Abstract
Integration of the nursing discipline within cooperative groups conducting pediatric oncology clinical trials provides unique opportunities to maximize nursing’s contribution to clinical care, and to pursue research questions that extend beyond cure of disease to address important gaps in knowledge surrounding the illness experience. Key areas of importance to the advancement of the nursing discipline’s scientific knowledge are understanding the effective delivery of patient/family education, and reducing illness-related distress, both of which are integral to facilitating parental/child coping with the diagnosis and treatment of childhood cancer, and to promoting resilience and well-being of pediatric oncology patients and their families.
Keywords: Children, malignancies, nursing, clinical trials, paediatric cancer
INTRODUCTION
A key research focus for the discipline of nursing is to develop a clear understanding of the human response to illness, with an emphasis on comfort and function as key outcomes [1–4]; in pediatric oncology, this focus extends to both child and family. Integration of the nursing discipline within cooperative groups conducting pediatric oncology clinical trials provides unique opportunities to maximize nursing’s contribution to clinical care, and to pursue research questions that extend beyond cure of disease to address important gaps in knowledge surrounding the illness experience, from diagnosis through survivorship or end-of-life. In this article, we will highlight key clinical and research contributions of the nursing discipline to pediatric oncology clinical trials, utilizing experiences from the Children’s Oncology Group and its legacy cooperative groups, and will identify critical issues currently facing pediatric oncology clinical trials nursing.
HISTORICAL PERSPECTIVE OF NURSING IN THE COOPERATIVE GROUPS
The discipline of nursing has been represented in oncology cooperative clinical trials groups in North America since the 1970s [5–7] and in Europe since the 1980s [5]. The initial focus for nursing in the North American pediatric cooperative groups (Children’s Cancer Group and Pediatric Oncology Group) was on improving implementation of clinical trials by providing education for nurses concerning the care of patients enrolled on protocols, developing protocol-specific patient education materials, and contributing nursing expertise to study development, execution, and evaluation [6]. In particular, nursing contributions greatly aided study implementation by providing practical recommendations related to therapy delivery and collection of protocol-specified specimens and data [6].
Nursing research evolved within the pediatric cooperative groups in the 1980s and 1990s, with nurses leading studies to address issues identified as high-priority by the discipline, including parental understanding of the study consent and randomization process [7] and parental caregiver burden [8], as well as studies addressing issues in late effects [9] and epidemiology [10]. The merger of the North American pediatric cooperative groups in 2000 provided an opportunity to align nursing research more directly with the scientific mission of the newly formed Children’s Oncology Group (COG) [4,11]. The organizational structure for nursing research within the COG was based on a scholar team model, designed to address the paucity of doctorally-prepared nurses within the cooperative group [4,12]. This model paired teams of PhD-level nurse scientists (who had limited cooperative group experience) with masters-level advanced practice nurses (APNs, who had extensive knowledge of cooperative group processes, but limited knowledge of the theoretical underpinnings of research). Each team focused on identified research priority areas for the nursing discipline [12], including fatigue [13], cognitive/behavioral/biological outcomes of central nervous system treatment [14], adolescent/young adult coping/adjustment [15], self-care/symptom management [16], treatment decision-making [17], complementary and alternative medicine [18], and end-of-life care [19]. Implementation of this model resulted in the unanticipated benefit of a significant number of APN nurses pursuing doctoral studies, yielding a core group of primarily PhD-prepared nurses, who are well-rounded with respect to both the theoretical and practical aspects of conducting research, and who are now well-integrated within the cooperative group. This has led to refinement of the original model beyond the scholar teams to include experienced researchers, trainees, and others interested in conducting nursing research within the cooperative group.
CLINICAL INITIATIVES IN COOPERATIVE GROUP PEDIATRIC ONCOLOGY NURSING
Nursing Role in Facilitating Staging/ Stratification and Delivery of Protocol-Based Treatment
Pediatric oncology nurses are pivotal to the delivery of protocol-directed therapy for children, adolescents, and young adults with cancer enrolled on clinical trials, and nursing’s integral role on the multidisciplinary team is well recognized. Nurses are involved at the point of care, and play a key role in educating patients and families regarding staging and stratification procedures, scheduling and carrying out diagnostic procedures, and assuring accurate and timely collection of specimens critical to patient staging and stratification across biological and therapeutic protocols. Nurses assume a crucial role in administration of therapeutic agents and supportive care regimens, delivery of nursing care for patients undergoing surgery and radiation, assessment and management of therapy-related complications, and provision of patient/family education regarding home medication administration, symptom management, and self-care.
Nursing Discipline Clinical Initiatives in the Cooperative Group
Within the COG, the discipline of nursing continues to make significant contributions to clinical trial implementation through nursing involvement on protocol committees (providing a nursing perspective during the protocol development process; assuring availability of a nursing liaison for each protocol to answer questions from nurses delivering protocol care, and to address clinical issues that arise during implementation of the trial), by developing clinical resource guides (including the Pediatric Oncology Palliative and End-Of-Life Care Resource [20] and the Long-Term Follow-Up Program Resource Guide [21]), and by developing and evaluating educational materials for patients and families (e.g., the COG Family Handbook [22], Family Protocol Summaries, and “Patients and Families” content for the COG Public Website [23]). These activities are supported by an organizational structure that distributes responsibilities among several key discipline leaders (Figure 1). Additionally, two priority areas have been identified by the nursing discipline to support continued facilitation of clinical trial conduct. These include development of evidence-based clinical summaries to guide protocol-related nursing care, and the development and dissemination of instructional programs to enhance nurses’ knowledge regarding care of patients participating in ongoing clinical trials.
Figure 1.
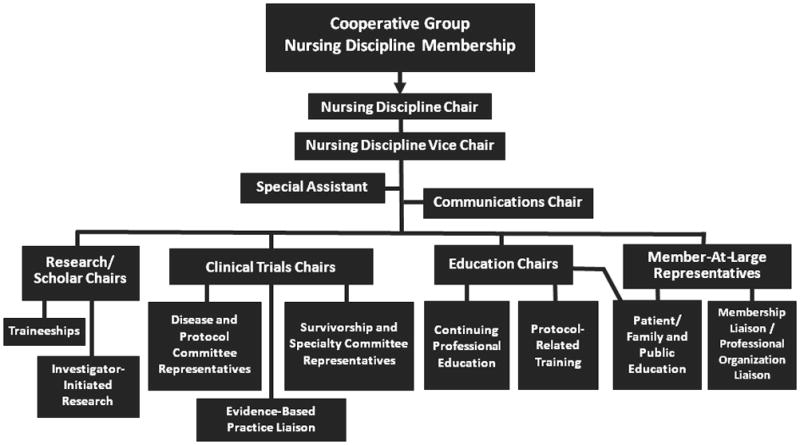
Nursing Discipline Organizational Structure within a Cooperative Group
Development of evidence-based clinical summaries
Principles of evidence-based practice (i.e., use of current best evidence in making patient care decisions [24]) are now evident in the setting of cooperative group nursing to address topics of high priority and relevance to the delivery of protocol-related nursing care. As a result, concise, clinically-focused summaries, synthesizing the latest evidence from multiple studies [25], are being developed by nurse-led teams through mentored experiences, to provide guidance for cooperative group nurses regarding best practices. These summaries will be updated every 5 years, to assure that recommendations remain aligned with current literature. Topics addressed are typically not broached in protocols, but pertinent to the provision of care to patients enrolled on clinical trials or in long-term follow-up after treatment (e.g., standardization of nursing practices related to chemotherapy administration; effective delivery of patient and family education). These topics are identified and prioritized in an iterative process involving the nursing discipline membership and leadership, and are vetted by the leadership of other relevant disciplines and focus areas within the cooperative group, such as hematology/oncology and adolescent/young adult oncology, prior to implementation. Development of evidence-based clinical summaries in the cooperative group setting has the potential to promote excellence in protocol-related nursing practice by standardizing care, reducing undesirable protocol-related nursing practice variation, and promoting best nursing practice based on scientific evidence whenever possible.
Development/dissemination of instructional programs for nurses
Providing education for nurses regarding the care of patients enrolled on protocols has been a priority for nursing since the discipline’s initial integration into cooperative group clinical trials [6]. Within the COG, the rapid dissemination of pertinent protocol information to nurses who share the responsibility for safe and effective administration and monitoring of therapy is accomplished through the ongoing development of instructional programs. These programs address pertinent protocol-related information, and are developed under the oversight of the nursing discipline leadership and disseminated to nurses in a variety of formats, including clinical trial-focused instructional sessions embedded in annual conferences of the pediatric oncology nursing professional organization (the Association of Pediatric Hematology/Oncology Nurses), multimedia educational modules accessible on a dedicated website, and educational presentations during nursing sessions held during annual cooperative group meetings. Additional strategies for dissemination of protocol-related instruction using web-based technologies (e.g., webinars) are currently being explored.
RESEARCH IN COOPERATIVE GROUP PEDIATRIC ONCOLOGY NURSING
Advances in the Field
Nurse-initiated studies
Nurse-initiated studies that have been successfully conducted within the COG over its first decade include an intervention aimed at increasing resilience and quality of life in adolescents and young adults undergoing hematopoietic stem cell transplant (ANUR0631; R01NR008583 [26–30]; evaluation of functional status and quality of life in children with medulloblastoma (ACNS0331) [31]; and comparison of parental caregiving demands between patients receiving inpatient versus outpatient methotrexate infusions (ACCL01P3).
Patient-Reported Outcome (PRO) Resource Center
As cancer survival rates continue to improve, measurement of patient-reported outcomes (e.g., symptom-related distress, quality of life, and behavioral outcomes) is increasingly being integrated into cooperative group clinical trials [32]. Measurement of PRO in pediatrics has lagged behind adult oncology as a result of methodological considerations and a paucity of available instrumentation that take into consideration patient age and developmental status [32–33]. However, progress with PRO instrument development in pediatrics continues [34], and the nursing discipline has been integrally involved in establishing a bank of validated PRO instruments for use in pediatric cooperative group research [35]. Instruments considered for the bank undergo a systematic review by a panel of experts in order to determine readiness of each instrument for use in pediatric oncology trials. For instruments determined by the expert panel as ready for implementation, a comprehensive description and details regarding the instrument’s psychometric properties, previous applications, limitations, and administration and scoring guidelines, are compiled and made available for use by investigators considering inclusion of PRO measures in protocols under development.
Nursing Research Traineeship Program
Developing the next generation of researchers committed to addressing the nursing discipline’s key knowledge gaps within the pediatric cooperative groups is essential. Within the COG, this issue is being addressed through a Nursing Research Traineeship program that provides mentored 2-year research experiences for two pre- or post-doctoral researchers every other year through a competitive application process [36].
Resilience-Focused Framework for COG Nursing Research
Based on experiences gained by nurse researchers in the cooperative group over the past decade, an organizing framework has recently been adopted to define the scope and sharpen the focus of nursing research within the COG. This framework, “Resilience in Individuals and Families Affected by Cancer” (Figure 2) is based on Haase’s Resilience in Illness Model [37]. Resilience is the ability to manage stressors in order to gain a positive outcome from a difficult experience [38]. The organizing framework is grounded in positive health constructs associated with resilience, such as courage, derived meaning, connectedness, spiritual perspective, hope, adaptability, and well-being. The framework also incorporates additional patient/family characteristics that may influence health outcomes (e.g., age, developmental level, illness and treatment-related characteristics, genetics), and depicts the theoretical and evidence-based relationships that exist between critical aspects of the patient/family cancer experience, including negative and positive influences on well-being. Negative influences include risk factors (e.g. illness-related distress, defensive coping), while positive influences include protective factors (e.g., family cohesion, courageous coping).
Figure 2.
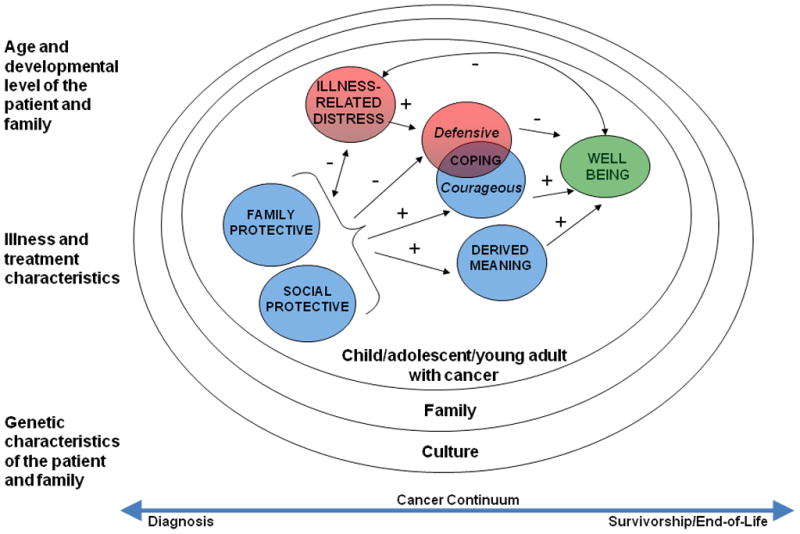
Framework for Nursing Research Adopted by the Children’s Oncology Group: Resilience in Individuals and Families Affected by Cancer (based on Haase’s Resilience in Illness Model [37]).
Constructs within circles represent key areas of influence on child and family resilience and well-being (red = negative influences; blue = positive influences; green = overall goal for care of children and families affected by cancer). Symbols depict positive (+) and negative (-) evidence-based relationships between each area of influence, based on current research findings.
The “Resilience in Individuals and Families Affected by Cancer” framework is aimed at the promotion of resilience and well-being for the child/adolescent/young adult with cancer and their family members across the cancer continuum. This framework will serve to guide the discipline’s development of new knowledge by focusing emerging research on understanding the ways individuals and their families sustain or regain optimal health in the midst of serious illness, and by grounding that research in positive health constructs, thus setting the stage for the development and testing of interventions designed to promote resilience and well-being for patients and families facing a pediatric cancer diagnosis.
KEY GAPS IN KNOWLEDGE OF IMPORTANCE TO THE FIELD
Key areas of importance to the advancement of the nursing discipline’s scientific knowledge are developing an understanding of the effective delivery of education to the patient/family, and reducing illness-related distress in children, adolescents, and young adults with cancer and their family caregivers. Both of these areas are integral to facilitating parental/child coping with the diagnosis and treatment of childhood cancer across the cancer continuum, and to promoting resilience and well-being of pediatric oncology patients and their families.
Understanding the Effective Delivery of Patient/Family Education
Providing patient/family education is a core responsibility of pediatric oncology nursing practice [39–40], and has been identified as the single most important factor in facilitating parental/child coping with the diagnosis and treatment of childhood cancer [41]. Over the past decade, much progress has been made in developing educational materials specifically for patients and families participating in pediatric oncology clinical trials. These include the COG Family Handbook for Children with Cancer (2005 [42]; 2011 [22]), content for the “Patients and Families” section of the COG public website (www.childrensoncologygroup.org), Family Protocol Summaries [23], and patient/family educational materials (“Health Links”) that accompany the COG Long-Term Follow-Up Guidelines [43]. These lay educational materials have been developed by pediatric oncology nursing experts who have capitalized on the multidisciplinary expertise available within the cooperative group to refine the content and message delivery. All materials have been rigorously reviewed (by members of multiple disciplines, including nursing, hematology/oncology, radiation oncology, patient advocates and survivorship experts) prior to dissemination. Nevertheless, to our knowledge, no hypothesis-driven research has been undertaken in the field of pediatric oncology to formally delineate the informational needs of patients/families, to describe the demographic, clinical, and psychosocial factors that affect the uptake of information, or to identify vulnerable sub-groups at highest risk for poor uptake of key information, all of which are necessary in order to develop targeted interventions to improve information uptake. Therefore, this is a key gap in knowledge and an area ripe for scientific inquiry.
Areas of potential focus for cooperative group nursing research that will address knowledge gaps related to delivering effective patient/family education in pediatric oncology include: (i) Describing the patient/family informational needs (i.e., understanding disease and treatment plan, informed consent/clinical trials, medication administration, symptom management/self-care) related to a cancer diagnosis during childhood/adolescence/young adulthood; (ii) Developing educational materials that address the informational needs of patients and family members across the cancer continuum; (iii) Describing demographic, clinical, and psychosocial factors (e.g., illness trajectory, emotional distress, learning style, literacy, culture, language, message formatting/delivery) that affect uptake of key information (i.e., disease, treatment, self-care) by patients/families, and identifying vulnerable sub-groups at highest risk for poor uptake of key information; (iv) Developing targeted interventions to improve patient/family uptake of key information related to a pediatric cancer diagnosis; (v) Developing the necessary infrastructure to standardize and disseminate state-of-the-art patient/family education materials; and (vi) Describing the impact of increased patient/family disease-, treatment-, and self-care-related knowledge on resilience and overall quality of life.
Knowledge gained from this work could be used to modify existing patient/family educational materials, to develop new materials as needed based on the findings, to determine the most effective use of these materials (e.g., method/timing of delivery [44], literacy level [45], use of technology [46]), and to develop the necessary cooperative group infrastructure to standardize and disseminate the materials. Potential trial endpoints could include disease and treatment-related knowledge, self (or parent/caregiver-delivered) care performance, patient/family coping, resilience, and quality of life. Potential opportunities within this line of research include the development of evidence-based educational interventions aimed at improving patient/family information uptake, coping, resilience and overall quality of life.
Reducing Illness-Related Distress
Management of illness-related distress (physiologic and emotional) is a key domain in pediatric oncology nursing practice [39] and has been a major focus area for nursing discipline research within the cooperative group over the past decade. The emerging field of cancer symptom science is also an area of high priority for the National Institute for Nursing Research, and the National Cancer Advisory Board has called for a significant increase in symptom-directed research [47]. Emerging methods include evaluation of patterns of treatment-related symptoms, genetic variance in cytokines and its relation with key symptoms, and the relationship between symptoms, cytokines, and neurotransmitters, which contribute to the understanding of factors (patient-treatment-environment) that may facilitate or inhibit symptom expression [48]. Several nurse researchers within the COG have expertise in symptom science, and many have conducted studies applicable to this domain [49–66]. Because symptoms are subjective in nature, incorporation of validated tools assessing patient-reported outcomes is a critical component of this work [33,35].
Currently, the nursing discipline within the COG is conducting an interventional trial (ANUR1131) aimed at reducing illness-related distress in adolescents and young adults with high-risk cancers and high potential for illness-related distress (including symptom distress). Additional areas of focus that will address knowledge gaps related to illness-related distress include: (i) Describing the symptoms (fatigue, pain, nausea, anxiety, depression, cognitive compromise) experienced by children/adolescents/young adults undergoing treatment for cancer; (ii) Understanding the host, clinical and biological basis for the symptoms experienced by children/adolescents/young adults undergoing treatment for cancer, and identifying vulnerable sub-groups at highest risk for symptom-related distress; (iii) Describing the impact of symptoms on resilience and overall quality of life; (iv) Describing the caregiver burden and distress experienced by the parents of children/adolescents/young adults undergoing treatment for cancer; (v) Developing targeted interventions to reduce symptoms, improve functional status, and promote resilience/overall well-being of children, adolescents, and young adults undergoing treatment for cancer and their families; and (vi) Developing the necessary cooperative group infrastructure to standardize the collection of patient-reported outcomes in order to describe, understand, and address illness-related distress.
Knowledge gained from this work could be used to identify vulnerable populations at high risk for symptom-related distress and to develop evidence-based interventions to prevent or alleviate treatment-related symptoms in those at highest risk; there are also significant opportunities for inclusion of PROs in symptom-focused trials. Advances in ameliorating symptom-related distress hold promise for the promotion of resilience and well-being for the child/adolescent/young adult with cancer and their family members across the cancer continuum.
CHALLENGES AND OPPORTUNITIES FOR NURSING WITHIN THE COOPERATIVE GROUPS
The focal point for nursing research and practice remains the human experience of and response to illness; this differs significantly from the survival-focused paradigm driving most cooperative group clinical trial endpoints. Yet, as differences in survival between treatment regimens continue to narrow, quality of survival is emerging as an important outcome across disciplines [67–68]. Thus, the nursing discipline is poised now more than ever to contribute its expertise toward exploring trial endpoints beyond survival, including reduction in illness-related distress, understanding delivery of effective patient/family education, and promotion of resilience for patients and families facing childhood cancer. Execution of these nursing research objectives is facilitated by the unique opportunities available within the cooperative group, including access to large and similarly-treated pediatric oncology patient populations, a biospecimen repository annotated with known treatment exposures at specific time points, and an infrastructure that facilitates data collection, protocol implementation, and close interdisciplinary collaboration. The discipline of nursing is thus well-positioned to continue to play a crucial role in implementation of pediatric cooperative group clinical trials, and to address gaps in scientific knowledge that will lead to an increased understanding of the amelioration of illness-related distress and the promotion of illness-related knowledge and self-care.
Acknowledgments
We thank the following individuals for their significant contributions to the strategic planning process for developing this Blueprint for Research for the COG Nursing Discipline: Joy Bartholomew, Joan E. Haase, Maureen Haugen, Pamela S. Hinds, Marilyn J. Hockenberry, Mary C. Hooke, Katherine P. Kelly, Carol Kotsubo, Margaret Kulm, Ida K. Moore, and Kathryn Murphy.
References
- 1.Donaldson SK, Crowley DM. The discipline of nursing. Nurs Outlook. 1978;26(2):113–120. [PubMed] [Google Scholar]
- 2.Richardson A. Sharing the research agenda: confronting what we believe to be the nursing contribution to research in cancer care. Eur J Oncol Nurs. 2006;10(2):91–92. doi: 10.1016/j.ejon.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.American Nurses Association. Nursing’s social policy statement: the essence of the profession. 2010. Silver Spring, MD: American Nurses Association; 2010. [Google Scholar]
- 4.Hinds PS, Baggott C, DeSwarte-Wallace J, et al. Online exclusive: functional integration of nursing research into a pediatric oncology cooperative group: finding common ground. Oncol Nurs Forum. 2003;30(6):E121–126. doi: 10.1188/03.onf.e121-e126. [DOI] [PubMed] [Google Scholar]
- 5.Arrigo C, Gall H, Delogne A, et al. The involvement of nurses in clinical trials. Results of the EORTC Oncology Nurses Study Group survey. Cancer Nurs. 1994;17(5):429–433. [PubMed] [Google Scholar]
- 6.Ruccione K, Kelly KP. Pediatric oncology nursing in cooperative group clinical trials comes of age. Semin Oncol Nurs. 2000;16(4):253–260. doi: 10.1053/sonu.2000.16576. [DOI] [PubMed] [Google Scholar]
- 7.Wiley FM, Ruccione K, Moore IM, et al. Parents’ perceptions of randomization in pediatric clinical trials. Children Cancer Group Cancer Pract. 1999;7(5):248–256. doi: 10.1046/j.1523-5394.1999.75010.x. [DOI] [PubMed] [Google Scholar]
- 8.Keegan-Wells D, James K, Moore IK. A pilot study to test the instrument: The care of my child with cancer. J Pediatr Oncol Nurs. 1998;15:136–137. [Google Scholar]
- 9.Fergusson J, Ruccione K, Waskerwitz M, et al. Time required to assess children for the late effects of treatment. A report from the Childrens Cancer Study Group. Cancer Nurs. 1987;10(6):300–310. [PubMed] [Google Scholar]
- 10.Ruccione KS, Waskerwitz M, Buckley J, et al. What caused my child’s cancer? Parents’ responses to an epidemiology study of childhood cancer. J Pediatr Oncol Nurs. 1994;11(2):71–84. doi: 10.1177/104345429401100207. [DOI] [PubMed] [Google Scholar]
- 11.Hinds PS, DeSwarte-Wallace J. Positioning nursing research to contribute to the scientific mission of the Pediatric Oncology Cooperative Group. Semin Oncol Nurs. 2000;16(4):251–252. doi: 10.1053/sonu.2000.16575. [DOI] [PubMed] [Google Scholar]
- 12.Ruccione KS, Hinds PS, Wallace JD, et al. Creating a novel structure for nursing research in a cooperative clinical trials group: the Children’s Oncology Group experience. Semin Oncol Nurs. 2005;21(2):79–88. doi: 10.1016/j.soncn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Hockenberry-Eaton M, Hinds PS. Fatigue in children and adolescents with cancer: evolution of a program of study. Semin Oncol Nurs. 2000;16(4):261–272. doi: 10.1053/sonu.2000.16577. discussion 272-268. [DOI] [PubMed] [Google Scholar]
- 14.Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16(4):279–290. doi: 10.1053/sonu.2000.16582. discussion 291-279. [DOI] [PubMed] [Google Scholar]
- 15.Hinds PS. Fostering coping by adolescents with newly diagnosed cancer. Semin Oncol Nurs. 2000;16(4):317–327. doi: 10.1053/sonu.2000.16590. discussion 328–336. [DOI] [PubMed] [Google Scholar]
- 16.Dodd MJ, Miaskowski C. The PRO-SELF Program: a self-care intervention program for patients receiving cancer treatment. Semin Oncol Nurs. 2000;16(4):300–308. doi: 10.1053/sonu.2000.16586. discussion 308–316. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JL, Pyke-Grimm KA, Kelly KP. Parental treatment decision making in pediatric oncology. Semin Oncol Nurs. 2005;21(2):89–97. doi: 10.1016/j.soncn.2004.12.003. discussion 98–106. [DOI] [PubMed] [Google Scholar]
- 18.Post-White J, Hawks RG. Complementary and alternative medicine in pediatric oncology. Semin Oncol Nurs. 2005;21(2):107–114. doi: 10.1016/j.soncn.2004.12.007. discussion 115–124. [DOI] [PubMed] [Google Scholar]
- 19.Nuss SL, Hinds PS, LaFond DA. Collaborative clinical research on end-of-life care in pediatric oncology. Semin Oncol Nurs. 2005;21(2):125–134. doi: 10.1016/j.soncn.2004.12.011. discussion 134–144. [DOI] [PubMed] [Google Scholar]
- 20.Ethier AM, Rollins J, Stewart JL, editors. Pediatric oncology palliative and end-of-life care resource. Glenview, IL: Association of Pediatric Hematology/Oncology Nurses; 2010. p. 140. [Google Scholar]
- 21.Landier W, editor. Long-term follow-up program resource guide: establishing and enhancing services for childhood cancer survivors. Arcadia, CA: Children’s Oncology Group; 2007. p. 236. [Google Scholar]
- 22.Murphy K, editor. The Children’s Oncology Group Family Handbook for Children with Cancer. Arcadia, CA: Children’s Oncology Group; 2011. [Google Scholar]
- 23.Kotsubo C, Murphy K. Nurturing Patient/Family Education through Innovative COG Resources. Association of Pediatric Hematology/Oncology Nurses 35th Annual Conference and Exhibit; Anaheim, CA. 2011. [Google Scholar]
- 24.Sackett DL, Strauss SE, Richardson WS, et al. Evidence-based medicine: How to practice and teach EBM. London: Churchhill Livingstone; 2000. [Google Scholar]
- 25.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing and healthcare: A guide to best practice. Philadelphia: Wolters Kluwer - Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 26.Docherty SL, Robb SL, Phillips-Salimi CR, et al. Parental perspectives on a behavioral health music intervention for adolescent/young adult resilience during cancer treatment: report from the Children’s Oncology Group. Journal of Adolescent Health. 2012 doi: 10.1016/j.jadohealth.2012.05.010. Epub ahead of print May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendricks-Ferguson VL, Cherven BO, Burns DS, et al. Recruitment Strategies and Rates of a Multi-Site Behavioral Intervention for Adolescents and Young Adults With Cancer. J Pediatr Health Care. 2012 doi: 10.1016/j.pedhc.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musick BS, Robb SL, Burns DS, et al. Development and use of a web-based data management system for a randomized clinical trial of adolescents and young adults. Comput Inform Nurs. 2011;29(6):337–343. doi: 10.1097/NCN.0b013e3181fcbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips-Salimi CR, Donovan Stickler MA, Stegenga K, et al. Principles and strategies for monitoring data collection integrity in a multi-site randomized clinical trial of a behavioral intervention. Res Nurs Health. 2011;34(4):362–371. doi: 10.1002/nur.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robb SL, Burns DS, Docherty SL, et al. Ensuring treatment fidelity in a multi-site behavioral intervention study: implementing NIH Behavior Change Consortium recommendations in the SMART trial. Psychooncology. 2011;20(11):1193–1201. doi: 10.1002/pon.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullen PL, Moore IM. Functional status, behavior, executive function and quality of life in children undergoing treatment for standard-risk medulloblastoma: the Children’s Oncology Group experience. Podium presentation at the International Symposium on Pediatric Neuro-Oncology; Toronto, Ontario, Canada. 2012. [Google Scholar]
- 32.Bruner DW, Bryan CJ, Aaronson N, et al. Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25(32):5051–5057. doi: 10.1200/JCO.2007.11.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinds PS. Patient-reported outcomes: a desirable specialty standard for oncology or an incomplete measurement approach? Cancer Nurs. 2008;31(4):259–260. doi: 10.1097/01.NCC.0000305736.26335.57. [DOI] [PubMed] [Google Scholar]
- 34.Hinds PS. Progress in quality of life in children and adolescents with cancer. Semin Oncol Nurs. 2010;26(1):18–25. doi: 10.1016/j.soncn.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Ruccione K, Hinds PS, Freyer DR. Patient-reported outcomes in the Children’s Oncology Group. APHON Counts. 2011;24(4):7. [Google Scholar]
- 36.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7):1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase JE. The adolescent resilience model as a guide to interventions. J Pediatr Oncol Nurs. 2004;21(5):289–299. doi: 10.1177/1043454204267922. discussion 300-284. [DOI] [PubMed] [Google Scholar]
- 38.Haase JE, Heiney SP, Ruccione KS, et al. Research triangulation to derive meaning-based quality-of-life theory: adolescent resilience model and instrument development. Int J Cancer Suppl. 1999;12:125–131. doi: 10.1002/(sici)1097-0215(1999)83:12+<125::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Association of Pediatric Hematology/Oncology Nurses. Scope and standards of pediatric oncology nursing practice. Glenview, IL: Association of Pediatric Hematology/Oncology Nurses; 2007. [Google Scholar]
- 40.Kelly KP, Porock D. A survey of pediatric oncology nurses’ perceptions of parent educational needs. J Pediatr Oncol Nurs. 2005;22(1):58–66. doi: 10.1177/1043454204272537. [DOI] [PubMed] [Google Scholar]
- 41.Walker C, Wright P, Curry D, et al. A Delphi study of pediatric oncology nurses’ facilitative behaviors. J Pediatr Oncol Nurs. 1993;10(4):126–132. doi: 10.1177/104345429301000402. [DOI] [PubMed] [Google Scholar]
- 42.Stormer K, editor. The Children’s Oncology Group Family Handbook for Children with Cancer. Arcadia, CA: Children’s Oncology Group; 2005. [Google Scholar]
- 43.Eshelman D, Landier W, Sweeney T, et al. Facilitating care for childhood cancer survivors: integrating children’s oncology group long-term follow-up guidelines and health links in clinical practice. J Pediatr Oncol Nurs. 2004;21(5):271–280. doi: 10.1177/1043454204268875. [DOI] [PubMed] [Google Scholar]
- 44.Aburn G, Gott M. Education given to parents of children newly diagnosed with acute lymphoblastic leukemia: a narrative review. J Pediatr Oncol Nurs. 2011;28(5):300–305. doi: 10.1177/1043454211409585. [DOI] [PubMed] [Google Scholar]
- 45.Betz CL, Ruccione K, Meeske K, et al. Health literacy: a pediatric nursing concern. Pediatr Nurs. 2008;34(3):231–239. [PubMed] [Google Scholar]
- 46.Baggott C. Patient education: to the internet and beyond. Pediatr Blood Cancer. 2011;57(1):6–7. doi: 10.1002/pbc.23123. [DOI] [PubMed] [Google Scholar]
- 47.Foley KM, Gelband H, et al., editors. National Cancer Policy Board, editor. Improving palliative care for cancer. Washington, D.C: National Academies Press; 2001. [PubMed] [Google Scholar]
- 48.Cleeland CS, Dunn AJ, Fisch MJ, editors. Cancer symptom science: measurement, mechanisms, and management. New York: Cambridge University Press; 2011. p. 349. [Google Scholar]
- 49.Baggott C. Biomarkers for mucositis assessment. Pediatr Blood Cancer. 2009;53(7):1169–1170. doi: 10.1002/pbc.22258. [DOI] [PubMed] [Google Scholar]
- 50.Baggott C, Cooper BA, Marina N, et al. Symptom cluster analyses based on symptom occurrence and severity ratings among pediatric oncology patients during myelosuppressive chemotherapy. Cancer Nurs. 2012;35(1):19–28. doi: 10.1097/NCC.0b013e31822909fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baggott C, Dodd M, Kennedy C, et al. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs. 2010;27(6):307–315. doi: 10.1177/1043454210377619. [DOI] [PubMed] [Google Scholar]
- 52.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallance K, Liu W, Mandrell BN, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46(10):1848–1855. doi: 10.1016/j.ejca.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallance K, Yang J, Li J, et al. Disturbed sleep in pediatric patients with leukemia: the potential role of interleukin-6 (-174GC) and tumor necrosis factor (-308GA) polymorphism. Oncol Nurs Forum. 2011;38(5):E365–372. doi: 10.1188/11.ONF.E365-E372. [DOI] [PubMed] [Google Scholar]
- 55.Caron JE, Krull KR, Hockenberry M, et al. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53(4):551–556. doi: 10.1002/pbc.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hockenberry MJ, Hooke MC, Gregurich M, et al. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009;31(9):664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- 57.Hockenberry MJ, Hooke MC, Gregurich M, et al. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37(1):E16–27. doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- 58.Hockenberry MJ, Hooke MC, McCarthy K, et al. Sickness behavior clustering in children with cancer. J Pediatr Oncol Nurs. 2011;28(5):263–272. doi: 10.1177/1043454211409586. [DOI] [PubMed] [Google Scholar]
- 59.Mandrell BN, Yang J, Hooke MC, et al. Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. J Pediatr Oncol Nurs. 2011;28(5):287–294. doi: 10.1177/1043454211418667. [DOI] [PubMed] [Google Scholar]
- 60.Moore IM, Miketova P, Hockenberry M, et al. Methotrexate-induced alterations in beta-oxidation correlate with cognitive abilities in children with acute lymphoblastic leukemia. Biol Res Nurs. 2008;9(4):311–319. doi: 10.1177/1099800407313268. [DOI] [PubMed] [Google Scholar]
- 61.Stenzel SL, Krull KR, Hockenberry M, et al. Oxidative stress and neurobehavioral problems in pediatric acute lymphoblastic leukemia patients undergoing chemotherapy. J Pediatr Hematol Oncol. 2010;32(2):113–118. doi: 10.1097/MPH.0b013e3181c9af84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooke MC, Garwick AW, Gross CR. Fatigue and physical performance in children and adolescents receiving chemotherapy. Oncol Nurs Forum. 2011;38(6):649–657. doi: 10.1188/11.ONF.649-657. [DOI] [PubMed] [Google Scholar]
- 63.Corey AL, Haase JE, Azzouz F, et al. Social support and symptom distress in adolescents/young adults with cancer. J Pediatr Oncol Nurs. 2008;25(5):275–284. doi: 10.1177/1043454208321117. [DOI] [PubMed] [Google Scholar]
- 64.Hockenberry M. Symptom management research in children with cancer. J Pediatr Oncol Nurs. 2004;21(3):132–136. doi: 10.1177/1043454204264387. [DOI] [PubMed] [Google Scholar]
- 65.Hockenberry M, Hooke MC. Symptom clusters in children with cancer. Semin Oncol Nurs. 2007;23(2):152–157. doi: 10.1016/j.soncn.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum. 2011;38(5):E382–393. doi: 10.1188/11.ONF.E382-E393. [DOI] [PubMed] [Google Scholar]
- 67.Calaminus G, Weinspach S, Teske C, et al. Quality of survival in children and adolescents after treatment for childhood cancer: the influence of reported late effects on health related quality of life. Klin Padiatr. 2007;219(3):152–157. doi: 10.1055/s-2007-973846. [DOI] [PubMed] [Google Scholar]
- 68.Bradley Eilertsen ME, Jozefiak T, Rannestad T, et al. Quality of life in children and adolescents surviving cancer. Eur J Oncol Nurs. 2012;16(2):185–193. doi: 10.1016/j.ejon.2011.08.001. [DOI] [PubMed] [Google Scholar]


