Abstract
We used the egg avidin gold complex as a polycationic probe for the localization of negatively charged sites in the secretory granules of mouse mast cells. We compared the binding of this reagent to mast cell granules in wild-type mice and in congenic brachymorphic mice in which mast cell secretory granules contained undersulfated proteoglycans. We localized anionic sites by post-embedding labeling of thin sections of mouse skin and tongue tissues fixed in Karnovsky's fixative and OsO4 and embedded in Araldite. Transmission electron microscopy revealed that the mast cell granules of bm/bm mice had a lower optical density than those of wild-type mice (P<0.001) and a lower adivin gold binding density (by approximately 50%, P<0.001). The latter result provides additional evidence that the contents of mast cell granules in bm/bm mice were less highly sulfated than in those of wild type mice. In both wild type and bm/bm mast cells, the distribution of granule equivalent volumes was multimodal, but the unit granule volume was approximately 19% lower in bm/bm cells than in wild type cells (P < 0.05). Thus, bm/bm mast cells develop secretory granules that differ from those of wild type mice in exhibiting a lower optical density and slightly smaller unit granules, however the processes that contribute to granule maturation and granule-granule fusion in mast cells are operative in the bm/bm cells.
Keywords: Secretory granules, Unit granule, Mast cell, Cationic gold, Quantitative cytochemistry, Mouse (C57BL/6JxC3HeB/FJ bm/bm)
Introduction
In the past, morphological approaches to detect intracellular anionic determinants (Thiéry and Ovtracht 1979) in mast cells were based primarily on methods in which the experimental animal was perfused with cationic dyes or these reagents were applied to tissue sections. For example, the detection of sulfated glycosaminoglycans (GAGs) in mast cell granules at the electron-microscopic level was carried out using cationic dyes such as Alcian blue (Röhlich and Csaba 1972; Scott 1996), ruthenium red (Gustafson and Pihl 1967; Lagunoff 1972; Hunziker et al 1983), cuprolinic blue (Juarranz et al 1987) and cupromeronic blue (Haigh and Scott 1986; Juarranz et al 1987; Erlinger et al 1990), which bind electrostatically to the anionic sites of the mast cell granules.
Skutelsky and Roth (1986) developed a preparation of polycationic colloidal gold (CCG), consisting of a complex between polylysine and colloidal gold, which can be used at various (including very low) values of ionic strength and pH, making it a flexible and highly sensitive probe for the localization of anionic cellular components of cells and tissues. A simple one-step incubation of sections with the cationic gold conjugate reveals subcellular sites having net negative charge (Skutelsky and Roth 1986) and the charge distribution can be examined with clear definition at a range of magnifications by employing cationic gold of various sizes (Skutelsky and Roth, 1986; Kashio et al 1992; Saga and Takahashi 1993; Shoichetman et al 2001). Accordingly, CCG has been used extensively for electron microscopic localization of extracellular and membrane-bound polyanionic constituents in a wide variety of cells and tissues (Vorbrodt 1989; Goode et al. 1991; Skutelsky et al.1992; Lawrenson et al. 1994).
At physiological pH, CCG binds primarily to sulfated GAGs and can be used for the characterization of proteoglycans in various cellular compartments, cell membranes, and the extracellular matrix (ECM; Skutelsky et al. 1995; Shoichetman et al 2001). Because of its high affinity for sulfated GAGs, we have used CCG for the post-embedding localization and quantification of sulfated GAGs in mouse and rat mast cell granules (Skutelsky et al 1995). We have observed the highest density of labeling in mast cell secretory granules (ca. 500-900 gold particles/μm2), whereas in the secretory granules of other peritoneal cells, this labeling is about 10 times lower (ca. 40-80 gold particles/μm2; Skutelsky et al 1995). Pretreatment of LR white sections with heparinase I or III resulted in a reduction of 97% or 72%, respectively, in the binding of the gold particles to the granules, indicating that the majority of the gold binding reactivity is attributable to heparin. Moreover, correlation of granule section profile area with labeling density revealed that the smaller granules were significantly more labeled when compared to the larger profiles (Skutelsky et al 1995). These observations indicate that a post-translational change (presumably, mainly sulfation of heparin) of secretory content influences the granule anionic charge, which may in turn affect the intra-granule buffer capacity. One possible explanation of the finding of the more extensive labeling of small versus large granules is that the process of sulfation occurs preferentially near the granule membrane, and that, in small granules, more of the granule content undergoing sulfation lies in proximity to the granule membrane.
In using CCG for our prior studies of anionic charge distribution in the secretory granules of mast cells (Skutelsky et al 1995) and pancreatic acinar cells (Shoichetman et al 2001), we have noticed that this reagent gives relatively high levels of staining of structures other than granules, presumably reflecting the negative charge of these structures, e.g., ribosomes and cellular membranes. Electron-microscopic studies have demonstrated that avidin binds highly preferentially to individual mast cell granules rather than to other cellular structures (Bussolati and Gugliotta 1983; Jones et al 1987; Tharp et al 1985), and rodent or human mast cells are known to be readily stained with avidin conjugated to horseradish peroxidase (Bussolati and Gugliotta 1983; Kasper and Tharp 1987) or to certain fluorochrome dyes (Tharp et al 1985; Markey et al 1989; MacBride 1998). Based on the results, we decided to use an adivin gold (AvG) complex as a cationic probe for detection of anionic sites in mast cell granules and other structures.
Avidin is a glycoprotein found in the egg white and tissues of birds, reptiles, and amphibians. Carbohydrate accounts for about 10% of the total mass of avidin. Avidin has a basic isoelectric point (pI) of 10-10.5 and is stable over a wide range of pH and temperature. Moreover, avidin can undergo extensive chemical modification without substantial loss of its ability to bind to anionic sites (Green 1975; Bayer and Wilchek 1994). In the current study, we conjugated avidin to gold particles to make a new cationic gold probe: avidin-gold conjugated particles (AvG). We found that this AvG probe bound strongly to mast cell granules, and gave less staining of other structures compared to the CCG method.
In addition to characterizing the binding of this AvG probe to the granules of normal mast cells, we have also used it to stain secretory granules of mast cells in homozygous brachymorphic (bm/bm) mice (Yamada et al 1984). Brachymorphic mice are characterized by disproportionately short stature (Vanky et al 2000; Pennypacker et al 1981; Orkin et al 1977) and by undersulphated mucopolysaccharides, reflecting a defect in the synthesis of the sulphate donor (3′-phosphoadenosine-5′-phosphosulphate: PAPS) (Schwartz et al 1978, 1998; ul Haque et al 1998). In bm/bm mice, connective tissue elements and components of epithelial tissues exhibited apparently weaker positive reactions for ester sulphate groupings of complex carbohydrates, as compared with those in the control mice (reviewed by Schwartz et al 1998). The mast cell granules of bm/bm mice were stained by Toluidine blue, but without metachromasia (Yamada et al 1984). These results suggest that undersulphation of complex carbohydrates is widespread throughout the tissues of these mutant mice, including their mast cells. We have used our new AvG method to compare the extent of anionic binding sites detected by this method in the mast cell secretory granules of wild type and bm/bm mice.
Materials and Methods
Tissue preparation
Two-month-old male brachymorphic mice (C57BL/6JxC3HeB/FeJ bm/bm; referred to herein as bm/bm mice) and their littermate control wild type mice (C57BL/6JxC3HeB/FeJ) (Jackson Laboratory, Bar Harbor, ME,) were used. The animal experiments were conducted in accordance with the Beth Israel Hospital Institutional Animal Care and Use Committee (the animal work was done when S. J. Galli and I. Hammel were working at Beth Israel Hospital [now Beth Israel Deaconess Medical Center] in Boston, Mass., USA) and with guidelines prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHHS publication No. 86-23, revised 1985). Animals were maintained on a mouse diet of standard rodent chow and tap water ad libitum. Mice were killed by inhalation of CO2 and 1-mm3 tissue blocks were removed from the ear pinnae and tongues. Tissues were washed once in phosphate-buffered saline (PBS) at pH 7.4, fixed with Karnovsky's fixative (Karnovsky 1965) for 1 h at room temperature, washed twice with PBS, and postfixed with 1% OsO4 in the same buffer. Dehydration was carried out with graded ethanols and propylene oxide, and tissues were embedded in either Araldite or LR White.
Avidin
Highly purified egg-white avidin, consisting of either the native cationic avidin (S.C. Belovo, Bastogne, Belgium) or modified avidin (Bayer and Wilchek 1994; called “NeutraLite Avidin” and referred to herein as “NL avidin”), which lacks the oligosaccharide moiety, exhibits a neutral pI, and bears free lysine groups, was obtained as a generous gift from Dr. E. A. Bayer (The Weizmann Institute of Science, Rehovot 76100, Israel) and used as lyophilized protein dissolved in double-distilled water (DDW).
Biotinylated bovine serum albumin (BSA)
Based on the work of Morris and Saelinger (1986) and Mayer and Rohde (1988), we used biotinyl-N-hydroxysuccinimid ester (BNHS) to prepare our bovine serum albumin (BSA; Skutelsky and Bayer 1979). BNHS (17 mg) was dissolved in dimethylformamide (DMF) and brought to a final volume of 1 ml with DDW. The BNHS solution (100 μl) was added to the BSA solution (10 mg in 5 ml 0.1 M NaHCO3), incubated for 4 h at room temperature, and then dialyzed at 4°C overnight against 1 L 0.15 M NaCl with one change and for 2 h against 1 L DDW. BSA-stabilized-gold and polyethylene-glycol (PEG)-stabilized gold particles were used as separate controls in all experiments.
Preparation of protein-gold complex
A monodispersed suspension of colloidal gold, with an average diameter of 15 nm, was prepared by reduction of HAuCl4 with Na citrate (Frens 1973; Roth 1984). The amounts of protein (either avidin or biotinylated BSA) required for stabilization of the gold colloids were determined by salt-stabilization tests (Horisberger et al.1975; Roth and Binder 1978).
In a previous study (Skutelsky et al. 1987), we found that egg-white AvG complex formation was most efficient at a relatively high pH (>9.0), but that we achieved a more uniform coating of the gold colloid, without clumping, at pH 8.5. To prepare a stable protein-gold complex, 1 mg native egg-white avidin, NL avidin, or biotinylated BSA were added to 10 ml gold solution at pH 8.5 (avidin) or at pH 7.4 (NL avidin and biotinylated BSA) at room temperature. After 5 min, the suspension was diluted 1:3 by the addition of PBS, pH 7.4, containing 0.01% PEG (molecular weight:20,000). The colloid solutions were then centrifuged at 100,000g (Optima TL ultracentrifuge, Beckman, USA) for 45 min, and the pellet containing the AvG complex was resuspended in 2 ml PBS containing 0.01% PEG.
Staining procedures
Ultrathin sections (0.075±0.015 μm) were prepared on an LKB III Ultratome by using a diamond knife (Diatome, Switzerland and SPI, USA), and the sections were mounted on Formvar-coated 200-mesh nickel grids. In order to block binding based on affinity for BSA alone, the sections were incubated with 1% BSA solution for 5 min. In a one-step reaction, the sections were labeled with AvG at a 1:100 dilution in PBS (pH 7.4) or in phosphate/citric acid buffer (pH 2.0 or pH 4.0). After being stained for 1 h, the sections were rinsed with DDW and post-stained for 40 min with saturated uranyl acetate in 50% methanol. In a two-step procedure, the sections were incubated with native avidin diluted in PBS (pH 7.4) or in phosphate/citric acid buffer (pH 2.0 or pH 4.0) for 1 h and than labeled with biotin-gold complex for 1 h before being stained with uranyl acetate. Controls consisted of NL AvG (one step) and NL avidin (two steps). In some experiments, prior to treatment with BSA solution and AvG, sections were incubated with 1U/ml heparinase I (Sigma, St. Louis, Mo., USA) dissolved in TRIS-buffered saline (TBS), pH 7.0, containing 0.04 M CaCl2 (Skutelsky et al. 1995). Treatment was carried out in a moisture chamber for 2 h at 37ºC, followed by a wash with TBS. All staining procedures were carried out at room temperature. The sections were examined in a JEOL-100CX transmission electron microscope, at 80 kV.
Quantitative microscopy
Morphometry of granules was performed on randomly obtained electron micrographs (×12,000) of secretory granules as previously described (Hammel et al. 1989; Skutelsky et al. 1995; Shoichetman et al. 2001). Briefly, organelle cross-sectional areas were measured directly on the transmission electron micrographs. For each experiment, three to five ultrathin sections taken from the two blocks of each tissue were placed on 200-mesh grids. The section that was the most technically adequate and the most clearly stained was selected. Generally, the grid pattern defined two to three complete section windows. All mast cells were photographed. Mature granule profile area (ai) measurements were carried out on the prints by using a graphic tablet (HP 9111A; Hewlett Packard Company, Palo Alto, Calif., USA) interfaced to a Power Macintosh 7100/66AV microcomputer for data transformation and analysis. For each granule, we also counted the number of gold particles (Ni). Gold particle mean density was calculated as ¼ P Ni =P ai (Hammel et al. 1989). In addition, granules were sorted by size, and the gold particle mean density was calculated for each bin size. The resulting data were analyzed by using the moving bin method and presented as a scattergram (Hammel et al. 1987). All data were plotted on an HP LaserJet 4050 N printer interfaced to the microcomputer. Statistical comparative analysis between the cumulative curves was performed by using the Kolmogorov-Smirnov (KS) test (Sokal and Rohlf 1995).
Granule electron density
Each electron micrograph negative (10.16 cm × 12.7 cm) was scanned at a resolution of 600 DPI and stored as a tagged image file format (tiff). Image analysis was performed by using ImageJ v3.91 software (http://rsb.info. nih.gov/ij). ImageJ is a public-domain Java-based image- processing freeware program developed at the National Institutes of Health, USA (Collins 2007). For each granule, the perimeter was marked, i.e. granule perimeter = region of interest (ROI), and an optical density histogram [gray levels/values = 1 (white)–256 (black)] was constructed. We did not filter the staining intensities in the images by applying a threshold value to remove low-intensity pixels that represent nonspecific/background values, since in all cases, a broad optical density zone (1-100) occurred in which no staining was observed (see below, for some examples). For each cell profile, all data were merged to generate the density histogram of all the granules within that image of the cell. In addition, all acquired images for each mouse type were grouped in a stack and subsequently analyzed by using the Microsoft Excel program.
Results
AvG binds primarily to secretory granules in the mast cells of wild-type or bm/bm mice
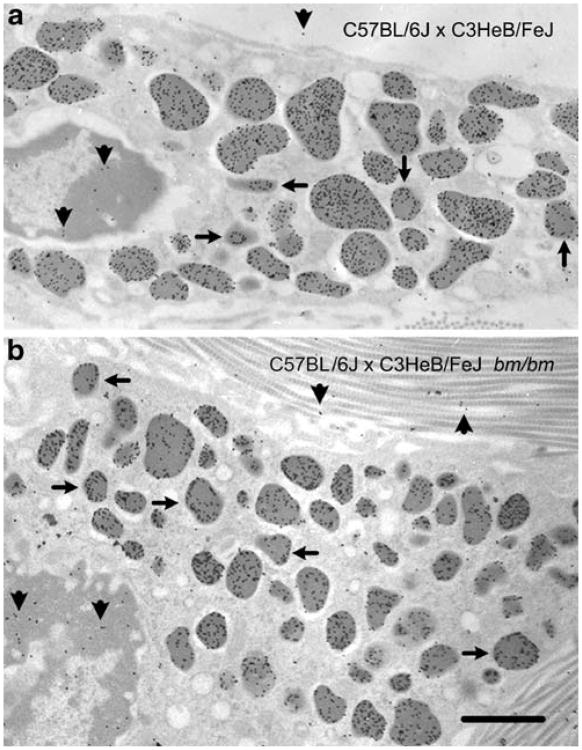
We used transmission electron microscopy to examine the labeling properties of mast cells in the ear pinnae and tongue in ultrathin Araldite sections that had been subjected to post-embedding labeling with avidin-colloidal gold (AvG) at physiological pH (7.4). In both wild-type and congenic bm/bm mice, labeling with AvG particles was restricted primarily to the mast cell secretory granules, but the density of labeling of mast cell granules in wild-type mice (Fig. 1a) was substantially higher than that in the mast cells of bm/bm mice (Fig. 1b). Notably, in some granules, labeling density was heterogeneous, with more label at the periphery of the granules than in central portions of the structure (see arrows in Fig. 1).
Fig. 1.
Araldite sections of ear mast cells of (a) wild-type (C57BL/6JxC3HeB/FeJ) and (b) bm/bm (C57BL/6JxC3HeB/FeJ bm/bm) mice. Tissues were fixed with aldehydes and OsO4, and sections were post- labeled with avidin gold (AvG) and counterstained with lead citrate. Note the differences in granules size and labeling densities between control and bm/bm mast cells (arrows granules labeled predominantly in the periphery). Gold particle density within all ear mast cell granule profiles was 334±148 particles/μm2 for wild-type mice versus 180±78 particles/μm2 for bm/bm mice; P<0.001. Some AvG binding to nuclear heterochromatin was seen. For wild-type mice, nuclear chromatin gold particle density (arrowheads left) was 0.9±0.4 particles/μm2 and extracellular matrix (arrowheads top) density was 0.4±0.3 particles/μm2. Bar 1 μm
In addition, some AvG binding occurred to nuclear heterochromatin (0.9±0.4 particles/μm2) or to the ECM of the connective tissue around the cells (0.4±0.3 particles/μm2 in ear tissue and 5.3±2.1 particles/μm2 in the tongue, P<0.05; arrowheads in Fig. 1). By contrast, other cellular constituents, such as the cytoplasm and plasma membranes, were practically devoid of labeling. The observation that the labeling of the connective tissue ECM was considerably higher in the tongue than in the ear probably reflected the presence of a different density of negatively charged sites in the ECM at those locations. We found that heparinase I treatment virtually eliminated the ECM labeling (0.2±0.1 particles/μm2 in tongue ECM, P<0.05 vs. values of specimens not treated with heparinase I). Others have also observed the binding of cationic probes to nuclear heterochromatin. For example, probes containing RNase, which is highly cationic (for a review, see Rosenberg 2008), bind both to nuclear heterochromatin and the granule matrix of human mast cells (Dvorak et al. 2000), and such staining is markedly reduced in specimens pretreated with heparinase I to block binding to heparin (Dvorak and Morgan 1998).
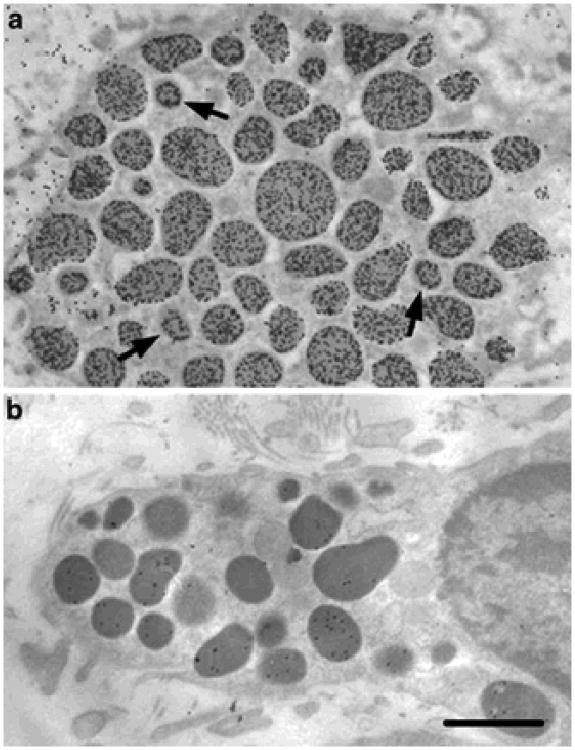
Nonspecific background staining (i.e., staining apparently not related to the presence of anionic constituents of the specimens) was apparently minimal. Pretreatment of the sections with BSA solution virtually eliminated staining of the cytoplasm by AvG, without reducing AvG binding to the secretory granules. However, after the use of either BSA-stabilized gold or PEG-stabilized gold particles alone as controls, granules labeled with one gold particle were observed rarely. Pretreatment of sections with heparinase I resulted in a 90%-95% reduction in AvG binding of the mast cell secretory granules (Fig. 2), and incubation with either NL AvG or NL avidin-biotinylated gold (BG) instead of AvG resulted in no labeling (data not shown). Similar results were obtained with the two-step labeling procedure, involving primary incubation of the section with avidin, followed by staining with BG. In avidintreated sections, the BG labeling was mainly restricted to the mast cell secretory granules (data not shown).
Fig. 2.
Araldite sections of tongue mast cells of wild-type mice. Tissues were fixed with aldehydes and OsO4, sectioned, post-labeled with AvG, and counterstained with lead citrate. Note the differences in labeling densities between controls (a) and heparinase-Itreated mast cells (b). Note the granules labeled predominantly in the periphery (arrows). Bar 1 μm
Mast cell granules of bm/bm mice have a lower optical density than those of wild-type mice
The ultrastructure of ear pinnae or tongue mast cells derived from the wild-type mice resembled that of resting connective tissue mast cells (for reviews, see Dvorak et al. 1983; Lagunoff 1972a, b). As shown in many previous studies of mouse mast cells, many mast cell secretory granules, in both wild-type and bm/bm mice, exhibited a homogeneous electron density; however, this intensity of staining varied from granule to granule (Fig. 1). In bm/bm mice, the electron density of the mast cell secretory granules appeared to be more heterogeneous than that in wild-type mast cells, with the cells containing a higher proportion of lightly stained granules. Contrast at the electron microscopy level (here referred to as “electron density”) is based on the interaction between cationic heavy metals and constituents of the structures imaged. Since mucopolysaccharides, including the GAGs in mast cell granules, are undersulfated in the bm/bm mouse, we expected that these components would appear less electron dense than those in the cells of wild-type mice.
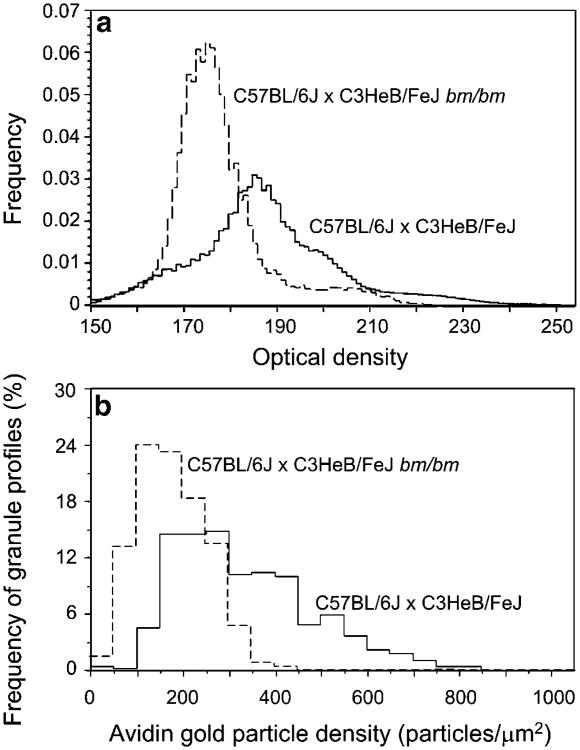
To quantify the electron density of mast cell granules objectively, we scanned all of the secretory granules in groups of ear pinnae mast cells from wild-type and bm/bm mice (490 granules in 22 wild-type mast cells and 719 granules in 17 bm/bm mast cells) and presented the data in optical density histograms (Fig. 3a). The mode of the secretory granule optical density in mast cells from bm/bm mice (175 OD units) was about 10 OD units less than that of the mast cell granules from the wild-type mice. The data shown in Fig. 3a (for granule optical density) and Fig. 3b (for granule AvG particle density) are derived from all the wild-type (n=22) and bm/bm (n=17) mast cells that we scanned.
Fig. 3.
Densitometry histograms showing the characteristics of all mast cell granules as measured by optical density (a) or by AvG (b). Each graph represents data summed from all granules in all cell profiles analyzed (derived from a total of 22 wild-type and 17 bm/bm mast cells). Granules of control wild-type mice (continuous line) are darker than those of the bm/bm mutant mice (dashed line); P<0.001 (Kolmogorov-Smirnov test). The frequency histogram shows that granule anionic site density (b), as measured by AvG particles, is also higher in the mast cells of control wild-type mice than in the bm/bm mice; P<0.001 (Kolmogorov-Smirnov test)
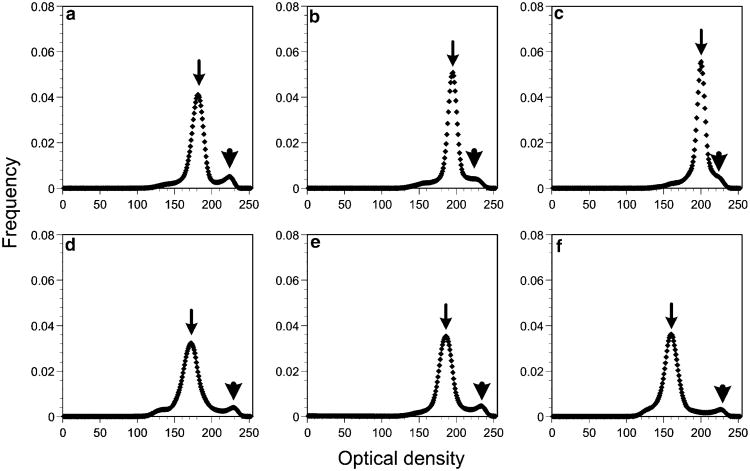
When we plotted data for each cell separately, a high variability was discerned in secretory granule optical density among all cells, independent of mouse origin, i.e., in both wild-type (Fig. 4a–c) and bm/bm (Fig. 4d–f) mast cells (the variability in the granule optical density measured by densitometry is greater than that evident to the naked eye; see Fig. 1).
Fig. 4.
Densitometry characteristics of control wild-type (a-c) and bm/bm (d-f) mast cell granules. Each graph represents data summed from all granules within a single mast cell profile (arrowheads peaks of the gold particles located at about the same density in all six examined cells). The granule “content density” mode (arrows), which is a measure of the density excluding the gold particles, is roughly the same for the three wild-type mast cells but is lower for the three bm/bm mast cells
Mast cell granules of bm/bm mice have a lower AvG binding density than those of wild-type mice
The AvG labeling density in the mast cell secretory granules exhibited considerable variation (Fig. 3b). The mean AvG labeling density in the bm/bm mice was 180±78 particles/μm2 (mean±SD, n=719 granule profiles), whereas in the wild-type mice, it was about twice that value, i.e.,334±148 particles/μm2 (mean±SD, n=490 mature granule profiles; P<0.001).
The data for AvG labeling density were analyzed for statistical significance by using the KS test, which compares two size distributions and is independent of any size distribution assumptions. The KS test compares the curves of two cumulative distributions by calculation of a variable (Dmax), which equals the largest observed distance between the curves. It is a sensitive test for the examination of differences in size distributions possibly reflecting such factors as location of peaks in the distribution and the dispersion and skewness of the population (Sokal and Rohlf, 1995). The present results indicate that the labeling density differs among individual granule profiles in a single mast cell, and that the range of variation in density of the mast cell granules in a single cell varies from one cell to another.
Mast cell granules of bm/bm mice have a lower unit granule volume than those of wild-type mice
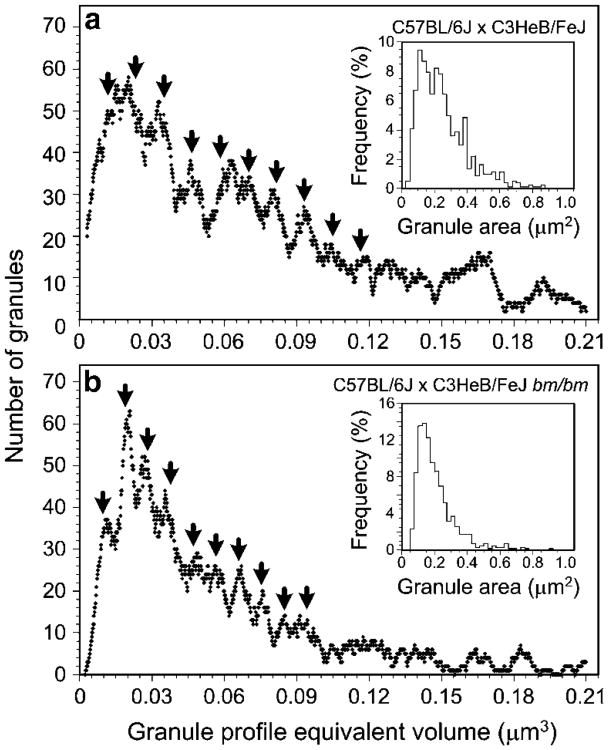
We wished to assess whether a correlation existed between AvG particle mean density for individual granules and the corresponding granule size (see below). First, we analyzed the distribution of mast cell secretory granule equivalent volumes and calculated the unit granule size (Hammel et al.1983). For both wild-type and bm/bm mouse mast cells, the secretory granule equivalent volume demonstrated a periodic multimodal distribution (Fig. 5). For wild-type mouse mast cells, the estimated unit granule volume was 0.0116±0.0003 μm3. For bm/bm mouse mast cells, the unit granule volume was about 19% lower (0.0094 ±0.0003 μm3; P<0.05 (Student's t-test, two-tailed).
Fig. 5.
Histograms of moving-bin analyses demonstrating multimodal frequency of mast granule equivalent volumes derived from control wild-type (a) and bm/bm mutant mouse (b) mast cells. The values for granule modes (indicating the calculated volumes of granules representing the unit granules (V1) and integral multimers of the unit granules from V2 to V10, are indicated by arrowheads (mast cell unit granule volume for wild-type mice = 0.0116 μm3 and for bm/bm mice = 0.0094 μm3; P<0.05, Student's t-test, two-tailed). The granule profile area histograms for each cell type are presented in the insets
Density of AvG binding to mast cell granules is lower in bm/bm than in wild-type mice but correlates with periodicity of multimodal distribution of equivalent volumes of granules
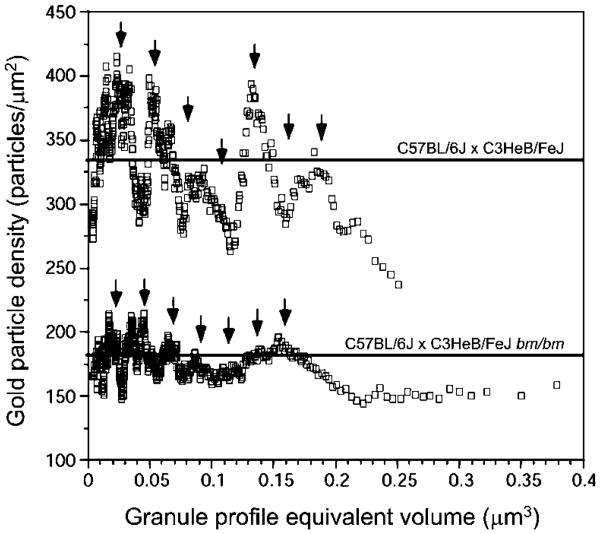
We then prepared a scattergram depicting the relationship between AvG particle mean density for individual granules and the corresponding granule sizes (Fig. 6). The results clearly suggested that the bm/bm mouse mast cell granules were less anionic than those in wild-type mice, since they had significantly reduced AvG binding density (wild-type value of 334±148 particles/μm2 vs. bm/bm value of 180±78 particles/μm2; P<0.001, Student's t-test, two-tailed). Moreover, when we superimposed, on the scattergram, arrows indicating the positions of cytplasmic granules whose equivalent volumes ranged from 1 to 7 unit granules (from Fig. 5), the positions of these arrows corresponded well to the peak location of AvG labeling density in the scattergram analysis of the gold bead histochemistry results (Fig. 6).
Fig. 6.
Scattergram analysis of the relationship between AvG particle density and mean granule profile equivalent volume. The locations of the arrows, indicating the calculated volumes of granules representing the unit granules (V1) and integral multimers of the unit granules from V2 to V7, are based on our calculation of the unit granule volume, as estimated in Fig. 5. The mean data, based on Fig. 3b, are indicated by horizontal solid lines
Discussion
Various cytochemical approaches have been used to define the ultrastructural localization of cell-surface and intracellular anionic sites. Most of these methods employ polycationic colloids of heavy metals, such as ruthenium red (Gustafson and Pihl 1967; Luft 1971; Lagunoff 1972a, b; Spicer et al. 1967), iron (Gasic et al. 1968) or thorium hydroxides (Roth and Binder 1978), or Alcian blue (Ruggeri et al. 1975; Enerbäck 1966). Such probes are generally introduced by immersion or perfusion, during or following aldehyde fixation of the tissue, and are used at acidic pH (pH 2) and low ionic strength. Under such conditions, their penetrability is poor, their labeling capacity is restricted to free surfaces, and they have a tendency to form micelles of various sizes and to alter the inherent distribution of anionic sites (Skutelsky and Danon 1975).
The introduction of cationized ferritin, a polycationic derivate of ferritin (Danon et al. 1972), represents an important improvement in anionic labeling capacity, since cationized ferritin can be used under physiological conditions on viable cells (Skutelsky and Danon 1975; Skutelsky and Hardy 1976). However, this marker is applicable only to free cells or to free surfaces of tissues and therefore cannot be used for quantitative characterization of most intracellular and extracellular polyanionic constituents. By contrast, colloidal gold densitometry can be used to characterize binding sites on the surface of cells and within intracellular compartments. Accordingly, colloidal gold has become the marker of choice for post- embedding cytochemistry and immunohistochemistry at the ultrastructural level of resolution (Faulk and Taylor1971; Horisberger et al. 1975; Roth 1984; Ueda et al.1998).
In the present study we designed and tested a new polycationic gold probe (AvG), as a histochemical marker for post-embedding visualization of anionic constituents in ultrathin sections. Native egg white avidin is a cationic glycoprotein, pI >9.0 (Green 1975; Bayer and Wilchek 1994) that exhibits high affinity for the naturally occurring biotin molecule (Bayer and Wilchek 1994). We therefore used egg-white avidin as the cationic determinant in the AvG probe. To test the binding capacity and specificity of the AvG, we applied it to thin sections of mast cells, cells in which we previously used CCG to demonstrate sulfated proteoglycans (Skutelsky et al 1995). In the absence of exogenous biotin, native avidin exhibits a high avidity for mast cells in tissue and suspensions (Bergstresser et al. 1984); this reaction is thought to be based on the ionic interaction between the negatively charged glycosaminoglycans (GAGs) of mast cell granules and the positively charged avidin molecule (Bergstresser et al 1984, Bussolati and Gugliotta 1983, Fritz et al.1986, Tharp et al. 1985). We found that AvG, like native avidin, stained mast cell granules with high selectivity.
We have used AvG in a simple one-step staining procedure to investigate the distribution of anionic sites in the secretory granules of wild-type and bm/bm mouse mast cells. The localization of the anionic sites has been performed by post-embedding labeling of thin sections of mouse skin or tongue tissue fixed in Karnovsky's fixative and OsO4 and embedded in Araldite. In mast cell secretory granules, we have observed the highest density of AvG labeling, i.e., 334±148 particles/μm2, in control mice versus 180±78 particles/μm2 in bm/bm mice (a reduction of about 47%). Interestingly, for each type of mouse, the histograms depicting mast cell granule optical density (Fig. 3a) or AvG labeling density (Fig. 3b) according to granule size have approximately the same shape. This result suggests that mast cell granule optical density and the density of anionic sites in the granules are roughly correlated.
The anionic contents of granules have long been thought to provide the main driving force for the post-Golgi condensation of immature granules (Reggio and Palade 1978; Farquhar and Palade 1981; Hammel et al. 1998). We have previously reported that the secretory granules of mast cells have a periodic multimodal size distribution in which the volumes of individual granules are integral multiples of the intermodal distance, a volume defined as the “unit granule” or V1 (Hammel et al. 1983, 1987, 1991). We also have provided evidence that the unit granule is formed via the condensation of immature granules formed in the Golgi (Lew et al. 1994; Hammel et al. 1998). In the current study, we have found that the unit granule volume in the bm/bm mutant mouse is slightly but significantly smaller than that in control wild-type mice. As noted in the Introduction, homozygous brachymorphic (bm/bm) mice are characterized by undersulfated proteoglycans because of a defect in the synthesis of the sulfate donor (PAPS); the under- sulfation of complex carbohydrates is widespread throughout the connective and epithelial tissues of the mutant mice (Yamada et al. 1984). Our finding that the unit granule volume is smaller in the mast cells of brachymorphic mice than in those of the corresponding wild-type mice indicates that the level of sulfation of the granule content influences the processes that regulate the size of the unit granule, perhaps including the condensation of the immature granule. However, the volume distribution of mast cell mature granule equivalent volumes is similar for bm/bm and wild-type mice, indicating that the formation of granules of higher volumes from unit granules (V2, V3, V4, etc.) can occur in the mutant animals. Our work thus supports the conclusion that mast cell granule condensation (resulting in the formation of a unit granule) can occur even in the face of significantly reduced granule “anionic content”, and that the process that results in the formation of granules of larger size can also occur.
We have been interested to observe that, in many profiles of secretory granules in both wild-type and bm/bm mice, the pattern of AvG distribution on the granule profile is heterogeneous, with higher densities of labeling by AvG near the granule membrane than in the center. We are tempted to hypothesize that the anionic site distribution within the granule itself is ordered in a way that results in the granule periphery having a higher anionic content than its core. However, we want to strike a note of caution concerning this possibility. Prior studies have used various cationic agents to identify anionic sites in mast cell granules, and these agents have exhibited either a uniform distribution of label over the entire granule profile (Tato et al. 1990) or have shown higher levels of binding at the granule periphery (Chi and Lagunoff 1975). The reason(s) for the variation in results obtained with these different cationic probes remain(s) to be determined.
References
- Bayer EA, Wilchek M. Modified avidins for application in avidin-biotin technology: an improvement on nature. In: Sim JS, Nakai S, editors. Egg uses and processing technologies. CAB International; Wallingford: 1994. pp. 158–176. [Google Scholar]
- Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol. 1984;83:214–218. doi: 10.1111/1523-1747.ep12263584. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Gugliotta P. Nonspecific staining of mast cells by avidin-biotin-peroxidase complexes (ABC) J Histochem Cytochem. 1983;31:1419–1421. doi: 10.1177/31.12.6195216. [DOI] [PubMed] [Google Scholar]
- Chi EY, Lagunoff D. Abnormal mast cell granules in the beige (Chédiak-Higashi syndrome) mouse. J Histochem Cytochem. 1975;23:117–122. doi: 10.1177/23.2.46876. [DOI] [PubMed] [Google Scholar]
- Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43(Suppl):25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972;38:500. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Morgan ES. Ribonuclease-gold labels heparin in human mast cell granules. New use for an ultrastructural enzyme affinity technique. J Histochem Cytochem. 1998;46:695–706. doi: 10.1177/002215549804600601. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Dvorak HF, Galli SJ. Ultrastructural criteria for identification of mast cells and basophils in humans, guinea pigs, and mice. Am Rev Respir Dis. 1983;128:S49–S52. doi: 10.1164/arrd.1983.128.2P2.S49. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Morgan ES, Lichtenstein LM, Weller PF, Schleimer RP. RNA is closely associated with human mast cell secretory granules, suggesting a role(s) for granules in synthetic processes. J Histochem Cytochem. 2000;48:1–12. doi: 10.1177/002215540004800101. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Erlinger R, Schumacher U, Welsch U. Ultrastructural localization of glycosaminoglycans in the human mammary gland. Acta Histochem Suppl. 1990;40:65–70. [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus (complex)- (1954-1981)—from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk WP, Taylor GM. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8:1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodispersed gold suspensions. Nat Phys Sci. 1973;241:20–22. [Google Scholar]
- Fritz P, Müller J, Reiser H, Saal JG, Hadam M, Tuczek HV, Wegner G, Laschner W. Avidin-peroxidase. A new mast cell staining method. Acta Histochem Suppl. 1986;32:235–239. [PubMed] [Google Scholar]
- Gasic GJ, Berwick L, Sorrentino M. Positive and negative colloidal iron as cell surface electron stains. Lab Invest. 1968;18:63–71. [PubMed] [Google Scholar]
- Goode NP, Shires M, Aparicio SR, Davison AM. Cationic colloidal gold—a novel marker for the demonstration of glomerular polyanion status in roetin renal biopsies. Nephrol Dial Transplant. 1991;6:923–930. doi: 10.1093/ndt/6.12.923. [DOI] [PubMed] [Google Scholar]
- Green NM. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Gustafson GT, Pihl E. Staining of mast cell acid glycosaminoglycans in ultrathin sections by ruthenium red. Nature. 1967;216:697–698. doi: 10.1038/216697a0. [DOI] [PubMed] [Google Scholar]
- Haigh M, Scott JE. A method of processing tissue sections for staining with Cu promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30:479–486. [PubMed] [Google Scholar]
- Hammel I, Lagunoff D, Bauza M, Chi E. Periodic, multimodal distribution of granule volumes in mast cells. Cell Tissue Res. 1983;228:51–59. doi: 10.1007/BF00206264. [DOI] [PubMed] [Google Scholar]
- Hammel I, Dvorak AM, Galli SJ. Defective cytoplasmic granule formation. I. Abnormalities affecting tissue mast cells and pancreatic acinar cells of beige mice. Lab Invest. 1987;56:321–328. [PubMed] [Google Scholar]
- Hammel I, Elmalek M, Castel M, Kalina M. Variability in gold bead density in cells. Quantitative immunocytochemistry. Histochemistry. 1989;91:527–530. doi: 10.1007/BF00492527. [DOI] [PubMed] [Google Scholar]
- Hammel I, Arizono N, Galli SJ. Mast cells in rat dermis and jejunal lamina propria show a five-fold difference in unit granule volume. Cell Tissue Res. 1991;265:329–334. doi: 10.1007/BF00398080. [DOI] [PubMed] [Google Scholar]
- Hammel I, Dvorak AM, Fox P, Shimoni E, Galli SJ. Defective cytoplasmic granule formation. II. Differences in patterns of radiolabeling of secretory granules in beige versus normal mouse pancreatic acinar cells after [3H] glycine administration in vivo. Cell Tissue Res. 1998;293:445–452. doi: 10.1007/s004410051136. [DOI] [PubMed] [Google Scholar]
- Horisberger M, Rosset J, Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975;31:1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Herrmann W, Schenk RK. Ruthenium hexamine trichloride (RHT)-mediated interaction between plasmalemmal components and pericellular matrix proteoglycans is responsible for the preservation of chondrocytic plasma membranes in situ during cartilage fixation. J Histochem Cytochem. 1983;31:717–727. doi: 10.1177/31.6.6341460. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Mosley SM, Jeffrey IJ, Stoddart RW. Elimination of the non-specific binding of avidin to tissue sections. Histochem J. 1987;19:264–268. doi: 10.1007/BF01675685. [DOI] [PubMed] [Google Scholar]
- Juarranz A, Ferrer JM, Tato A, Cañete M, Stockert JC. Metachromatic staining and electron dense reaction of glycosaminoglycans by means of cuprolinic blue. Histochem J. 1987;19:1–6. doi: 10.1007/BF01675286. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137a. [Google Scholar]
- Kashio N, Tsuyama S, Ihida K, Murata F. Cationic colloidal gold—a probe for light- and electron-microscopic characterization of acidic glycoconjugates using poly–lysine gold complex. Histochem J. 1992;24:419–430. doi: 10.1007/BF01089104. [DOI] [PubMed] [Google Scholar]
- Kasper CS, Tharp MD. Quantification of cutaneous mast cells using morphometric point counting and a conjugated avidin stain. J Am Acad Dermatol. 1987;16:326–331. doi: 10.1016/s0190-9622(87)70044-9. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Contributions of electron microscopy to the study of mast cells. J Invest Dermatol. 1972a;58:296–311. doi: 10.1111/1523-1747.ep12540314. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Vital staining of mast cells with ruthenium red. J Histochem Cytochem. 1972b;20:938–944. doi: 10.1177/20.11.938. [DOI] [PubMed] [Google Scholar]
- Lawrenson JG, Reid AR, Allt G. Molecular characterization of anionic sites on the luminal front of endoneural capillaries in sciatic nerve. J Neurocytol. 1994;23:29–37. doi: 10.1007/BF01189814. [DOI] [PubMed] [Google Scholar]
- Lew S, Hammel I, Galli SJ. Cytoplasmic granule formation in mouse pancreatic acinar cells. Evidence for formation of immature granules (condensing vacuoles) by aggregation and fusion of progranules of unit size, and for reductions in membrane surface area and immature granule volume during granule maturation. Cell Tissue Res. 1994;278:327–336. doi: 10.1007/BF00414176. [DOI] [PubMed] [Google Scholar]
- Luft JH. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971;171:347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MacBride RG. Potential use of embalmed cadavers to study mast cell presence. Anat Rec. 1998;250:117–120. doi: 10.1002/(SICI)1097-0185(199801)250:1<117::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Markey AC, Churchill LJ, MacDonald DM. Human cutaneous mast cells—a study of fixative and staining reactions in normal skin. Br J Dermatol. 1989;120:625–631. doi: 10.1111/j.1365-2133.1989.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Mayer F, Rohde M. Analysis of dimensions and structural organization of proteoliposomes. Methods Bacteriol. 1988;20:283–292. [Google Scholar]
- Morris RE, Saelinger CB. Problems in the production and use of 5 nm avidin-gold colloids. J Microsc. 1986;143:171–176. doi: 10.1111/j.1365-2818.1986.tb02775.x. [DOI] [PubMed] [Google Scholar]
- Orkin RW, Williams BR, Cranley RE, Poppke DC, Brown KS. Defects in the cartilaginous growth plates of brachymorphic mice. J Cell Biol. 1977;73:287–299. doi: 10.1083/jcb.73.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkening TA, Pal D. GnRH receptor site increase on the surface of cultured gonadotropes of senescent C57BL/6NNia mice. J Gerontol A Biol Sci Med Sci. 1995;50:B342–B350. doi: 10.1093/gerona/50a.6.b342. [DOI] [PubMed] [Google Scholar]
- Pennypacker JP, Kimata K, Brown KS. Brachymorphic mice (bm/bm): a generalized biochemical defect expressed primarily cartilage. Dev Biol. 1981;81:280–287. doi: 10.1016/0012-1606(81)90291-8. [DOI] [PubMed] [Google Scholar]
- Reggio HA, Palade GE. Sulfated compounds in the zymogen granules of the guinea pig pancreas. J Cell Biol. 1978;77:288–314. doi: 10.1083/jcb.77.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P, Csaba G. Alcian blue-safranine staining and ultrastructure of rat mast cell granules during degranulation. Acta Biol Acad Sci Hung. 1972;23:83–89. [PubMed] [Google Scholar]
- Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol. 2008;83:1079–1087. doi: 10.1189/jlb.1107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Light and electron microscopic localization of antigens with protein A-gold (pAg) technique. In: DeLellis RA, editor. Diagnostic immunochemistry. Vol. 2. Masson; New York: 1984. pp. 43–65. [Google Scholar]
- Roth J, Binder M. Colloidal gold, ferritin and peroxidase as markers for electron microscopic double labeling lectin techniques. J Histochem Cytochem. 1978;26:163–169. doi: 10.1177/26.3.632554. [DOI] [PubMed] [Google Scholar]
- Ruggeri A, Dell'orbo C, Quacci D. Electron microscopic visualization of proteoglycans with Alcian blue. Histochem J. 1975;7:187–197. doi: 10.1007/BF01004562. [DOI] [PubMed] [Google Scholar]
- Saga K, Takahashi M. Demonstration of anionic sites in human eccrine and apocrine sweat glands in post-embedded ultrathin sections with cationic colloidal gold: effect of enzyme digestion on these anionic sites. J Histochem Cytochem. 1993;41:1197–1207. doi: 10.1177/41.8.8331283. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Domowicz M. Chondrodysplasias due to proteoglycan defects. Glycobiology. 2002;12:57R–68R. doi: 10.1093/glycob/12.4.57r. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Ostrowski V, Brown KS, Pratt RM. Defective PAPS-synthesis in epiphyseal cartilage from brachymorphic mice. Biochem Biophys Res Commun. 1978;82:173–178. doi: 10.1016/0006-291x(78)90592-2. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Lyle S, Ozeran JD, Li H, Deyrup A, Ng K, Westley J. Sulfate activation and transport in mammals: system components and mechanisms. Chem Biol Interact. 1998;109:143–151. doi: 10.1016/s0009-2797(97)00129-4. [DOI] [PubMed] [Google Scholar]
- Scott JE. Alcian blue. Now you see it, now you don't. Eur J Oral Sci. 1996;104:2–9. doi: 10.1111/j.1600-0722.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Shoichetman T, Skutelsky E, Lew S, Hammel I. Changes in the distribution of anionic constituents in secretory granules of mouse pancreatic acinar cells after pilocarpine-induced degranulation. J Histochem Cytochem. 2001;49:1199–1204. doi: 10.1177/002215540104901001. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Bayer EA. The ultrastructural localization of cell surface glycoconjugates: affinity cytochemistry via avidin-biotin complex. Biol Cell. 1979;36:237–252. [Google Scholar]
- Skutelsky E, Danon D. Redistribution of surface anionic sites on the luminal front of blood vessel endothelium after interaction with polycationic ligand. J Cell Biol. 1975;71:232. doi: 10.1083/jcb.71.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E, Hardy B. Regeneration of plasmalemma and surface properties in macrophages. Exp Cell Res. 1976;101:337. doi: 10.1016/0014-4827(76)90386-4. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Roth J. Cationic colloidal gold—a new probe for the detection of anionic cell surface sites by electron microscopy. J Histochem Cytochem. 1986;34:693–696. doi: 10.1177/34.5.3701033. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Goyal V, Alroy J. The use of avidin-gold complex for light microscopic localization of lectin receptors. Histochemistry. 1987;86:291–295. doi: 10.1007/BF00490261. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Bar-Shira B, Maymon R, Shalgi R. Histochemical characterization of anionic constituents in oocyte-cumulus complex of rats. Histochemistry. 1992;98:299–304. doi: 10.1007/BF00270013. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Shoichetman T, Hammel I. An histochemical approach to characterization of anionic constituents in mast cell secretory granules. Histochem Cell Biol. 1995;104:453–458. doi: 10.1007/BF01464335. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman; New York: 1995. [Google Scholar]
- Spicer SS, Horn RG, Leppi TJ. Histochemistry of connective tissue mucopolysaccharides. In: Wanger BM, Smith DE, editors. The connective tissue. Williams and Wilkins; Baltimore: 1967. pp. 251–303. [Google Scholar]
- Tato A, Ferrer JM, Quintana E, Romero JB, Del Castillo P, Stockert JC. Observations on the contrasting reaction of some electron dense stains applied on epoxy-embedded tissue sections. Z Mikrosk Anat Forsch. 1990;104:337–348. [PubMed] [Google Scholar]
- Tharp MD, Seelig LL, Jr, Tigelaar RE, Bergstresser PR. Conjugated avidin binds to mast cell granules. J Histochem Cytochem. 1985;33:27–32. doi: 10.1177/33.1.2578142. [DOI] [PubMed] [Google Scholar]
- Thiéry JP, Ovtracht L. Differential characterization of carboxyl and sulfate groups in thin sections for electron microscopy. Biol Cell. 1979;36:281–288. [Google Scholar]
- Ueda H, Kato Y, Ohno S. Quantitative detection of anionic sites in rat femoral cartilage using cationic colloidal gold at low pH levels. Histol Histopathol. 1998;13:1001–1009. doi: 10.14670/HH-13.1001. [DOI] [PubMed] [Google Scholar]
- ul Haque MF, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, Superti-Furga A, Haque S, Abbas H, Ahmad W, Ahmad M, Cohn DH. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat Genet. 1998;20:157–162. doi: 10.1038/2458. [DOI] [PubMed] [Google Scholar]
- Vanky P, Brockstedt U, Nurminen M, Wikström B, Hjerpe A. Growth parameters in the epiphyseal cartilage of brachymorphic (bm/bm) mice. Calcif Tissue Int. 2000;66:355–362. doi: 10.1007/s002230010073. [DOI] [PubMed] [Google Scholar]
- Vorbordt AW. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J Neurocytol. 1989;18:359–368. doi: 10.1007/BF01190839. [DOI] [PubMed] [Google Scholar]
- Winograd E, Sherman IW. Characterization of a modified red cell membrane protein expressed on erythrocytes infected with the human malaria parasite Plasmodium falciparum: possible role as a cytoadherent mediating protein. J Cell Biol108. 1989:23–30. doi: 10.1083/jcb.108.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Shimizu S, Brown KS, Kimata K. The histochemistry of complex carbohydrates in certain organs of homozygous brachymorphic (bm/bm) mice. Histochem J. 1984;16:587–599. doi: 10.1007/BF01003387. [DOI] [PubMed] [Google Scholar]