Abstract
To establish the diagnosis of virtually any disease, the clinician must combine a variety of information. Often emphasised in this context is thorough medical history-taking including information on exposure to factors leading to or being associated with the disease in question. Continuous assessment of all available information is of utmost importance, as fixation on single details can be misguiding with inappropriate consequences in both the diagnostic and therapeutic approach. This case report presents how an atypical medical history led to a delay in the diagnosis of malignant pleural mesothelioma due to a low a priori likelihood of the disease because of non-exposure to asbestos. We highlight the fact that postrationalisation and attempts to renew a diagnostic approach must be carried out each time diagnostic dilemmas emerge, and when some or all diagnostic clues disagree.
Background
To establish the diagnosis of virtually any disease, the clinician must combine a variety of information.1 Often emphasised in this context is thorough medical history-taking, including information on exposure to factors leading to or being associated with the disease. Continuous assessment of all available information is of utmost importance, as fixation on single details can be misguiding with inappropriate consequences in both the diagnostic and therapeutic approach.
This case report presents how an atypical medical history led to a delay in the diagnosis of malignant pleural mesothelioma due to a low a priori likelihood of the disease because of non-exposure to asbestos.
Case presentation
A middle-aged woman with no previous medical history was admitted to hospital due to fever, dyspnoea, productive cough, minor weight loss and night sweats. Haemoptysis, chest pain and ankle oedema were not reported, and on physical examination she did not appear to be in major distress. Diminished breath sounds over the left hemithorax were noticed, but otherwise the physical examination was unremarkable. The temperature was 37.8°C, blood pressure was 130/82 mm Hg, pulse was 104/min and oxygen saturation was 96% on room air. On detailed questioning, the patient and her relatives had no history of exposure to asbestos.
Investigations
Biochemical analyses were notable only for moderate elevation of C reactive protein. Blood cultures were without the growth of bacteria or fungus. A chest x-ray and CT of the chest are shown in figures 1 and 2, respectively, and revealed left-sided pleural thickening and basal pleural effusion. Only sparse pleural fluid (biochemically characterised as an exudate) with jelly-like consistency was drained. On more occasions, analyses were negative for bacterial and fungal growth, but sufficient exfoliative cytology examination was not possible due to degeneration and necrosis. On suspicion of malignant pleural mesothelioma, a diagnostic left-sided video-assisted thoracoscopic surgery (VATS) was conducted because of the lack of response to antibiotics and pleural drainage. This procedure, performed approximately 1 month after first hospital contact, uncovered white and thick pleural surfaces from which biopsies showed biphasic malignant mesothelioma (figure 3).
Figure 1.
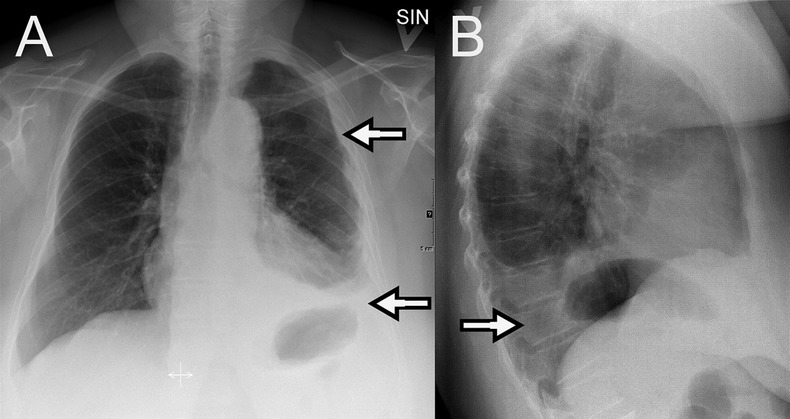
Chest x-ray at admission in posterior-anterior projection (A) and lateral projection (B). The findings are typical for pleural effusion (arrows) and pleural thickening which is surrounding the entire left lung. Courtesy of Department of Radiology, Odense University Hospital.
Figure 2.
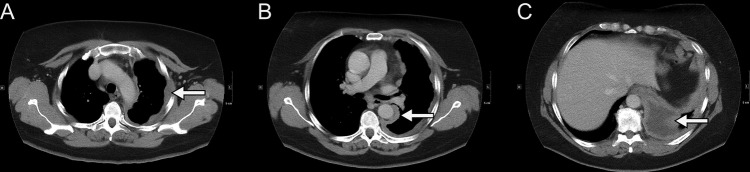
CT of the chest with axial image at aortic arch level (A), at pulmonary trunk level (B) and at the lung bases (C). The pleura is thickened and lobulated at all levels (arrows) and at the base of the left lung a minor effusion is seen. Courtesy of Department of Radiology, Odense University Hospital.
Figure 3.
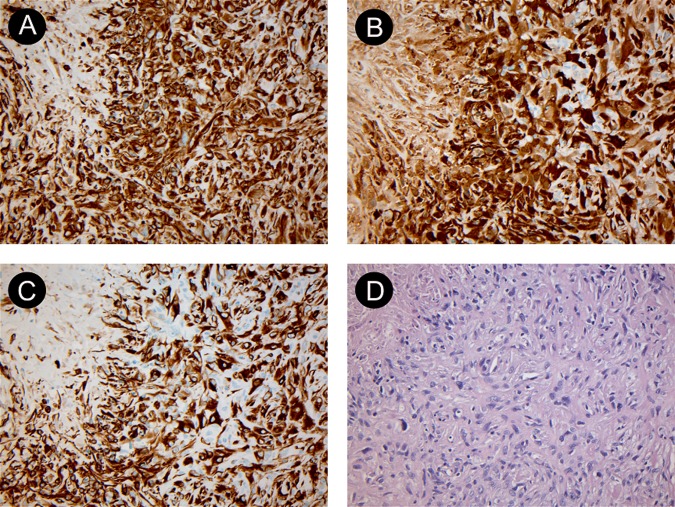
Histology specimens from video-assisted thoracoscopic surgery showing biphasic malignant pleural mesothelioma. (A) shows a vimentin stain ×200, (B) shows calretinin stain ×200, (C) shows cytokeratin stain ×200 and (D) shows H&E stain ×200. The specimens were examined by the Department of Pathology at our institution and reviewed by the national reference centre for malignant pleural mesothelioma. Courtesy of Department of Pathology, Odense University Hospital.
Differential diagnosis
The imaging studies were interpreted clinically as indicative of either empyema or malignant pleural mesothelioma. Because of fever, elevated C reactive protein and the lack of asbestos exposure, empyema was initially considered the most likely diagnosis. However, treatment failure of otherwise appropriate antibiotics and pleural drainage lead to the consideration of malignant pleural mesothelioma.
Treatment
Initial, when empyema was considered likely, treatment comprised of the administration of intravenous broad-spectrum antibiotics (meropenem and metronidazole) and insertion of a chest tube. Later, when the diagnosis of malignant pleural mesothelioma was established, the patient was treated with carboplatin and pemetrexed.
Outcome and follow-up
Despite treatment, the general condition was further impaired, and the patient died 5 months after the initial hospital admission. No autopsy was performed.
Discussion
Asbestos has been identified as the single most important carcinogen associated with malignant pleural mesothelioma, with a gap between exposure and disease development of 20–50 years.2 3 The importance of thorough interviewing about asbestos is emphasised in current guidelines.4
Often, the delay in diagnosing malignant pleural mesothelioma is due to non-specific symptoms like dyspnoea and chest pain, and often the first finding is unexplained unilateral pleural effusion. Typical differential diagnosis comprises pleural infection, benign asbestos-related pleural disease and other malignant conditions with pleural dissemination. The overall median survival time of malignant pleural mesothelioma approximates 12 months.5 Despite this disappointing fact, rapid and early diagnosis is crucial to identify the minority of patients who are candidates for intended curative treatment before declining overall performance status, and disease progression hinders this.6 7
In this case report, the diagnosis of malignant pleural mesothelioma or empyema was suggested by initial imaging studies, but malignant pleural mesothelioma was considered less likely as a consequence of non-exposure to asbestos. Instead, the patient was treated for empyema. As the information from the imaging studies disagreed with the negative culture of pleural fluid, the lack of biochemical improvement, and especially the inappropriate clinical response to otherwise rational treatment for empyema, the correctness of the fixation on the unlikely link between non-exposure to asbestos and malignant pleural mesothelioma was questioned and led to a diagnostic VATS biopsy.
The objective of this case report is to illustrate the importance and complexity of correct diagnosis generation and to underline the importance of reassessing a diagnosis when a patient does not respond to otherwise effective therapy.
Preoccupation with positive or negative associations or findings during the diagnostic process, leading to delay in malignant lung disease, has recently been described in this journal.8 Timely and correct diagnostic workup is a complex discipline familiar to most practitioners of clinical medicine. It depends on multiple elements including (1) patient factors (eg, nature and urgency of symptoms, social and psychological traits, test outcomes and on exposure to factors leading to or being associated with the diagnosis under consideration), (2) societal/socioeconomic factors (eg, accessibility of care facilities, economy, health insurance, political demands and degree of defensive medicine practice) and (3) physician factors (eg, experience, diligence, training and talent).1
In conclusion, this case report emphasises the importance of postrationalisation and importantly points out that attempts to renew a diagnostic approach must be carried out each time diagnostic dilemmas emerge, and when some or all diagnostic clues disagree. In an effort to assemble all details to a correct diagnosis, the physician should not put all weight on one or few positive or negative findings, but consider whether each counter agrees with, and fits into, the diagnostic puzzle.
Learning points.
When planning diagnostic work-up for pleural disease, malignant pleural mesothelioma should be considered even when no exposure to asbestos can be established.
When malignant pleural mesothelioma is suspected, prompt diagnosis and treatment is warranted before the general condition of the patient hinders this.
When diagnosing and treating virtually all diseases, a lack of response to otherwise effective treatment should result in reconsideration of the diagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mark BM. Descion-making in clinical medicine. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, eds. Harrison's principles of internal medicine. 16th edn New York: McGraw-Hill, 2005:6–13 [Google Scholar]
- 2.Kao SCH, Reid G, Lee K, et al. Malignant mesothelioma. Intern Med J 2010;2013:742–50 [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis and pathogenesis. Curr Treat Options Oncol 2008;2013:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Thoracic Society Standards of Care Committee BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;2013:ii1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson BWS, Lake RAL. Advances in malignant mesothelioma. N Engl J Med 2005;2013:1591–603 [DOI] [PubMed] [Google Scholar]
- 6.Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer Experience. J Clin Oncol 1998;2013:145–52 [DOI] [PubMed] [Google Scholar]
- 7.Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965–1985. J Clin Oncol 1988;2013:147–53 [DOI] [PubMed] [Google Scholar]
- 8.Revannasiddaiah S, Madabhavi I, Thakur P, et al. Undue delay in the diagnosis of lung cancer due to the clinician's preoccupation with pre-existing tuberculosis. BMJ Case Rep. Published: 1 Dec 2011. doi:10-1136/bcr.10.2011.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]