Abstract
Cathepsin D (CD) is a lysosomal aspartyl protease which plays an important role in α-synuclein degradation, and neuronal survival. CD knockout mice die by post-natal day 25 ± 1 due to intestinal necrosis. We analyzed the young adult male heterozygous mice, and found no behavior abnormalities in the heterozygous mice compared to wildtype littermates. LC3-II, p62, and α-synuclein levels are similar, while LAMP1 is higher in the striatum in CD heterozygous compared to wildtype mice. Interestingly, we found that dopamine and metabolites in the striatum and olfactory bulbs are at higher levels than wildtype littermates, while the DOPAC/DA and HVA/DA ratio remain similar between wildtype and CD heterozygous mice. In response to sub-chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration, dopamine, DOPAC, and HVA are depleted to similar levels in the striatum in both heterozygous and wildtype mice. Dopamine synthesizing enzyme tyrosine hydroxylase, metabolic enzyme monoamine oxidase, and catechol-O-methyltransferase (COMT) levels are similar in the striatum in wildtype and heterozygous mice. These studies provide valuable information regarding how lysosomal function may contribute to neurochemical homeostasis in animal models.
Keywords: MPTP, Monoamine, Lysosome, Heterozygotes, Behavioral assessment
1. Introduction
The autophagy lysosome pathway has come to the forefront of research in recent years as a signaling pathway which plays an important role in protein and organelle turnover. Perturbations in autophagic function are thought to underlie many disease phenotypes, particularly neurodegenerative disease. Cathepsin D (CD) is a lysosomal aspartyl protease which plays an integral role in maintaining cellular and lysosomal homeostasis. Homozygous CD knockout mice die by post-natal day 25 ± 1 from pathological events which take place in various tissues including intestinal necrosis thromboembolia, lymphopenia and neurodegeneration [15,9]. For this reason, we have focused our studies on comparisons between the CD wild type and heterozygous mice. CD heterozygotes are viable and fertile, exhibiting no gross physiological defects compared to wild type mice.
Our lab and others have shown that CD is capable of degrading the α-synuclein protein as well as reducing its aggregation and toxicity in both in vitro and in vivo models [17,14]. Aberrant accumulation of α-synuclein is thought to underlie the pathogenesis of several synucleinopathies, the most common of which is Parkinson’s disease (PD). In sporadic PD, the α-synuclein protein is thought to be at the root of disease pathogenesis. Through mechanisms which are not yet fully understood, the dopaminergic neurons in the substantia nigra pars compacta (SNpc) exhibit protein aggregates composed primary of α-synuclein [19] and eventually, these neurons degenerate and die.
Because CD is capable of decreasing α-synuclein toxicity, we also wanted to investigate whether CD haploinsufficiency would result in increased pathology in response to PD models which are dependent on the presence of α-synuclein. One such model employs the use of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) compound. MPTP is a PD mimetic which selectively targets the nigrostriatal dopaminergic pathway, causing the death of SN neurons. When given systemically via i.p. injection, MPTP crosses the blood brain barrier within minutes where it is rapidly metabolized by endogenous monoamine oxidase (MAO) to 1-methyl-4-phenylpyridinium (MPP+), the active metabolite [2]. Due to its size and charge, MPP+ can selectively cross into dopaminergic neurons via the dopamine transporter (DAT) [12], where it causes toxicity by binding to mitochondrial complex I, causing increased reactive oxygen species (ROS) and depletion of ATP levels [13]. In lieu of entering the mitochondria, MPP+ can also interact with vesicular monoamine transporters [11], translocate into synaptosomal vesicles [11], or remain in the cytosol where it can aberrantly interact with various cytosolic enzymes [8]. The effects of MPTP toxicity have been shown to be dependent on the presence of α-synuclein, as α-synuclein null mice were found to be highly resistant to MPTP toxicity [4].
We wanted to test whether CD haploinsufficiency results in increased toxicity to MPTP administration compared to wild type controls. To this end, we have employed the use of a sub-chronic MPTP regiment after which striatal biogenic amines were analyzed to assess lesion size in intoxicated animals. We have also used a battery of behavioral assays to determine whether CD+/+ and CD+/− mice exhibit any overt cognitive differences.
2. Materials and methods
2.1. Mice
All animals used in this study were male on a C57BL/6 background and between the ages of 2 and 6 months. Breeding was from both CD+/+ × CD+/− and CD+/− × CD+/− parents. Genotyping was performed using the following primers to distinguish the wildtype allele and the knockout allele: CD WT 5′: AGA CTA ACA GGC CTG TTC CC; CD WT 3′: TCA GCT GTA GTT GCT CAC ATG; CD KO 5′:CTC GTC CTG CAG TTC ATT CA; CD KO 3′: CCC CTC AGC TGT AGT TGC TC. For striatal monoamine analysis, n = 10 MPTP-treated mice per genotype were used, and n = 8 sham mice per genotype were used. For the striatal monoamine studies which combined sham wild type and heterozygous animals from several studies, a total of n = 27 mice per genotype were used. For monoamine studies in the olfactory bulb, n = 8 sham mice per genotype were used. For the Morris water maze testing, n = 20 mice per genotype were used. We used n = 5 mice per genotype in the open field test, and n = 8 mice per genotype for the rotarod. Behavioral methods can be found in the Supplemental materials. All mouse experiments were done in compliance with the University of Alabama at Birmingham Institutional Animal Care and Use Committee guidelines.
2.2. MPTP regimen
MPTP-HCl (Sigma) was diluted in saline and a total volume of ~200 μl was administered per animal per injection. Mice were injected once per day for five consecutive days with 30 mg/kg MPTP-HCl [20]. Control animals were injected with saline on the same schedule as MPTP-injected animals. With this chronic injection schedule, it has been determined that the nigrostriatal lesion is stable 21 d after the last injection [20], at which time animals were sacrificed via pentobarbital overdose. One half of the striatum was removed and flash frozen in isopentane and dry ice. This tissue was sent to the Vanderbilt Neurochemistry Core for HPLC analysis of striatal monoamine content. The other half of the striatum was flash frozen and saved for Western blot analysis. In some studies, olfactory bulb tissue was collected from mice and processed for monoamine analysis.
2.3. HPLC analysis
Striatal and olfactory bulb biogenic amine analysis was performed at the Vanderbilt Neurochemistry Core facility. Tissue Extraction. The brain sections are homogenized, using a tissue dismembrator, in 100–750 μl of 0.1 M TCA, which contains 10−2 M sodium acetate, 10−4 M EDTA, 5 ng/ml isoproterenol (as internal standard) and 10.5% methanol (pH 3.8). Samples are spun in a microcentrifuge at 10,000 × g for 20 min. The supernatant is removed and stored at −80° [3]. The pellet is saved for protein analysis. Supernatant is then thawed and spun for 20 min. Samples of the supernatant are then analyzed for biogenic monoamines. Biogenic amines: Biogenic amines are determined by a specific HPLC assay utilizing an Antec Decade II (oxidation: 0.4) electrochemical detector operated at 33 °C. Twenty μl samples of the supernatant are injected using a Water 717+ autosampler onto a Phenomenex Kintex (2.6u, 100A) C18 HPLC column (100 mm × 4.60 mm). Biogenic amines are eluted with a mobile phase consisting of 89.5% 0.1 M TCA, 10−2 M sodium acetate, 10−4 M EDTA and 10.5% methanol (pH 3.8). Solvent is delivered at 0.6 ml/min using a Waters 515 HPLC pump. Using this HPLC solvent the following biogenic amines elute in the following order: noradrenaline, MHPG, adrenaline, DOPAC, dopamine, 5-HIAA, HVA, 5-HT, and 3-MT [10]. HPLC control and data acquisition are managed by Empower software.
2.4. Western blot analysis
Frozen tissue for Western blot analysis was transferred to a glass tube and homogenized by hand with a plastic pestle in RIPA lysis buffer containing the following: 50 mM Tris pH 7.8, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS. Protease (Roche) and phosphatase (Sigma) cocktail inhibitors were added to fresh samples. Tissues were incubated on ice for 30 min prior to centrifugation at top speed (~16,700 × g) in a microcentrifuge at 4 °C. The supernatant was kept for BCA protein analysis and subsequent separation by SDS PAGE. The following primary antibodies were used for protein detection: rabbit anti-TH (Pelfreeze), goat anti-MAOB (C17, Santa Cruz), mouse anti-COMT (BD Biosciences), rabbit anti-LC3 (Sigma), mouse anti-p62 (Abnova), rat anti-LAMP1 (1D4B, Developmental Studies Hybridoma Bank), and rabbit anti-α-synuclein (Santa Cruz). The following secondary antibodies were obtained from Santa Cruz: goat anti-rabbit, goat anti-mouse, donkey anti-rat and donkey anti-goat. Image J was used to quantify the Western band intensities.
2.5. Statistical analysis
All data were analyzed using either two way analysis of variance (ANOVA) (data which required grouping) or t-tests (paired data sets).
3. Results
3.1. Both CD+/+ and CD+/− mice exhibit MPTP-induced decrease of striatal TH and monoamine levels
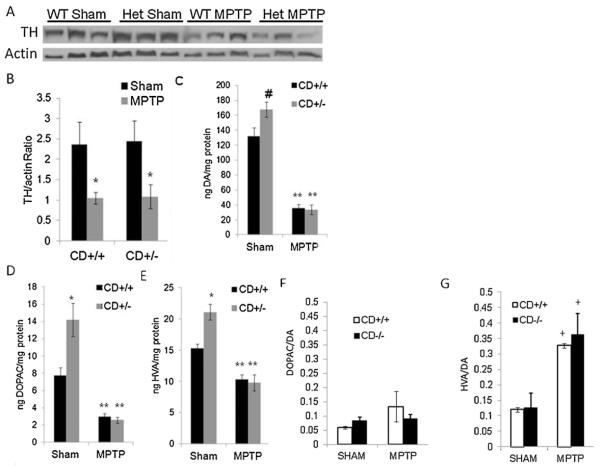
Substantia nigra dopaminergic neurons project into the striatum and TH is expressed in the processes of these neurons. We found that the striatal TH levels in both CD+/+ and CD+/− mice are decreased after mice are exposed to sub-chronic MPTP. Western blot analyses showed that MPTP administration resulted in a significant lesion in the nigrostriatal pathway, as evidenced by the significant decrease in striatal TH levels between sham and MPTP-treated mice (Fig. 1A and B, p < 0.01 sham > MPTP). Despite evidence for the significant lesion, there did not appear to be any differences in TH levels between CD+/+ and CD+/− mice post-MPTP, with each group experiencing a similar decline compared to sham animals (Fig. 1A and B, p > 0.05 CD+/+ = CD+/− for sham and MPTP groups).
Fig. 1.
Both CD+/+ and CD+/− mice exhibit MPTP-induced decrease in TH in the striatum. (A) Western blot analyses of TH protein levels in striatal protein extracts after sham or MPTP treatment. (B) Quantification of Western blot data from (A) showing that TH levels are significantly reduced in both CD+/+ and CD+/− mice in response to MPTP treatment. Data shown are mean ± SEM (*p < 0.01 sham > MPTP). There was no significant difference in TH levels between CD+/+ and CD+/− mice in the sham or MPTP-treated groups (p > 0.05). (C). Striatal DA levels are significantly higher in CD+/− mice than CD+/+ mice and are significantly reduced in both CD+/+ and CD+/− mice after MPTP. Data shown are mean ± SEM (#p < 0.05 sham CD+/− > sham CD+/+ and **p < 0.001 sham > MPTP). (D) Striatal DOPAC levels are significantly higher in CD+/− mice compared to CD+/+ mice and are significantly reduced in both CD+/+ and CD+/− mice after MPTP. Data shown are mean ± SEM (*p < 0.01 sham CD+/− > sham CD+/+ and **p < 0.001 sham > MPTP). (E) Striatal HVA levels are significantly higher in sham CD+/− mice compared to sham CD+/+ mice and are significantly reduced in both CD+/+ and CD+/− mice after MPTP. Data shown are mean ± SEM (*p < 0.05 sham CD+/− > sham CD+/+ and +p < 0.05 MPTP > SHAM in CD+/+). (F) Striatal DOPAC/DA ratio levels are similar between CD+/− and CD+/+ mice in sham mice, or mice after MPTP. Data shown are mean ± SEM (two way ANOVA was used to examine the effects of group (MPTP vs. sham) and genotype (+/+ vs.+/−). (G) Striatal HVA/DA ratio are similar between CD+/+ and CD+/− mice in sham and after MPTP, and are increased after MPTP in both genotypes. Data shown are mean ± SEM (+p < 0.05 sham < MPTP).
Both CD+/+ and CD+/− mice that were exposed to MPTP exhibited decreases of striatal DA, DOPAC, and HVA levels compared to sham animals. This decrease further provided evidence of dopaminergic neurodegeneration in MPTP-lesioned mice (Fig. 1C–E, p < 0.001 sham > MPTP). There were no significant differences in levels of striatal DA, DOPAC, or HVA between CD+/+ and CD+/− animals after MPTP (Fig. 1C–E, p > 0.05 CD+/+ MPTP = CD+/− MPTP). However, we noticed that in CD+/− mice, striatal levels of DA, DOPAC, and HVA were consistently–higher than in CD+/+ controls (Fig. 1C, p < 0.05 CD+/+ sham < CD+/− sham; D, p < 0.01 CD+/+ < CD+/−; E, p < 0.01 CD+/+ < CD+/−). Fig. 1F and G shows the ratio of DOPAC/DA and HVA/DA. Sriatal DOPAC/DA ratio levels are similar in CD+/− and CD+/+ mice in sham and MPTP-treated mice. Striatal HVA/DA ratio are similar between CD+/+ and CD+/− mice in sham and after MPTP, but both increased after MPTP.
3.2. CD+/− mice have significantly higher levels of DA, DOPAC and HVA than CD+/+ mice
Due to the pattern we observed in our sham animals, we used additional CD+/+ and CD+/− sham mice for analysis of striatal monoamines. The differences in DA, DOPAC, and HVA were all highly significant between the two genotypes (Fig. 2A, p < 0.01 CD+/+ < CD+/−; B, p < 0.001 CD+/+ < CD+/−).
Fig. 2.
CD+/− mice have significantly higher levels of DA and its metabolites than CD+/+ mice in the striatum and the olfactory bulbs. Striatal monoamines are higher in CD+/− mice than in CD+/+ controls (A and B). (A) DA levels are significantly higher in CD+/− mice than in CD+/+ controls. Data = mean ± SEM (*p < 0.01 CD+/+ < CD+/−). (B) DOPAC and HVA levels are significantly higher in CD+/− mice than in CD+/+ controls. Data = mean ± SEM (**p < 0.001 CD+/+ < CD+/−). Monoamine levels in the olfactory bulb are also higher in CD+/− mice than in CD+/+ controls (C and D). (C) Olfactory DA levels are significantly higher in CD+/− mice. Data = mean ± SEM (*p < 0.01 CD+/+ < CD+/−). (D) DOPAC levels in the olfactory bulb are significantly higher in CD+/− mice. Data = mean ± SEM (#p < 0.05 CD+/+ < CD+/−). HVA levels were consistently higher in the olfactory bulb of CD+/− mice than in CD+/+ mice but did not reach statistical significance. Data = mean ± SEM (p = 0.06 CD+/+ vs. CD+/−). (E) Striatal DOPAC/DA and HVA ratio are similar between CD+/+ and CD+/− mice. Data shown are mean ± SEM. (F) Olfactory DOPAC/DA and HVA/DA ratio are similar between CD+/+ and CD+/− mice. Data shown are mean ± SEM, p > 0.05 Student t-test.
Another brain region in mice which is rich in DA and DA metabolites is the olfactory bulb. In order to test whether the differences in dopaminergic monoamines was regional, we also tested levels of DA, DOPAC, and HVA in the olfactory bulb of CD+/+ and CD+/− mice. In this brain region, CD+/− mice also had higher levels of DA and DOPAC compared to CD+/+ controls (Fig. 2C, p < 0.01 CD+/+ < CD+/−; D, p < 0.05 CD+/+ < CD+/−). HVA levels in the olfactory bulb were consistently higher in CD+/− than in CD+/+ mice but the difference did not reach statistical significance (Fig. 2D, p = 0.06 CD+/+ vs. CD+/−). The difference in olfactory monoamine levels between CD+/+ and CD+/− mice was not as dramatic as that seen in the striatum. This could be due to the smaller sample size of this group (n = 8 mice per genotype for olfactory bulb comparisons) than in the striatal samples (n = 27 mice per genotype), or the less abundant total dopamine and metabolites in this region, or the phenomenon may simply be less apparent in this brain region. The ratio of DOPAC/DA and HVA/DA are similar between CD+/+ and CD+/− mice in both striatum and olfactory bulbs (Fig. 2E and F).
3.3. No difference in MAOB and COMT levels between CD+/+ and CD+/− mice
After we observed the striking differences in DA, DOPAC, and HVA in CD+/− mice compared to CD+/+ controls, we decided to determine whether there were any differences in levels of the enzymes responsible for generating these biogenic amines. MAOB, which generates DOPAC from DA [6] was not different between CD+/+ and +/− mice (Supplemental Figure 1A and B, p > 0.05). COMT, which converts DA to 3-methoxytyramine (3-MT) and DOPAC to HVA [1], exists in two forms. A 28 kDa form is membrane-bound and known as COMT-MB, and a 24 kDa form is soluble and known as COMT-S [7,21]. From whole cell extract of the striatal tissue, neither COMT-S nor COMT-MB levels were significantly different in striatal extracts between wild type and heterozygous CD mice (Supplemental Figure 1C and D, p > 0.05), CD+/− mice have higher levels of LAMP1: since cathepsin D has been shown to be involved in α-synuclein degradation [17] and that a CD knock-out mouse presented a high increase of α-synuclein in neurons [14], we wondered whether such an increase would be detectable in a CD haploinsufficiency model. Nevertheless, we did not detect any difference in monomer, dimer or high molecular weight α-synuclein. We then wondered whether the autophagosome/lysosome pathway (ALP) would be deregulated following decreased CD levels. In our Western blot experiments, we did not detect any difference in autophagic protein markers such as p62, LC3-I or LC3-II but we observed a significant increase in LAMP1 levels (Supplemental Figure 2).
3.4. There are no differences in cognitive function between CD+/+ and CD+/− mice
After discovering the differences in biogenic amines between wild type and heterozygous CD mice, we next subjected animals to a battery of behavioral testing to test various aspects of cognition. Surprisingly, we saw no differences in any of the following parameters: learning/memory, anxiety-like behavior, or balance/motor coordination (Supplemental Figures 3–5).
4. Discussion
We have shown that CD haploinsufficiency results in increased levels of the biogenic amines DA, DOPAC and HVA, and that MPTP administration abolishes these observed differences. Interestingly, the ratio of DOPAC/DA and HVA/DA are similar between CD+/+ and CD+/− mice. Of the proteins we examined, TH, MAOB or COMT levels were not significantly altered between CD+/+ and +/− mice. Whether their cell type distribution, intracellular location, or enzymatic activities were changed due to CD heterozygosity would need future investigation. Although TH levels are similar, stereological analyses of dopaminergic cell number and densities of dopaminergic processes may also provide potential insights into how DA, DOPAC and HVA level increases in CD+/− mice compared to CD+/+ mice.
The importance of autophagic function in neurodegenerative diseases such as PD has become increasingly clear in recent years. Lysosomal depletion has been shown to be a pathogenic mechanism of MPTP toxicity in vivo, and inducing lysosomal biogenesis in this model attenuated MPTP toxicity [5], so it stands to reason that slight perturbations in the autophagy pathway (i.e., CD haploinsufficiency) may predispose animals to experience increased MPTP toxicity. The present study tested the striatal LC3, p62, α-synuclein and LAMP1 levels. LC3, p62, and α-synuclein are unchanged in the striatal extract, whereas LAMP1 is increased, potentially as a compensatory mechanism for the decreased lysosomal proteolysis associated with decreased CD levels. We speculate that haploinsufficiency of CD would lead to less autophagy flux activities, especially when cells are under stress. Future studies will need to determine the autophagic flux activities in vivo.
Despite having hypothesized that CD+/− mice would be more sensitive to MPTP-induced neurotoxicity than CD+/+ mice, we found that the heterozygous mice experienced comparable lesion size compared to control mice. This might be because that the levels of DA and its metabolites were significantly elevated in CD+/− mice. It will be interesting to investigate whether CD overexpression via adeno-associated viral (AAV) delivery may change the response to MPTP toxicity. In CD homozygous knockout mice, it was shown that exogenous CD delivered to the brain via AAV was able to prevent both neuronal and systemic pathologies associated with CD deficiency, suggesting that CD was transported from the brain to extracranial tissues in the body [18]. A mouse over expressing CD may also more effective in dealing with increases in α-synuclein levels induced by genetic over expression or MPTP treatment.
The striking differences in DA, DOPAC, and HVA in CD+/− mice compared to CD+/+ controls did not lead to any discernable changes in behavior or cognitive function as determined by Morris water maze, open field, and rotarod testing. It is possible that the biochemical alterations we observed were not drastic enough to elicit behavioral alterations in mice with the behavioral paradigms we employed. Additionally, any physiological changes brought about by CD haploinsufficiency may be compensated for by other genes/proteins in the autophagy lysosome pathway. For instance, the transcription factor EB (TFEB) has been shown to be a master regulator of lysosomal biogenesis and is able to upregulate the expression of many genes in the autophagy pathway [16]. It is possible that TFEB activity is greater in CD+/− mice than in CD+/+ controls. Because the CD knockout mice fail to thrive, future studies will need to investigate the effects of CD deficiency in response to MPTP treatment or α-synuclein over expression would be through the use of conditional knockouts in which CD has been ablated from subpopulations of neurons, such as the dopaminergic neurons of the SNpc or other neuron groups that are sensitive to α-synuclein over expression-induced pathology.
5. Conclusions
CD haploinsufficiency results in increased levels of DA, DOPAC, and HVA compared to CD+/+ mice. Having increased levels of dopaminergic monoamines does not afford protection in an MPTP model, as CD+/+ and CD+/− mice had similar levels of DA, DOPAC and HVA in MPTP-lesioned striatal tissue. Increased levels of soluble COMT in CD+/− animals may be one contributing factor to the observed differences in monoamine levels between genotypes. These biochemically divergent phenomena did not lead to any observed behavioral alterations in CD+/− mice.
Supplementary Material
HIGHLIGHTS.
▶CD+/− mice have higher levels of striatal dopamine and metabolites than CD+/+ mice.
▶MPTP treatment abolished the observed differences in striatal monoamines.
▶There were no discernable behavioral differences between CD+/+ and CD+/− mice.
Acknowledgments
We thank members of the Zhang Laboratory for technical help and discussions. We thank Dr. Paul Saftig for the cathepsin D knockout mice, Dr. Thomas Van Groen for his help and expertise with the behavioral assessments. This work was supported by Michael J Fox Foundation, NIHR01-NS064090 and a VA merit award (to JZ), and in part by P30 NS47466 (UAB Behavioral Assessment Core).
Abbreviations
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- 3-MT
3-methoxytyramine
- MHPG
3-methoxy-4-hydroxyphenylglycol
- DOPAC
3,4-dihydroxymethylphenylacetic acid
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine
- AAV
adeno-associated virus
- COMT
catechol-O-methyltransferase
- CD
cathepsin D
- HVA
homovanillic acid
- MAO
monoamine oxidase
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SNpc
substantia nigra pars compacta
- TFEB
transcription factor EB.
Footnotes
Author contributions DC contributed to the main body of the experiments and wrote the manuscript. XO and MBG performed some of the Western blot analyses and some of the mouse maintenance and genotyping. NF assisted on data analyses. JZ directed the study and finalized the manuscript.
Conflict of interest We do not have any conflict of interest.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet. 2013.01.035.
References
- [1].Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. Journal of Biological Chemistry. 1958;233:702–705. [PubMed] [Google Scholar]
- [2].Chiba K, Trevor AJ, Castagnoli N., Jr. Active uptake of MPP+, a metabolite of MPTP, by brain synaptosomes. Biochemical and Biophysical Research Communications. 1985;128:1228–1232. doi: 10.1016/0006-291x(85)91071-x. [DOI] [PubMed] [Google Scholar]
- [3].Cransac H, Cottet-Emard JM, Pequignot JM, Peyrin L. Monoamines (norepinephrine, dopamine, serotonin) in the rat medial vestibular nucleus: endogenous levels and turnover. Journal of Neural Transmission. 1996;103:391–401. doi: 10.1007/BF01276416. [DOI] [PubMed] [Google Scholar]
- [4].Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the Parkinsonian neurotoxin MPTP. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson’s disease. Journal of Neuroscience. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Glover V, Sandler M, Owen F, Riley GJ. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977;265:80–81. doi: 10.1038/265080a0. [DOI] [PubMed] [Google Scholar]
- [7].Kaakkola S, Mannisto PT, Nissinen E. Striatal membrane-bound and soluble catechol-O-methyl-transferase after selective neuronal lesions in the rat. Journal of Neural Transmission. 1987;69:221–228. doi: 10.1007/BF01244343. [DOI] [PubMed] [Google Scholar]
- [8].Klaidman LK, Adams JD, Leung AC, Jr., Kim SS, Cadenas E. Redox cycling of MPP+: evidence for a new mechanism involving hydride transfer with xanthine oxidase, aldehyde dehydrogenase, and lipoamide dehydrogenase. Free Radical Biology and Medicine. 1993;15:169–179. doi: 10.1016/0891-5849(93)90056-z. [DOI] [PubMed] [Google Scholar]
- [9].Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, Schulz-Schaeffer W, Watanabe T, Waguri S, Kametaka S, Shibata M, Yamamoto K, Kominami E, Peters C, von Figura K, Uchiyama Y. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. Journal of Neuroscience. 2000;20:6898–6906. doi: 10.1523/JNEUROSCI.20-18-06898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lindsey JW, Jung AE, Narayanan TK, Ritchie GD. Acute effects of a bicyclophosphate neuroconvulsant on monoamine neurotransmitter and metabolite levels in the rat brain. Journal of Toxicology and Environmental Health. 1998;54(Part A):421–429. doi: 10.1080/009841098158827. [DOI] [PubMed] [Google Scholar]
- [11].Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mayer RA, Kindt MV, Heikkila RE. Prevention of the nigrostriatal toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by inhibitors of 3,4-dihydroxyphenylethylamine transport. Journal of Neurochemistry. 1986;47:1073–1079. doi: 10.1111/j.1471-4159.1986.tb00722.x. [DOI] [PubMed] [Google Scholar]
- [13].Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. Journal of Neurochemistry. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- [14].Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, Xie ZL, Speake LD, Parks R, Crabtree D, Liang Q, Crimmins S, Schneider L, Uchiyama Y, Iwatsubo T, Zhou Y, Peng L, Lu Y, Standaert DG, Walls KC, Shacka JJ, Roth KA, Zhang J. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Molecular Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Koster A, Hess B, Evers M, von Figura K. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO Journal. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di MC, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- [17].Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxyterminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shevtsova Z, Garrido M, Weishaupt J, Saftig P, Bahr M, Luhder F, Kugler S. CNS-expressed cathepsin D prevents lymphopenia in a murine model of congenital neuronal ceroid lipofuscinosis. American Journal of Pathology. 2010;177:271–279. doi: 10.2353/ajpath.2010.091267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- [20].Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- [21].Tilgmann C, Melen K, Lundstrom K, Jalanko A, Julkunen I, Kalkkinen N, Ulmanen I. Expression of recombinant soluble and membrane-bound catechol O-methyltransferase in eukaryotic cells and identification of the respective enzymes in rat brain. European Journal of Biochemistry. 1992;207:813–821. doi: 10.1111/j.1432-1033.1992.tb17112.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.