ASM and bone parameters at the tibia and radius in men remained significant after further adjusting for physical activity (Table 3).
Relative ASM is associated with trabecular microarchitecture in men and women
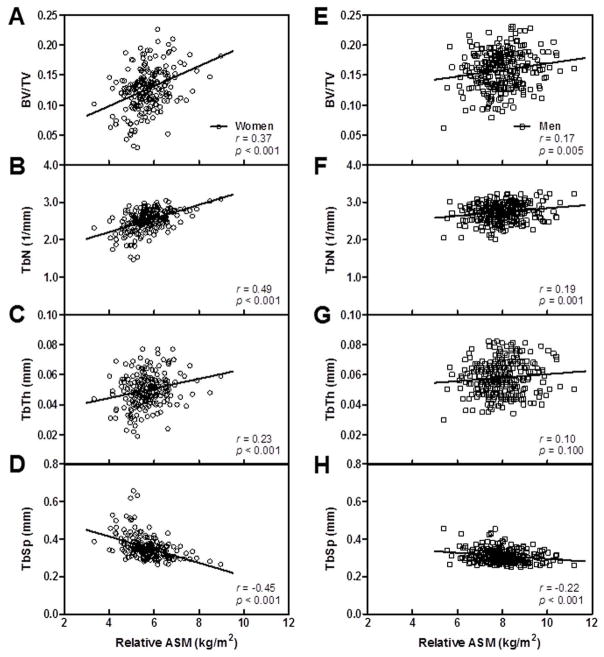
Assessment of bone microarchitecture at the radius using HRpQCT revealed strong positive unadjusted and age-adjusted relationships between relative ASM and BV/TV and TbN in women (Table 3 and Figures 3A and B). Significant relationships were also observed between relative ASM and TbTh (Figure 3 C). Correspondingly, strong inverse unadjusted and age-adjusted correlations were observed with TbSp (Figures 3A and B). The significant age-adjusted relationships between relative ASM and the parameters of bone microarchitecture at the radius remained after further adjusting for physical activity in the women (Table 3).
Figure 3.
Relation of relative appendicular skeletal muscle mass (ASM) to trabecular microarchitecture of the ultradistal radius in women (A–D) and men (E–H).
In men, the relationships between relative ASM and trabecular microarchitecture were not as consistent as those observed in women (Table 3). Following age-adjustment, there were significant relationships between relative ASM and TbN, but not BV/TV. After adjusting for age, relative ASM surprisingly showed a negative association with TbTh. Additional analyses of the association between relative ASM and TbTh in all subjects revealed a significant interaction with gender (p = 0.021) and showed that women had a steeper slope than men. As in the women, relative ASM had a significant inverse association with TbSp in the men. All of the age-adjusted correlations between relative ASM and the bone microarchitectural parameters in the men remained significant following adjustment for physical activity.
Relative ASM is associated with circulating biomarkers
To further assess the interaction between skeletal muscle and bone, we examined the association between relative ASM and mediators of skeletal homeostasis. Significant inverse unadjusted and age-associated associations were observed in women between relative ASM and the bone turnover markers, osteocalcin, CTX and TRAP (Table 4). The associations between relative ASM and the latter two markers were no longer significant after adjusting for both age and physical activity levels. In the men, OC was associated with relative ASM after adjusting for age, but not age and physical activity (Table 4). PINP and TRAP were also associated with relative ASM; however, no relationships were observed with these or the other bone turnover markers after adjusting for age.
Table 4.
Pearson correlation coefficients (rp-value unadjusted/rp-value age-adjusted/rp-value age- and physical activity adjusted) between relative ASM and bone turnover markers, sex steroids, and components of the IGF system in women and men. Statistics in bold are significant at p < 0.05.
| Variable | Women | Men |
|---|---|---|
| Bone turnover markers | Unadjusted/age-adjusted/age- and physical activity adjusted | Unadjusted/age-adjusted/age- and physical activity adjusted |
| Osteocalcin | −0.20<0.001/−0.20<0.001/−0.150.016 | 0.010.904/−0.110.044/−0.100.093 |
| PINP | −0.090.153/−0.090.128/−0.070.271 | 0.20<0.001/0.000.935/−0.020.705 |
| CTX | −0.150.011/−0.130.037/−0.080.196 | 0.070.185/−0.060.313/−0.050.380 |
| TRAP | −0.20<0.001/−0.130.040/−0.100.092 | −0.160.006/−0.060.327/−0.050.424 |
| Sex steroids | ||
| Mass spec E2 | 0.26<0.001/0.130.036/0.080.182 | 0.090.122/0.060.269/0.050.426 |
| Mass spec Bio E2 | 0.33<0.001/0.22<0.001/0.180.006 | 0.34<0.001/0.120.030/0.110.051 |
| Mass spec E1 | 0.25<0.001/0.150.012/0.120.054 | −0.000.963/0.040.466/0.030.621 |
| Mass spec T | −0.050.384/−0.110.071/−0.100.123 | −0.080.157/−0.150.010/−0.130.027 |
| Mass spec Bio T | 0.21<0.001/0.190.002/0.180.003 | 0.34<0.001/−0.020.790/0.010.796 |
| SHBG | −0.180.003/−0.26<0.001/−0.22<0.001 | −0.42<0.001/−0.20<0.001/−0.20<0.001 |
| IGF system | ||
| IGF-I | 0.150.013/0.001.000/0.070.288 | 0.37<0.001/0.150.009/0.150.008 |
| IGF-II | 0.080.176/0.060.326/0.050.392 | 0.22<0.001/0.120.040/0.130.023 |
| IGFBP-3 | 0.140.023/0.060.361/0.110.065 | 0.33<0.001/0.110.053/0.130.020 |
| IGFBP-2 | −0.34<0.001/−0.25<0.001/−0.170.006 | −0.47<0.001/−0.26<0.001/−0.24<0.001 |
In the women, relative ASM was significantly associated with E2 and Bio E2 before and after adjusting for age (Table 4). E1 and SHBG also demonstrated positive and negative associations with relative ASM, respectively. Relative ASM exhibited unadjusted and age-adjusted associations with Bio T. After adjusting for age and physical activity, the relationships between relative ASM and E2 and E1 were no longer significant. In men, relative ASM was positively associated with Bio E2 and inversely associated with T (Table 4). Correspondingly, relative ASM was also associated with SHBG; however, no age-adjusted associations were observed with Bio T in men. After further adjusting for physical activity, the relationships between relative ASM and Bio E2 were no longer significant.
In women, IGFBP-2 was inversely associated with relative ASM before and after adjusting for age (Figure 4A) and physical activity (Table 4). No other significant relationships between relative ASM and components of the IGF system in women were observed after adjusting for age (Table 4). Among the panel of circulating biomarkers, IGFBP-2 demonstrated the strongest unadjusted, age-adjusted, and age- and physical activity-adjusted inverse association with relative ASM in the men (Table 4, Figure 4B). Relative ASM was also positively associated with IGF-1 and IGF-2 in the men. These age-adjusted associations also remained significant even after further adjusting for physical activity.
Figure 4.
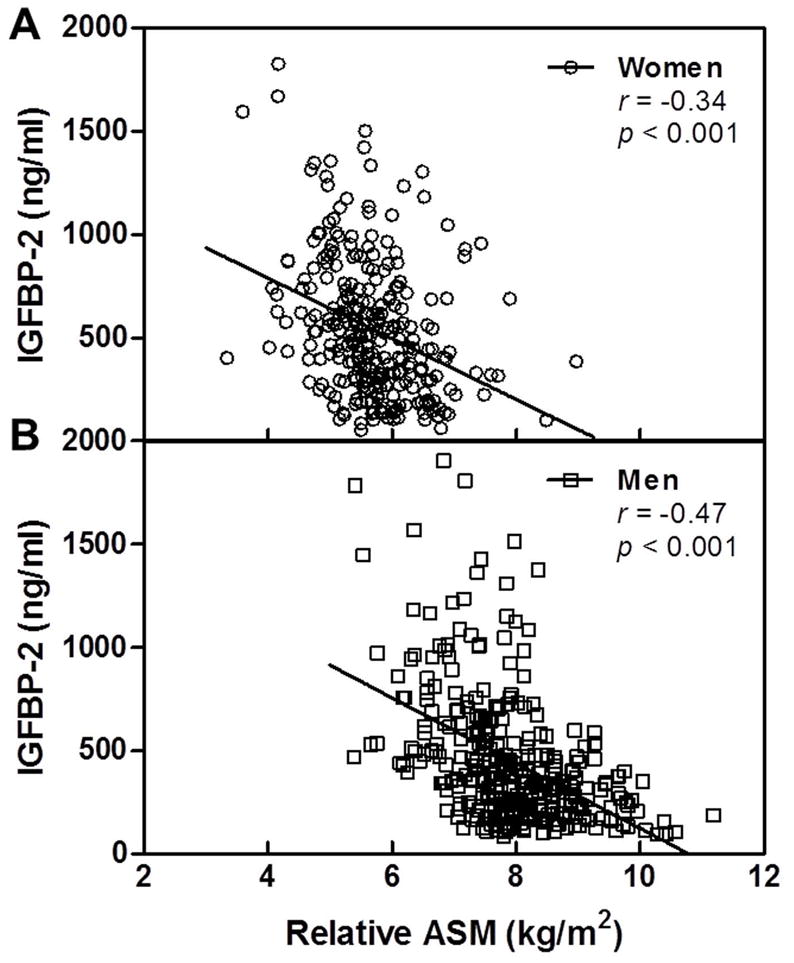
Relation of IGFBP-2 to relative appendicular skeletal muscle mass (ASM) in women (A) and men (B).
DISCUSSION
The major finding of the present study is that skeletal muscle mass relative to body size is significantly associated with cortical and trabecular bone geometry and microstructure at multiple skeletal sites in adult women and men. In addition, we observed that IGFBP-2, a factor whose circulating concentrations we previously reported to be associated with low BMD and high bone resorption markers (26,45), was associated with low relative ASM in both sexes. These data add to the growing body of evidence supporting the highly integrated nature of skeletal muscle and bone, and they provide new insights into potential biomarkers that reflect the health of the musculoskeletal system.
Relative ASM was predictive of cortical bone parameters, and in particular, CtTh, in women and men. The mechanical influence of skeletal muscle on bone has long been recognized, and may in part mediate the relationships we observed between it and cortical bone, particularly at load-bearing sites such as the femoral neck, lumbar spine and tibia. However, we observed that the age-adjusted relationships between relative ASM and CtTh (in the women and men), cortical vBMD (in the women), and, importantly, proximal femur strength (in women and men) remained significant even after adjusting for physical activity. Indeed, recent studies have challenged the prevailing notion that skeletal loading is a major determinant of BMD. Thus, in adult men aged 20 to 72 years, Blain et al. reported that relative ASM was the strongest factor associated with DXA-derived BMD at the femoral neck, but independent of habitual skeletal loads assessed by measures of physical activity and muscle strength (46). Similarly, Melton and colleagues concluded that age-related changes in parameters of bone strength at multiple skeletal sites were not determined by indices of skeletal loading in women or men ranging in age from 20 to 90 years (43). In line with these studies, we found that the relationship between relative ASM and CtTh at the radius, a non-load-bearing skeletal site, was also quite strong. These findings raise new questions regarding the underlying biological mechanisms that, in addition to mechanotransduction, regulate parameters of skeletal muscle and bone health in what appears to be a coordinated fashion.
The strong relationship between relative ASM and trabecular microstructure at the radius is of particular interest. The advent of HRpQCT (“non-invasive bone biopsy”) has permitted more sensitive assessments of bone quality not encompassed by either DXA BMD measurements or pQCT trabecular and cortical vBMD measurements, and revealed that trabecular bone loss starts in young adulthood in both sexes and continues throughout life (16). In the present study, we observed that relative ASM was highly correlated with trabecular number and equally, but inversely, associated with trabecular separation at the radius in women and to a slightly lesser extent in men. A number of studies have now associated fewer and more separated trabeculae with the incidence of distal forearm (Colles’) fracture in postmenopausal women (47,48). Whether or not the higher relative ASM of men contributes to their greater TbN and TbTh that cooperatively increase their bone strength and fracture resilience is unknown (16). Moreover, whether or not interventions that increase relative ASM, such as resistance training, confer benefit upon trabecular microarchitecture in women at an elevated risk for fracture is an important area for future investigation.
It should be noted that following adjustment for age and physical activity, relative ASM was not associated with trabecular BV/TV at the radius in the men, but was positively associated with trabecular number. It seems this apparently paradoxical result was due to the concomitant inverse association of relative ASM with trabecular thickness. Thus, higher relative ASM was associated with an increased number of thinner trabeculae, resulting in no net association with BV/TV. This was not the case in women, where relative ASM as positively associated with TbN, TbTh, and BV/TV. The underlying reasons for these discrepant findings regarding the relationship of relative ASM with trabecular microstructure in men versus women are unclear.
The mechanisms that may underlie the coordinated regulation of relative ASM and bone geometry and microstructure remain poorly defined, but at least three distinct possibilities are noteworthy. First, similar regulatory factors and associated pathways may mediate both skeletal muscle and bone health. As noted earlier, several endocrine hormones play a key role in each tissue, and these are thought to contribute to changes across the lifespan. Clearly, sex steroid deficiency is an important factor in cortical and trabecular bone loss in postmenopausal women (16,19,49), and we observed modest associations between relative ASM and serum E1, Bio E2 and, in particular, SHBG levels in women even after adjusting for age. In the men, relative ASM was positively associated with Bio E2, but inversely associated with both T, and more impressively, SHBG levels, and unassociated with Bio T after adjusting for age. T has long been recognized for its anabolic effects on skeletal muscle (50); however, it is not evident that the age-related declines in muscle mass or in bone parameters are causally related to the parallel decrease in Bio T levels. Serum Bio E2 has been shown to fall below the threshold that accelerates bone loss in men in the seventh and eight decades; however, how low levels of Bio E2 may impact skeletal muscle health in older men, or even younger men, is unknown. Second, physical activity encompasses mechanical, metabolic, hormonal and neural stimuli that have the potential to impact both skeletal muscle and bone. The extent to which the effects of physical activity on bone are mediated by skeletal muscle remains unclear. The use of more objective and discriminating metrics of physical activity than self-report such as accelerometry may provide greater insight. Third, there is strong evidence that a communication network exists between skeletal muscle and bone, which includes muscle- and bone-derived secreted factors now commonly referred to as myokines and osteokines, respectively (12). For example IGF-1 and fibroblast growth factor-2 (FGF-2) represent potent myokines that promote bone formation. While both are produced in and secreted by skeletal muscle, their receptors are localized to periosteum at the muscle-bone interface (51). In addition, very recent evidence suggests that osteocyte-derived Wnt3a, one component of the Wnt signaling axis that is critical for bone development and function, augments myogenesis and myofiber size in culture (13). Identifying myokines and osteokines and understanding their regulation in the context of aging, injury, physical activity and disease are promising areas for future investigation.
Of particular interest in the context of coordinated regulation of skeletal muscle and bone health was the significant inverse relationship between relative ASM and serum IGFBP-2 levels. Components of the IGF signaling axis have attracted considerable attention in studies of aging, cardiovascular disease, diabetes, and osteoporosis (e.g., (26,52–54)). Serum concentrations of IGF-1 and IGFBP-3 decrease with advancing age, while IGFBP-2 levels increase. The regulation of IGFBP-2 is not well understood. It has been reported to be a component of the senescence-associated secretory phenotype, or SASP (55), that has been strongly supported to be a fundamental driver of age-related tissue dysfunction (56). In addition to being a product of senescent cells, IGFBP-2 levels have also been reported to be inversely associated with those of insulin in women and men (though not acutely regulated by either glucose or insulin administration) (57), and levels of growth hormone in men (58). Previously, we reported that high IGFBP-2 levels were the strongest predictor of low BMD by DXA, particularly among men and postmenopausal women, independent of age and bioavailable sex steroids (26). We also observed that high serum IGFBP-2 concentrations were associated with bone resorption markers, including urine and serum cross-linked N-telopeptides (NTX) in these same cohorts, as well as serum CTX levels that were only assessed in women (45). In the present study, we discovered that circulating concentrations of IGFBP-2 were inversely associated with relative ASM in both women and men. High IGFBP-2 levels have been linked to osteoporotic fractures in men (59); and, intriguingly, they have also been associated with poor physical performance and disability in older men (60). Additional work is warranted to determine whether or not elevated levels of IGFBP-2 mediate and/or serve as a biomarker of deleterious changes in skeletal muscle that lead to impairments in strength and functional decline in later life. To date, a circulating biomarker of skeletal muscle health has remained elusive.
In summary, using state-of-the-art imaging technologies, we observed that relative ASM is associated with parameters of cortical and trabecular bone geometry, strength and microarchitecture in women and men. In part, these observations raise new questions regarding the apparently coordinated regulation of skeletal muscle and bone. In line with this query, we identified IGFBP-2, a factor we previously associated with lower BMD and increased bone resorption, as a biomarker of lower relative ASM in both sexes. How IGFBP-2 directly, or indirectly through modulation of the IGF signaling axis, contributes to the maintenance and/or degradation of the musculoskeletal system and/or accurately reflects its health, requires further study.
Acknowledgments
Funding: This work was supported by NIH Grants AR027065 and UL1-RR24150 (Center for Translational Science Activities), funds from Mayo Clinic and a generous gift from Robert and Arlene Kogod
We would like to thank Mr. James Peterson for help with manuscript preparation and Ms. Elizabeth Atkinson for advice regarding statistical analyses.
Footnotes
Study design: NKL, SJA, LMJ, SA, SK. Study conduct: SK. Data collection: SK, SA. Data analysis: NKL, SJA, SK. Data interpretation: NKL, SJA, LMJ, SA, SK. Drafting manuscript: NKL, SK. Revising manuscript content: NKL, SK, LJM. Approving final version of manuscript: NKL, SJA, LMJ, SA, SK. NKL takes responsibility for the integrity of the data analysis.
Contributor Information
Nathan K. LeBrasseur, Email: lebrasseur.nathan@mayo.edu.
Sara J. Achenbach, Email: achenbach.sara@mayo.edu.
L. Joseph Melton, III, Email: melton.j@mayo.edu.
Shreyasee Amin, Email: amin.shreyasee@mayo.edu.
Sundeep Khosla, Email: khosla.sundeep@mayo.edu.
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of Low Femoral Bone Density in Older U.S. Adults from NHANES III. Journal of Bone and Mineral Research. 1997;12(11):1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of Applied Physiology. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 11.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamrick MW. A Role for Myokines in Muscle-Bone Interactions. Exercise and Sport Sciences Reviews. 2011;39(1):43–47. doi: 10.1097/JES.1090b1013e318201f318601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Suarez S, Mo C, Lara N, Jaehn K, Johnson M, Bonewald L, Brotto M. The β-Catenin Activating Factor, Wnt3a, Stimulates Skeletal Myogenesis. J Bone Miner Res. 2011;26(Suppl 1) Available at http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007458-11007429. [Google Scholar]
- 14.Proctor DN, Melton LJ, Khosla S, Crowson CS, O’Connor MK, Riggs BL. Relative influence of physical activity, muscle mass and strength on bone density. Osteoporos Int. 2000;11 (11):944–952. doi: 10.1007/s001980070033. [DOI] [PubMed] [Google Scholar]
- 15.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., 3rd Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11(6):857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 16.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 18.Khosla S, Melton LJ, 3rd, Riggs BL. Clinical review 144: Estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87(4):1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26 (3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh M, Hunter G, Livingstone M. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporosis International. 2006;17(1):61–67. doi: 10.1007/s00198-005-1900-x. [DOI] [PubMed] [Google Scholar]
- 21.Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Molecular Aspects of Medicine. 2005;26(3):181–201. doi: 10.1016/j.mam.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Greenlund LJS, Nair KS. Sarcopenia--consequences, mechanisms, and potential therapies. Mechanisms of Ageing and Development. 2003;124(3):287–299. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 23.Patrick G, Elisabeth S-R, Pierre DD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355(9207):898–899. doi: 10.1016/s0140-6736(99)05463-x. [DOI] [PubMed] [Google Scholar]
- 24.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 25.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu J-L, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. The Journal of Clinical Investigation. 2002;110(6):771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin S, Riggs BL, Atkinson EJ, Oberg AL, Melton LJ, 3rd, Khosla S. A potentially deleterious role of IGFBP-2 on bone density in aging men and women. J Bone Miner Res. 2004;19 (7):1075–1083. doi: 10.1359/JBMR.040301. [DOI] [PubMed] [Google Scholar]
- 27.Amin S, Riggs BL, Melton LJ, 3rd, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(6):799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- 28.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 29.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71 (3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 30.Melton LJ, 3rd, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clin Proc. 2000;75(Suppl):S10–12. discussion S12–13. [PubMed] [Google Scholar]
- 31.Camp JJ, Karwoski RA, Stacy MC, Atkinson EJ, Khosla S, Melton LJ, Riggs BL, Robb RA. A system for the analysis of whole-bone strength from helical CT images. Proceedings of SPIE. 2004;5369:74–88. [Google Scholar]
- 32.Cann CE. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166(2):509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 33.Kalender WA, Felsenberg D, Genant HK, Fischer M, Dequeker J, Reeve J. The European spine phantom: a tool for standardization and quality control in spinal bone mineral measurements by DXA and QCT. Eur J Radiol. 1995;20:83–92. doi: 10.1016/0720-048x(95)00631-y. [DOI] [PubMed] [Google Scholar]
- 34.Keaveny TM, Kopperdahl DL, Melton LJ, 3rd, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2010;25 (5):994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., III Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller R, Hahn M, Vogel M, Delling G, Ruegsegger P. Morphometric analysis of noninvasively assessed bone biopsies: comparison of high-resolution computed tomography and histologic sections. Bone. 1996;18:215–220. doi: 10.1016/8756-3282(95)00489-0. [DOI] [PubMed] [Google Scholar]
- 37.Laib A, Hilderbrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: a parameter for in vivo bone structure analysis. Bone. 1997;21(6):541–546. doi: 10.1016/s8756-3282(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 38.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6(5–6):329–337. [PubMed] [Google Scholar]
- 39.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24(1):35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 40.Parfitt AM, Mathews CHE, Villaneuva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19:1465–1471. doi: 10.1007/s00198-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83(7):2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 43.Melton LJ, 3rd, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S. Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res. 2006;21(12):1847–1855. doi: 10.1359/jbmr.060908. [DOI] [PubMed] [Google Scholar]
- 44.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Amin S, Riggs BL, Melton LJ, 3rd, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res. 2007;22(6):799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- 46.Blain H, Jaussent A, Thomas E, Micallef JP, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Rossi M, Picot MC. Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Experimental Gerontology. 2010;45(9):679–684. doi: 10.1016/j.exger.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Melton LJ, 3rd, Riggs BL, van Lenthe GH, Achenbach SJ, Muller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22(9):1442–1448. doi: 10.1359/jbmr.070514. [DOI] [PubMed] [Google Scholar]
- 48.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22 (3):425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 49.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 50.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. Journal of Clinical Endocrinology & Metabolism. 1996;81(10):3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 51.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- 52.Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. European Journal of Endocrinology. 2009;160(1):25–31. doi: 10.1530/EJE-08-0452. [DOI] [PubMed] [Google Scholar]
- 53.Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. Journal of the American Geriatrics Society. 2009;57(7):1213–1218. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359(9319):1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 55.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature cell biology. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CLEMMONS DR, SNYDER DK, BUSBY WH. Variables Controlling the Secretion of Insulin-Like Growth Factor Binding Protein-2 in Normal Human Subjects. Journal of Clinical Endocrinology & Metabolism. 1991;73(4):727–733. doi: 10.1210/jcem-73-4-727. [DOI] [PubMed] [Google Scholar]
- 58.Mûnzer T, Rosen CJ, Harman SM, Pabst KM, Clair CS, Sorkin JD, Blackman MR. Effects of GH and/or sex steroids on circulating IGF-I and IGFBPs in healthy, aged women and men. American Journal of Physiology - Endocrinology And Metabolism. 2006;290(5):E1006–E1013. doi: 10.1152/ajpendo.00166.2005. [DOI] [PubMed] [Google Scholar]
- 59.Meier C, Nguyen TV, Center JR, Seibel MJ, Eisman JA. High IGFBP-2 levels are associated with an increased risk of osteoporotic fractures in elderly men. J Bone Miner Res. 2005;20(S1; S36) doi: 10.1359/JBMR.041207. [DOI] [PubMed] [Google Scholar]
- 60.van den Beld AW, Blum WF, Pols HA, Grobbee DE, Lamberts SW. Serum insulin-like growth factor binding protein-2 levels as an indicator of functional ability in elderly men. Eur J Endocrinol. 2003;148(6):627–634. doi: 10.1530/eje.0.1480627. [DOI] [PubMed] [Google Scholar]