Abstract
Cytoplasmic domains of gap junction proteins (connexins) are involved in channel gating, voltage and pH sensitivity, and contain binding sites for partner proteins. However, their secondary structure is incompletely characterized and comparisons among the connexins is totally lacking. Circular dichroism (CD) was used to study the conformational properties of synthetic peptides corresponding to the highly divergent amino acid sequences of cytoplasmic domains of connexin (Cx)32, Cx36, and Cx43. We report that whereas peptides were largely unstructured in aqueous buffer, certain peptides in 30% trifluoroethanol (TFE) showed considerable helical content. These structured peptides correspond to analogous regions in each of the three connexin cytoplasmic domains. This first comparative study of conformational properties of connexin cytoplasmic domains reveals protein domains that may play similar roles in channel function and protein-protein interactions.
Keywords: gap junctions, secondary structure, peptides, spectroscopy, circular dichroism
Introduction
The connexin family of gap junction proteins (with more than 20 murine and human members1) form channels that interconnect cells in almost all vertebrate tissues, allowing intercellular passage of current-carrying ions and signaling molecules. Sequence variability between connexins results in a diversity of gap junction channel properties (such as ion selectivity and sensitivity to gating stimuli: see Ref. 2) as well as binding partners (including enzymes responsible for posttranslational processing and cytoskeletal and scaffolding molecules: see Ref. 3).
Connexin proteins display characteristic topology, with four transmembrane domains separating cytosolic NH2— (NT) and COOH-termini (CT), two extracellular loops and a cytosolic loop (CL) region connecting the second and third transmembrane domains (Supporting Information Figure 1). The domain most divergent among connexins is CT while the most conserved domains are the transmembrane segments. References 4 and 5 showed that for two very different connexins, liver connexin26 (Cx26) and connexin43 (Cx43), the most abundant connexin in heart, the transmembrane domains are helical in nature and may reflect a property shared by other connexin transmembrane domains. The only structural study involving extracellular domains (EL) focused on Cx32, abundantly expressed in liver, and concluded that EL domains likely contain beta sheet structures, based on mutagenesis studies.6 Intracellularly, the only study of an NT involved Cx26, which was shown to have helical features,7 while the structures of the other two cytoplasmic domains, CL and CT, which vary the most in size and sequence among connexins, have been elucidated for Cx43. Structural studies on the CT and CL domains of Cx43 have shown that they contain helical segments that may be modified by pH or protein-protein interactions.8–11 These studies provide a mosaic overview of connexin structure obtained on different connexins using various techniques, and issues that remain unexplored include whether different connexins contain homologous structured motifs and the degree to which such structures are dynamic and modifiable, especially in the CL and CT domains, where diversity is highest.
As an initial step to characterize structural features of cytoplasmic domains of different connexins, we have examined synthetic peptides corresponding to the NT, CL, and CT segments of three phylogenetically distinct connexins (Cx43, expressed predominantly in astrocytes and cardiac myocytes and encoded by gene Gja1; Cx32, found mainly in liver hepatocytes and myelinating glia and encoded by Gjb1; and Cx36, found in neurons and pancreatic islet cells, encoded by Gja9). For each peptide (see Table I and Supporting Information Figure 1), we determined circular dichroism (CD) spectra in aqueous solvent and in the presence of TFE, which we have used to probe helical propensity as it is believed to stabilize secondary protein structure, in particular helical configurations, and protects against denaturation. In the presence of this cosolvent, certain peptides were demonstrated to contain helical structure; topological location of several of the structured domains roughly corresponded in the three distantly related connexins. Such demonstration for helical propensity in analogous structured regions irrespective of amino acid sequence suggests a convergence in connexin secondary structure most likely for proper folding and protein-protein interactions.
Table I. Peptide Information and Summary of Results.
| Peptide | Residues | aaa | Peptide Sequence | Buffer % Helixb | 30% TFE % Helixb |
|---|---|---|---|---|---|
| Cx32-NT | 1–22 | 22 | MNWTGLYTLLSGVNRHSTAIGR | 6 | 63 |
| Cx32-CL1 | 97–116 | 20 | HQQHIEKKMLRLEGHGDPLH | 2 | 19 |
| Cx32-CL2 | 112–130 | 19 | GDPLHLEEVKRHKVHISGT | 5 | 59 |
| Cx32-CT1 | 208–230 | 23 | EVVYLIIRACARRAQRRSNPPSR | 7 | 61 |
| Cx32-CT2 | 215–234 | 20 | RACARRAQRRSNPPSRKGSG | 4 | 15 |
| Cx32-CT3 | 232–251 | 20 | GSGFGHRLSPEYKQNEINKL | 5 | 24 |
| Cx32-CT4 | 252–271 | 20 | LSEQDGSLKDLRRSPGTGA | 5 | 41 |
| Cx32-CT5 | 263–283 | 21 | LRRSPGTGAGLAEKSDRCSAC | 3 | 10 |
| Cx36-NT | 1–24 | 24 | MGEWTILERLLEAAVQQHSTMIGR | 18 | 90 |
| Cx36-CL1 | 99–119 | 21 | HQSAKQRERRYSTVFLALDRD | 6 | 24 |
| Cx36-CL2 | 135–154 | 20 | SGGGKREDKKLQNAIVNGVLW | 5 | 55 |
| Cx36-CL3 | 175–195 | 21 | TPHPSGLRTASKSKLRRQEGIW | 2 | 46 |
| Cx36-CT1 | 272–292 | 21 | LNHLGWRKIKLAVRGAQAKRK | 2 | 68 |
| Cx36-CT2 | 300–321 | 21 | KDLPRVSVPNFGRTQSSDSAYV | 2 | 7 |
| Cx43-NT | 1–23 | 23 | MGDWSALGKLLDKVQAYSTAGGK | 4 | 74 |
| Cx43-CL1 | 95–114 | 20 | HVFYVMRKEEKLNKKEEELK | 2 | 24 |
| Cx43-CL2 | 119–144 | 26 | DGVNVEMHLKQIEIKKFKYGIEEHGK | 13 | 53 |
| Cx43-CT0 | 227–248 | 22 | ELFYVFFKGVKDRVKGKSDPYH | 6 | 31 |
| Cx43-CT1 | 232–245 | 14 | CFFKGVKDRVKGRSD | 9 | 28 |
| Cx43-CT2 | 241–260 | 20 | KGRSDPYHATTGPLSPSKDC | 6 | 10 |
| Cx43-CT3 | 255–276 | 22 | SPAKDCGSQKYAYFNGCSSPTA | 5 | 7 |
| Cx43-CT4 | 271–287 | 17 | CSSPTAPLSPMSPPGYK | 7 | 10 |
| Cx43-CT5 | 285–306 | 22 | GYKLVTGDRNNSSCRNYNKQAS | 2 | 8 |
| Cx43-CT6 | 302–321 | 20 | NKQASEQNWANYSAEQNRMG | 4 | 24 |
| Cx43-CT7 | 312–336 | 25 | NYSAEQNRMGQAGSTISNSHAQPFD | 4 | 20 |
| Cx43-CT8 | 336–354 | 19 | DFPDDNQNAKKVAAGHELQC | 5 | 35 |
| Cx43-CT9 | 346–360 | 16 | CKVAAGHELQPLAIVD | 3 | 16 |
| Cx43-CT10 | 363–382 | 20 | PSSRASSRASSRPRPDDLEI | 4 | 16 |
Number of amino acids in peptide.
Percent of peptide in helical conformation, obtained from analysis of CD spectra.
Results
Peptide Spectra in Aqueous Solvent
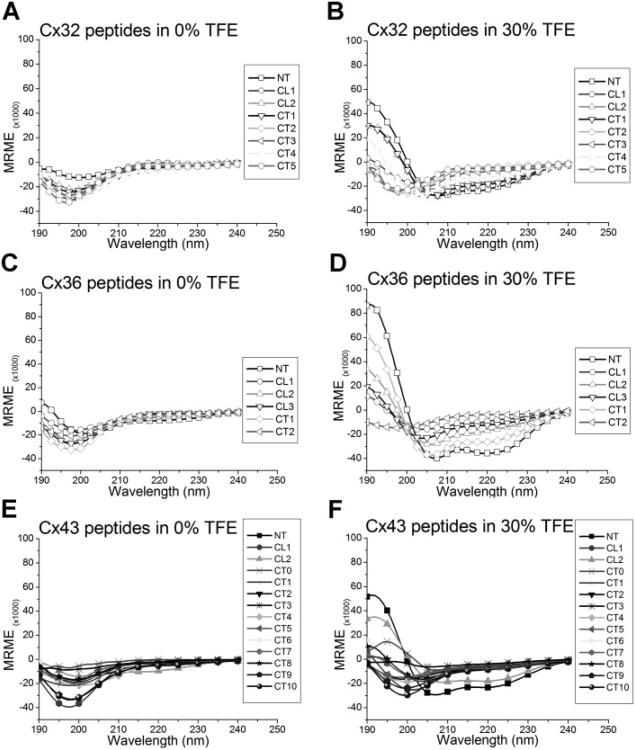
Helical content is characterized in CD spectra by a peak maximum at 190 nm and two peak minima around 208 and 222 nm. Random or flexible state of proteins is characterized by a peak minimum centered at 198 nm. When peptides were scanned in aqueous solution, almost all of the 26 connexin peptides examined had a peak minimum at 198 nm and little ellipticity at 222 nm (Figure 2A–2C). Two exceptions were spectra of Cx36-NT (Figure 2B) and Cx43-CL2 (Figure 2C), both of which demonstrated peak minima wavelength shifts from 198 to 202 nm, as well as more negative ellipticity values at 222 nm. Together, these results suggest there is a larger helical contribution to the CD spectra of these two peptides in aqueous solution than the other peptides examined. The lack of detectable structure in most peptides tested likely reflects the low probability that these short strands of amino acids attain a folded state in aqueous solvent.
Figure 2.
CD spectra of Cx32, Cx36, and Cx43 cytoplasmic peptides in aqueous solution (A, B, C, respectively) or supplemented with 30% TFE (D, E, F, respectively). Most peptides in buffer alone show a peak minimum at 198 nm, characteristic of a flexible, random structure. In 30% TFE, several peptides demonstrate complex secondary structure profiles, with many showing helical characteristics (typically, a peak maximum at 190 nm and two peak minima at 208 and 222 nm). Some peptides retain their random structure as evidenced by the presence of a peak minimum near 198 nm.
Effects of TFE on Peptide Folding
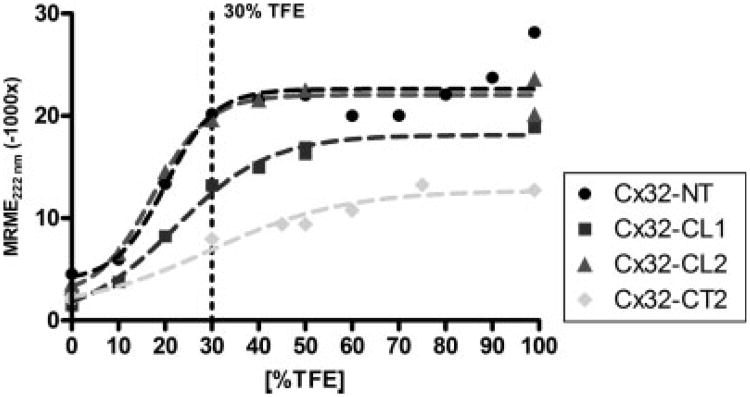
As observed above, peptides in solution lack long-distance protein interactions for proper folding and as a result maintain a flexible nature that obscures the study of inherent secondary structural information. To probe the peptides for helical secondary structures, the lipophile TFE (2,2,2-trifluoroethanol) was added to the solvent, generally at a concentration of 30% as suggested by previous studies (For examples see Refs. 12 and 13). As illustrated in Figure 1, we evaluated the TFE stabilization effect on helical conformations, represented by an increase in the negative mean residue molar ellipticity (MRME) values at 222 nm, for peptides Cx32-NT, Cx32-CL1, Cx32-CL2, and Cx32-CT2 at various TFE concentrations (0–99%). Very low TFE concentrations provided minimal helix stability consistent with similar experiments reported in the literature (e.g. myelin basic protein peptides).14 However, the helical content increased approaching 30% TFE for most of these peptides, with little additional structural stability being achieved at higher concentrations (EC50 values were 18–21% TFE).
Figure 1.
Comparison of peptide helical structure content in four peptides at 0–99% TFE concentration. The titration curves demonstrate that for three peptides their inherent structure begins stabilizing at low TFE concentrations and reach half maximal structures around 20% TFE. Nonlinear regressions of mean residue molar ellipticity (MRME) values for Cx32-NT, -CL1, -CL2, and CT2 shows half-maximal values (EC50) at 20.1, 21.3, 18.1, and 29.5% of TFE. Note that Cx32-CT2 peptide demonstrates little structure even at high TFE. The dotted line marks 30% TFE.
Overall Characteristics of Peptide Structures in 30% TFE
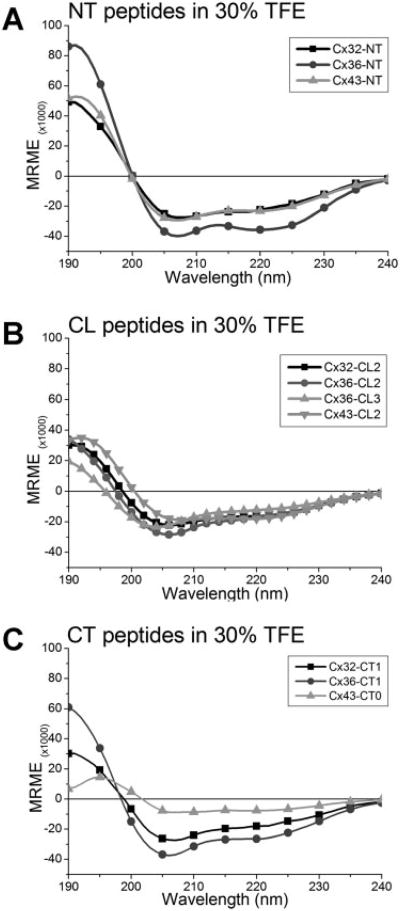
The spectra of most cytoplasmic connexin peptides in 30% TFE (Figure 2, panels D–F) showed dramatic differences compared to aqueous solution alone. The spectra of several peptides which appeared disordered in aqueous buffer, characterized with a peak minimum at 198 nm, obtained more ordered structures, demonstrated by the appearance of a peak maximum at 190 nm and a marked minimum around 208 nm, when exposed to TFE. Specifically, peptide spectra from Cx32-NT, -CL2, -CT1, -CT4; from Cx36-NT, -CL2, -CL3, and -CT1 (Figure 2E); and Cx43-NT and -CL2 (Figure 2F) showed more negative ellipticity values at 222 nm, a determinant of more helical structure profiles. It is interesting to note that certain peptides which showed little helical tendencies in buffer alone, suddenly presented highly ordered states with TFE, such as the NT peptides of Cx32 and Cx43, several CL peptides and a few CT peptides of all three connexins. Comparison of these spectra in 30% TFE highlights the similarities contained in peptides representing analogous domains (see Figure 3). Thus, the NT peptides (Figure 3A), the second half of the cytoplasmic loop (CL) of all three connexins (Figure 3B), and the proximal region of the carboxyl terminus for peptides from Cx32 and Cx36 (Figure 3C), demonstrated highly comparable CD spectra, with peak maxima at 190 nm and peak minima at 208 and 222 nm, as expected for a structure with substantial helical content. All NT peptides had similar spectral profiles suggesting a homologous structural component. This was similar for the Cx32 and Cx36 CT1 peptides. An exception to similarity is noted in Cx43-CT0 peptide which did not appear to have as much helical content as the other two connexin peptides.
Figure 3.
Comparison between CD spectra of Cx32, Cx36, and Cx43 peptides in 30% TFE. (A) All three connexin-NT peptides showed spectral profiles closely matching typical helical reference spectra. (B) Similarly, peptides from the distal cytoplasmic loop (CL) region of all three connexins demonstrated comparable CD spectra. Proximal CL (CL1) peptides demonstrated little structural complexity and are therefore not shown. (C) The proximal domain of all three connexins (Cx32-CT1, Cx36-CT1, and Cx43-CT0) showed that Cx32 and Cx36 share similar helical structure profiles, whereas a more flexible structure was observed for Cx43. Other peptides had little highly ordered structure and were therefore not compared.
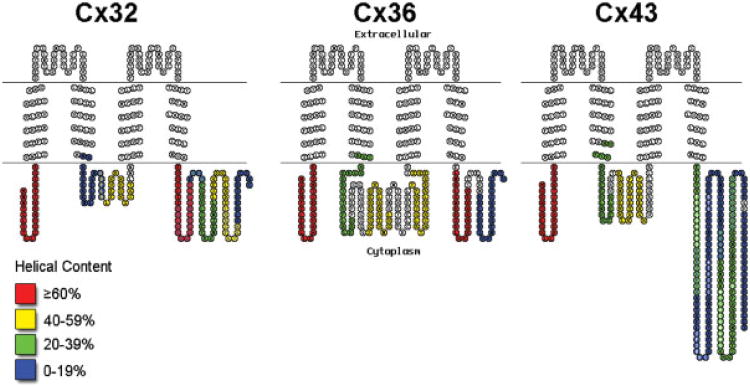
Results from spectral fitting of CD spectra for each peptide (Table I) revealed that a large number of peptides in 30% TFE exhibited greater than 40% helical structure. Other peptides showed moderate (20–39%) helical content. The 10 peptides that did not show moderate (<20%) helical content in 30% TFE had spectra with a peak minimum around 198 nm (indicative of a random coil) with little characteristics of any other secondary structure, suggesting a lack of helical propensity in these peptides. The estimation of helical content for each peptide studied is plotted as pseudo-color displays superimposed on the topology of connexins (see Figure 4), providing a global view of the three connexins and the homologous presence of helical structure. It is clear using this perspective that the NT and parts of the CL and CT domain contain considerable helical structural content in all three connexins and at relatively similar locations, even though the lengths (and amino acid sequences) of these domains are not the same.
Figure 4.
Modified TOPO2 diagrams of Cx32, Cx36, and Cx43 color-coded with the helical content from CD analysis of peptides observed in 30% TFE. Overlapping peptide regions with different color codes are denoted by circles filled with the preceding peptide's color and letters colored for the following peptide's code. Where two peptides with same helical content (color code) overlap, white letters mark the overlapping residues between peptides.
Discussion
Electrophysiological and imaging studies have provided detail about similarities and differences in functional properties of gap junctions formed by different connexins,2 biochemical studies have examined posttranslational modifications and revealed binding partners,15 and molecular biology has provided insights into interactions of connexin genes and genes encoding other proteins.16–18 By contrast, there have been few reports detailing structural features of cytosolic connexin domains, and virtually none comparing one connexin with another. The main conclusion from the present work is that TFE was able to reveal helical tendencies in similarly located regions within Cx32, Cx36, and Cx43.
NH2-Terminus
The current study presents, for the first time, data on the structure of the NT of Cx32, Cx36, and Cx43, and also on the relative similarities of these highly ordered domains. The only previously studied amino terminal domain is that of Cx26, studied by Purnick et al., applying proton 2D NMR to elucidate the structure of a peptide corresponding to the first 15 amino acids.7 The authors concluded that a helical structured terminal region, containing mostly hydrophobic residues, was present and was 9 residues long. The Cx26 NT sequence shares 59% sequence identity with Cx32, which led Purnick et al. to suggest that Cx32-NT may assume a similar structure as Cx26-NT. Certainly, the sequence conservation is much higher, at 82% (Supporting Information Figure 2), reiterating the likelihood of a common structure in these two connexins. This extrapolation is not as certain for Cx36-NT and Cx43-NT, which share only 38 and 39% identity, respectively, with Cx26 (Supporting Information Figure 2), although sequence conservation is higher at 58 and 52%, respectively. Secondary structure prediction programs such as Jpred and PORTER predict helical structures near the amino end for Cx32, Cx36, and Cx43 (Supporting Information Figure 3) (Cx36 having the highest predicted percentage of helical content, followed by Cx43 and then Cx32). Thus, we expected to observe relatively high helical content for our NT peptides. The CD data presented here, albeit with longer peptides encompassing virtually the entire NT domain, indicate that all three connexins present substantial helical structure propensity (63, 90, and 74% helical structure in this domain for Cx32, Cx36, and Cx43 respectively), and suggests a common structure-function relationship for this domain in all four connexins. The secondary structure present in this domain would result in the alignment of residues along a helical facet, an important feature given that the NT domain is believed to play a role in sensing voltage change across the channel and may interact with residues lining the transmembrane and extracellular domains.19,20 For the NT to enter the channel pore, a hinge region has been hypothesized to exist, which in Cx26 is marked by a flexible double glycine motif. Our finding of a higher helical content for Cx36-NT suggests a longer helix that could modify NT binding affinities and could explain the low voltage sensitivity observed for this connexin.21
Full-length secondary structure predictions (not shown) demonstrate a likely second helix in the NT near its site of entry into the membrane. If this is the case, then TFE may stabilize conformations that require long range interactions normally present in intact proteins.
Cytoplasmic Loop
Our CD measurements indicate that the second half of this domain has a high helical propensity in all three connexins: 59, 55, and 53% for Cx32, Cx36, and Cx43, respectively. This similarity is striking given that the CL2 peptide from Cx32 is only 17 and 23% identical to Cx36 and Cx43 (29 and 39% conserved, respectively), while Cx36 is 23% identical to Cx43 (33% conserved; see Supporting Information Figure 2). Thus, even though the peptides share similar helical content, their sequence identity and conservation is low, suggesting that the structures formed might be more closely linked to function rather than to primary sequence. Of the three connexin cytoplasmic loops, only Cx43 has been studied in detail by NMR8 showing two alpha helices (11 residues out of 26, or 42% helical content, in segments aa122–127 and aa139–143) in the region covered by Cx43-CL2 (aa119–144) whose structure and binding affinity to the Cx43-CT was markedly affected by lowering pH.22
The first half of this domain has not been studied before and our results demonstrate a low helical content for all three connexins (19–25%); this is in contrast to secondary structure prediction software which suggests helical content in the first and not the second half of the CL.
COOH-Terminus
The CT domain is the least conserved among the three connexin proteins and may play a role in determining the connexin- and cell type-specific functions of gap junction channels formed of these proteins. If we allow for some gaps, Cx32 full-length CT is 27% and 21% identical to Cx36-CT and Cx43-CT, respectively; while Cx43-CT and Cx36-CT are 11% identical. Although the divergent sequences in all three connexins result in their functional characteristics, such as unique protein partners and biophysical properties, nevertheless, the fact that the CT domain plays a role in gating and pH sensitivity in several connexins2 suggests that some structural conservation remains. Our CD studies indicate that this domain contains two distinctive regions with helical propensity: one near the membrane (peptides covering juxtamembranal regions, i.e. Cx32-CT1), with 61, 68, and 31% helical content in Cx32, Cx36, and Cx43, respectively; and a second located on the distal part of the tail (with the highest helical content for all CT peptides of Cx32 and Cx43 at 35%). The similarity in helical propensity for these peptides is once again contrasted by the low sequence identity between the juxtamembranal peptides Cx32-CT1 and Cx36-CT1 (17% identical, 35% conserved) or Cx32-CT1 and Cx43-CT0 (22% identical, 48% conserved), while Cx36-CT1 and Cx43-CT0 are 18% identical and 46% conserved (Supporting Information Figure 2). The two distal peptides from Cx32 and Cx43 that show similar high helical propensity also have low sequence similarity (20%), and interestingly they both contain a number of charged residues, which would align along a face of a helical wheel.
Sorgen et al.9 have described alpha helical structures in their 3D NMR study of the CT domain of Cx43, involved in CT dimerization and pH-dependent binding to the CL domain.22 These domains are believed to play a role in channel gating by interacting with one another and this phenomenon is regulated by such environmental cues as changes in pH. Given that pH-sensitivity is a prominent feature of gap junction channels, it is therefore of interest to determine if high order structure is part of the mechanism of response. In agreement with the Sorgen et al. study, peptides Cx43-CT6, Cx43-CT7, and Cx43-CT8, which encompass these helical regions observed by NMR, are the only peptides to demonstrate high helical propensity. The correlation between the low pH structure by NMR and the TFE-stabilized helices by CD indicate that the CD spectra on peptides in cosolvent can predict regions of propensity for helical structure. Although a topologically similar region of structure was detected in our CD studies of Cx32 peptides, it is unknown whether it is involved in pH sensitivity. Furthermore, a proximal region of Cx43-CT is missing from the study by Sorgen et al., covered by peptides -CT0 and -CT1, and warrants further study as we have observed high helical structure propensity and it has been shown to bind to tubulin.23
Helical structure obtained from CD experiments using 30% TFE have generally been found to be similar to that observed in the protein by other methods; however, our interpretation of these findings critically depends on the assumption that TFE minimally induces non-native structure in the peptides analyzed and faithfully reports structural features observed with other spectroscopic techniques. We are aware that the interpretation of results obtained using 2,2,2-Trifluoroethanol (TFE) merits caution. Since introduced as a cosolvent in spectroscopic studies by Goodman and Listowsky,24 TFE is commonly used to enhance NMR spectra and to optimize both NMR and CD structural studies.25,26 TFE at moderate concentrations (generally <40%) has been shown to stabilize helical folding of peptides and proteins that are unfolded in aqueous solution. Published reports show that in 30% TFE many peptides and proteins form structures similar to those predicted from secondary structure programs.27,28 and closely match structures obtained by X-ray crystallography or NMR spectroscopy in solutions without TFE.28–30 Both of the latter techniques take advantage of high protein concentration, highly ordered lattices (crystals) or selection of the most probable ordered structures NMR to obtain the conformational states of proteins. It is important to point out that the stabilization of helical conformation in peptides and proteins by TFE is not indiscriminate, but rather reflects underlying structural properties of the amino acid residues within the peptides.31–33 Nevertheless, there are reported studies using full length proteins that contain beta sheet segments, as observed by crystallography or NMR, where beta sheets have been observed to fold into helical conformation under high concentrations of TFE, due to the disruption of long range hydrophobic interactions,34–36 thus resulting in overestimation of the helical content and underestimating the contributions of beta sheets. Thus, evidence favors the interpretation that TFE may stabilize inherent helical structures that may be unordered in aqueous solutions due to unstable hydrophobic interactions and the lack of the contributions of neighboring residues in the full length proteins.
We have successfully used CD to determine the helical propensity of cytoplasmic domains of connexins using corresponding short peptides in 30% TFE. Although other techniques such as NMR yield higher resolution maps than CD, we believe CD allows rapid screening of regions of interest that may predict propensity for structural complexity. The helical domains predicted by CD correspond to homologous regions in the cytoplasmic domains of all three connexins examined. It is conceivable that the structurally homologous domains mediate folding or binding by similar mechanisms, thereby fulfilling requirements for trafficking, gating and interactions with other proteins in the gap junction complex. For example, future studies should determine, as with Cx43, whether Cx32-CL and -CT domains interact, given the structural similarities and pH-sensitivity of these two connexins.37 Residue differences in homologous locations and variability in the length of their domains between connexin isoforms (as exemplified by the three connexins studied here) could result in different folding kinetics or binding affinities that could endow the macromolecular complex with a variety of properties depending on the composition of the gap junction channels (The Nexus3). Application of CD on remaining connexin isoforms may further determine similar structural characteristics.
Mutations
It is yet unknown whether diseases caused by mutations in Cx32, like X-linked Charcot-Marie-Tooth (CMTX),38 and oculodentodigital dysplasia (ODDD), due to mutations in Cx43,39 are a result of aberrant secondary structures, leading to the disruption of gap junction function and loss of signaling pathways necessary for cell function. Several human single-point mutations occur in sequences covered by peptides which we have found to exhibit structure, and it is possible that some may disrupt function through misfolding. To speculate that given amino acids glycine (G), serine (S), proline (P), asparagine (N), and aspartic acid (D) favor disorder, CMTX mutants lying in the NT domain, such as W3S, T8P, G12S, H16P, I20S, would be expected to decrease helical content in this peptide; K124N, V125D, S128P in the Cx32-CL2 peptide; E208G and R220G are the most likely to disrupt helices in Cx32-CT1 peptide. We have observed similar predictions using secondary structure prediction programs (not shown) highlighting these mutations as likely for future structural studies. CD is ideal for screening the effects of these mutations on native structural conformations and could complement more precise techniques such as NMR. Meanwhile, what is known for these mutants is that W3S and G12S do not form channels, as well as a man-made mutant similar to E208G (E208K) which does not reach the plasma membrane.40–42 Mutation R220G was observed to go to the plasma membrane but channel function was not determined.43 Cx43 ODDD mutants that lie on structured peptides reported in this study and that are predicted to cause considerable structural change would be Y17S and S18P in the NT domain, and G143S in Cx43-CL2. Interestingly, Y17S was shown to traffic to the cell surface but does not form cell-cell channels44,45 and neither does G143S.46 It is important that we study the effects mutations have on protein structure if we are to design effective therapeutic strategies that target reversing or bypassing protein misfolding.
In conclusion, we believe further use of CD and TFE to probe the effect of these disease-causing mutations and changes in the physico-chemical environment (pH or the presence of other domains) on connexin structure may provide a tool to study and enrich our knowledge of connexins and determine both the presence of high order structures and the susceptibility of these structured domains to mutations.
Materials and Methods
Chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Assignment of extracellular, cytoplasmic and transmembrane domains to the primary sequences was obtained from public sequence annotations in combination with predictions from a transmembrane hidden Markov modeling program, TMHMM,47 and from alignments (see below) of Cx32, Cx36, and Cx43 with regard to residues with similar physico-chemical characteristics.
Synthetic Peptides
Cx32 (Swissprot accession number P08034), Cx36 (Q9UKL4/O70610) and Cx43 (P08050) sequences were used for the design and synthesis of 26 corresponding peptides (see Table I and Supporting Information Figure 1) using F-moc chemistry, as described previously,48 at the Albert Einstein laboratory for macromolecular analysis and proteomics (LMAP). The length of peptides were chosen to cover domains of interest from previous studies and regions known to play a role in protein-protein interactions. Several of these regions also were predicted to contain secondary structures from prediction programs. Some peptides contain extra cysteine or tryptophan residues to facilitate quantification. Peptides were centrifuged at 20,000 rpm for 30 min at 4°C after dilution to remove precipitates.
CD Measurements
CD spectra of synthetic peptides were obtained at 100 to 200 μM concentration. Peptides were dissolved in 20 mM sodium/potassium phosphate/Tris buffers, pH 7.4, at 23°C or supplemented with 30% TFE. Five accumulated spectra were obtained using a J-720 spectropolarimeter (JASCO, Easton, MD) with a xenon light source, a 0.5 mm path length cell, over a 185–255 nm range, at 0.5 nm resolution, 20 mdeg sensitivity, 2 sec response and at 50 nm/min scan rate.
CD Spectral Analysis
CD spectra between 185–255 nm were subtracted from background (buffer without peptide) and converted from CD intensity to mean residue molar ellipticity (deg cm2/dmol.residue, MRME). Most peptide spectra obtained in aqueous solvent were smoothed. For full spectral analysis, CDPro49 was used to calculate helical content of peptides and predict the number of amino acid residues.50 The percentage of regular and distorted alpha helices and beta sheets, beta turns and random structures, which totaled 100% ± 2% for all peptides (and were accepted only if the root mean square deviation (rmsd) between the observed and calculated value was below 0.25) was combined and averaged across experiments, however, predicted beta sheet, beta turn and random coil structure content are not presented here. Helical content is predicted with higher correlation coefficient and less RMS deviation than for other secondary structures and given that beta sheets appear mostly unordered in aqueous solution51 (e.g. Figure 2, no CD spectra show peak maxima at about 198 nm and peak minima about 215 nm, characteristic of beta sheet content) we therefore emphasize only the extent of helical structure in each peptide. We obtained similar helical content percentages using MRME values at 222 nm (not shown, Ref. 52).
Programs
Alignments (Supporting Information Figure 2) were performed with ClustalW,53 EMBOSS54 and Peptool demo software (Biotools, Edmonton, Canada). TOPO2 diagrams were obtained online (Johns SJ, http://www.sacs.ucsf.edu/TOPO-run/wtopo.pl). Jpred (ver. 3) was used to obtain secondary structure predictions (Supporting Information Figure 3) of peptides55 together with PORTER.56 Microsoft Excel (Microsoft Corp., Redmond, WA), Graphpad Prism ver4 (GraphPad Software, San Diego, CA), DOS ver6 (Microsoft Corp., Redmond, WA), Jasco software (JASCO, Easton, MD) and Microcal Origin 5.0 (OriginLab Corp., Northampton, MA) were used to calculate, tabulate, analyze and graph the predictions and spectra.
Supplementary Material
Acknowledgments
The authors are grateful for the assistance of Mr. Edward Nieves, Dr. Glen McLachlan, Mrs. Lisa Mintz, Dr. Heather Duffy, and Dr. Ruth Angeletti. The authors thank Dr. Paul Sorgen, Dr. Irving Listowsky, Dr. Elliot Hertzberg, and Dr. Steven Almo for comments on earlier drafts of this manuscript.
Contract grant sponsor: NIH
Contract grant numbers: DK41918, MH65495, NS41282
Footnotes
Additional Supporting Information may be found in the online version of this article.
Electronic Supplementary Material: We have included three Supporting Information figures that complement the manuscript by providing additional information on the peptides used (FORT_SupplFig1.tif) or add to the discussion of our results by providing pairwise alignments of homologously structured peptides, with the number of identical and conserved (similar physico-chemical properties) residues (FORT_SupplFig2.tif) or secondary structure predictions (FORT_SuppFig3A/B/C.tif).
Publisher's Disclaimer: This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at biopolymers@wiley.com
References
- 1.Sohl G, Willecke K. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 2.Harris AL. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 3.Duffy HS, Fort AG, Spray DC. Adv Cardiol. 2006;42:1–17. doi: 10.1159/000092550. [DOI] [PubMed] [Google Scholar]
- 4.Unger VM, Kumar NM, Gilula NB, Yeager M. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 5.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Proc Natl Acad Sci USA. 2007;104:10034–10039. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foote CI, Zhou L, Zhu X, Nicholson BJ. J Cell Biol. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Arch Biochem Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 8.Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- 9.Sorgen PL, Duffy HS, Cahill SM, Coombs W, Spray DC, Delmar M, Girvin ME. J Biomol NMR. 2002;23:245–246. doi: 10.1023/a:1019892719979. [DOI] [PubMed] [Google Scholar]
- 10.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 11.Sorgen PL, Duffy HS, Spray DC, Delmar M. Biophys J. 2004;87:574–581. doi: 10.1529/biophysj.103.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez-Alvarado M, Blanco FJ, Niemann H, Serrano L. J Mol Biol. 1997;273:898–912. doi: 10.1006/jmbi.1997.1347. [DOI] [PubMed] [Google Scholar]
- 13.Reiersen H, Rees AR. Protein Eng. 2000;13:739–743. doi: 10.1093/protein/13.11.739. [DOI] [PubMed] [Google Scholar]
- 14.Martenson RE, Park JY, Stone AL. Biochemistry. 1985;24:7689–7695. doi: 10.1021/bi00347a028. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Hertzberg EL, Spray DC. J Biol Chem. 2005;280:7941–7948. doi: 10.1074/jbc.M410548200. [DOI] [PubMed] [Google Scholar]
- 16.Iacobas DA, Iacobas S, Li WE, Zoidl G, Dermietzel R, Spray DC. Physiol Genomics. 2005;20:211–223. doi: 10.1152/physiolgenomics.00229.2003. [DOI] [PubMed] [Google Scholar]
- 17.Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC. Biochim Biophys Acta. 2005;1711:183–196. doi: 10.1016/j.bbamem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Spray DC, Iacobas DA. J Membr Biol. 2007;218:39–47. doi: 10.1007/s00232-007-9049-5. [DOI] [PubMed] [Google Scholar]
- 19.Verselis VK, Ginter CS, Bargiello TA. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 20.Oh S, Abrams CK, Verselis VK, Bargiello TA. J Gen Physiol. 2000;116:13–31. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. J Biol Chem. 2007;282:5801–5813. doi: 10.1074/jbc.M605233200. [DOI] [PubMed] [Google Scholar]
- 23.Giepmans BN, Verlaan I, Moolenaar WH. Cell Commun Adhes. 2001;8:219–223. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- 24.Goodman M, Listowsky I. J Am Chem Soc. 1962;84:3770–3771. [Google Scholar]
- 25.Alexandrescu AT, Ng YL, Dobson CM. J Mol Biol. 1994;235:587–599. doi: 10.1006/jmbi.1994.1015. [DOI] [PubMed] [Google Scholar]
- 26.Olofsson S, Baltzer L. Fold Des. 1996;1:347–356. doi: 10.1016/S1359-0278(96)00050-8. [DOI] [PubMed] [Google Scholar]
- 27.Lehrman S, Tuls J, Lund M. Biochemistry. 1990;29:5590–5596. doi: 10.1021/bi00475a025. [DOI] [PubMed] [Google Scholar]
- 28.Luidens M, Figge J, Breese K, Vajda S. Biopolymers. 1996;39:367–376. doi: 10.1002/(SICI)1097-0282(199609)39:3%3C367::AID-BIP8%3E3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Sönnichsen F, Van Eyk J, Hodges R, Sykes B. Biochemistry. 1992;31:8790–8798. doi: 10.1021/bi00152a015. [DOI] [PubMed] [Google Scholar]
- 30.Howard M, Smales C. J Biol Chem. 2005;280:22582–22589. doi: 10.1074/jbc.M501480200. [DOI] [PubMed] [Google Scholar]
- 31.Kemmink J, Creighton T. Biochemistry. 1995;34:12630–12635. doi: 10.1021/bi00039a019. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Buck M, Pitkeathly M, Kotik M, Haynie D, Dobson C, Radford S. J Mol Biol. 1995;252:483–491. doi: 10.1006/jmbi.1995.0513. [DOI] [PubMed] [Google Scholar]
- 33.Schönbrunner N, Wey J, Engels J, Georg H, Kiefhaber T. J Mol Biol. 1996;260:432–445. doi: 10.1006/jmbi.1996.0412. [DOI] [PubMed] [Google Scholar]
- 34.Fregeau Gallagher N, Sailer M, Niemczura W, Nakashima T, Stiles M, Vederas J. Biochemistry. 1997;36:15062–15072. doi: 10.1021/bi971263h. [DOI] [PubMed] [Google Scholar]
- 35.Najbar L, Craik D, Wade J, Salvatore D, McLeish M. Biochemistry. 1997;36:11525–11533. doi: 10.1021/bi970730s. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Modig K, Halle B. Biochemistry. 2003;42:13708–13716. doi: 10.1021/bi035330l. [DOI] [PubMed] [Google Scholar]
- 37.Morley GE, Taffet SM, Delmar M. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- 39.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Am J Hum Genet. 2003;72(2):408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin PE, Mambetisaeva ET, Archer DA, George CH, Evans WH. J Neurochem. 2000;74:711–720. doi: 10.1046/j.1471-4159.2000.740711.x. [DOI] [PubMed] [Google Scholar]
- 41.Abrams CK, Freidin MM, Verselis VK, Bennett MV, Bargiello TA. Brain Res. 2001;900:9–25. doi: 10.1016/s0006-8993(00)03327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang HL, Chang WT, Yeh TH, Wu T, Chen MS, Wu CY. Neurobiol Dis. 2004;15:361–370. doi: 10.1016/j.nbd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Yum SW, Kleopa KA, Shumas S, Scherer SS. Neurobiol Dis. 2002;11:43–52. doi: 10.1006/nbdi.2002.0545. [DOI] [PubMed] [Google Scholar]
- 44.Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Circ Res. 2005;96:e83–e91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- 45.Lai A, Le DN, Paznekas WA, Gifford WD, Jabs EW, Charles AC. J Cell Sci. 2006;119(Part 3):532–541. doi: 10.1242/jcs.02770. [DOI] [PubMed] [Google Scholar]
- 46.Dobrowolski R, Sommershof A, Willecke K. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- 47.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 48.Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- 49.Sreerama N, Woody RW. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 50.Sreerama N, Venyaminov SY, Woody RW. Anal Biochem. 2000;287:243–251. doi: 10.1006/abio.2000.4879. [DOI] [PubMed] [Google Scholar]
- 51.Viguera AR, Jimenez MA, Rico M, Serrano L. J Mol Biol. 1996;255:507–521. doi: 10.1006/jmbi.1996.0042. [DOI] [PubMed] [Google Scholar]
- 52.Chen YH, Yang JT, Chau KH. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JD, Higgins DG, Gibson TJ. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice P, Longden I, Bleasby A. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 55.Cole C, Barber JD, Barton GJ. Nucleic Acids Res. 2008;36(Web Server issue):W197–201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bau D, Martin AJ, Mooney C, Vullo A, Walsh I, Pollastri G. BMC Bioinformatics. 2006;7:402. doi: 10.1186/1471-2105-7-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.