Abstract
A 25-year-old woman presented with severe dyspnoea at an emergency care unit on her postpartum day 7. Her O2 saturation level was low. Blood tests showed a high blood D-dimer level; echocardiography showed a high pulmonary artery pressure. Initially, heparin was administered for suspicion of pulmonary embolism. After transfer to the intensive care unit, she suffered respiratory failure. A three-dimensional (3D) reconstruction CT angiography then revealed a giant patent ductus arteriosus. Extracorporeal membrane oxygenation was performed owing to low O2 saturation after ventilator use. After 1 month, she died of multiple organ failure. In postpartum patients with congenital heart disease, a diagnosis of pulmonary embolism should be immediately confirmed by 3D reconstruction CT angiography to rule out patent ductus arteriosus.
Background
The 25-year-old woman reported in this case was from mainland China and had been previously diagnosed with an unspecified congenital heart disease. Interviews with her husband revealed that they had been married since 1 year and that, during her pregnancy, she had experienced no dyspnoea, no dyspnoea on exertion (DOE) or orthopnoea. During the previous week, the patient had given birth by natural spontaneous vaginal delivery at a local clinic. During the week after delivery, she suffered progressively worsening symptoms of tachycardia, dyspnoea, DOE and intermittent chest pain. She was sent to a regional hospital, where she received blood tests showing a high D-dimer level. However, chest CT showed no obvious thrombus over the pulmonary artery trunk. She was then transferred to the emergency care unit, where a low O2 saturation under non-rebreathing mask was noted. Physical examination then revealed jugular vein engorgement and orthopnoea. Her D-dimer was 1294 ng/ml, and her cardiac enzyme level was normal. ECG showed right ventricular hypertrophy with qR pattern over V1 (figure 1B) and right axis deviation. Transthoracic echocardiography showed a high peak systolic pressure gradient of 140 mm Hg across the tricuspid valve and moderate right ventricular systolic dysfunction. She was then admitted to the cardiac intensive care unit under a strong suspicion of pulmonary embolism.
Figure 1.
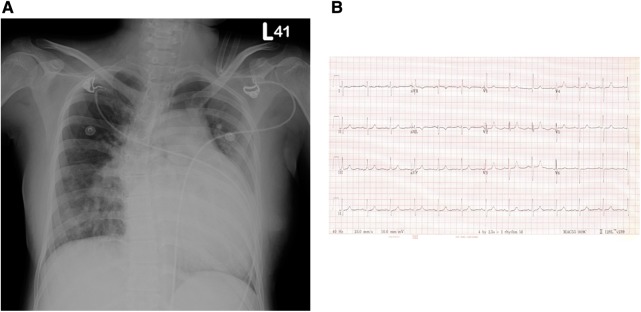
(A) Chest x-ray revealed a prominent enlarged pulmonary trunk and cardiomegaly with right ventricular hypertrophy. (B) ECG revealed right ventricular hypertrophy (qR pattern over V1) and right axis deviation.
Case presentation
A 25-year-old woman presented at an emergency care unit on her postpartum day 7 with severe dyspnoea, orthopnoea and chest pain.
Chest x-ray (CXR; figure 1A) revealed a prominent enlargement of the pulmonary trunk and cardiomegaly with right ventricular hypertrophy. Her D-dimer was 1294 ng/ml and her cardiac enzyme levels were normal. The risk of deep vein thrombosis is increased during pregnancy, and further accrual and formation of new clots may increase the risk of pulmonary embolism. The patient denied any history of systemic disease except for a previous diagnosis with an unspecified congenital heart disease.
The patient was afebrile, her blood pressure was 130/70 mm Hg, regular pulse 87 bpm and respiratory rate 20 breaths/min with O2 saturation of 94% under nasal cannula 2 l/min. Jugular venous pressure had increased due to engorgement. Mild pitting oedema of the lower extremities was noted. Heart sounds were normal with no obvious murmurs and lungs were clear on auscultation. Abdominal examination was unremarkable.
Investigations
Blood tests by latex immunoassay revealed an abnormally high D-dimer of 1294 ng/ml (a normal D-dimer of <500 ng/ml; rules out pulmonary embolism in 95% of cases), troponin I level of 0.106 ng/ml (0–0.1 ng/ml is considered normal), arterial blood gas PaO2 of 66 mm Hg (nasal cannula 2 l/min O2), haemoglobin 12.1 g% (12–16 g% is considered normal for females) and haematocrit 37.1% (38–47% is considered normal for females). ECG (figure 1B) showed qR pattern over V1 and right axis deviation. CXR (figure 1A) revealed a prominent enlarged pulmonary trunk and cardiomegaly owing to right ventricular hypertrophy. Echocardiography showed a high peak systolic pressure gradient of 140 mm Hg across the tricuspid valve, moderate right ventricular systolic dysfunction and paradoxical motion of the interventricular septum with the presence of D-shaped left ventricle. Therefore, the patient was suspected with acute pulmonary embolism. Further three-dimensional (3D) reconstruction CT angiography revealed a giant patent ductus arteriosus and no obvious thrombus over pulmonary artery (figure 2). The postpartum patient was diagnosed with a giant patent ductus arteriosus with Eisenmenger syndrome.
Figure 2.
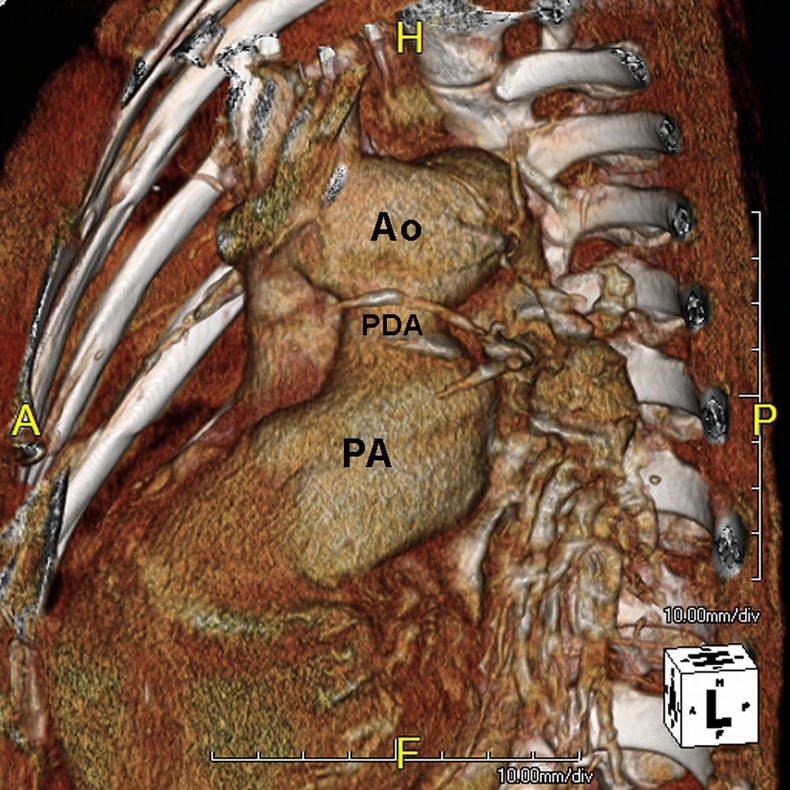
Chest CT angiography three-dimensional reconstruction showed a giant patent ductus arteriosus connecting the pulmonary artery to the aortic arch.
Differential diagnosis
Pulmonary embolism
Patent ductus arteriosus
Pulmonary valve stenosis
Atrial septal defect
Ventricular septal defect
Treatment
After admission to the intensive care unit, the patient revealed a low O2 (88–90%) saturation under the non-rebreathing mask (100% O2). Heparin was administered as an initial treatment for pulmonary embolism. However, her dyspnoea persisted and progressively worsened. Eventually, hypoxaemia and respiratory failure occurred. After the insertion of an endotracheal tube and ventilation, O2 saturation reached 50%. Further, 3D reconstruction CT angiography showed a giant patient ductus arteriosus and no obvious thrombus over pulmonary artery. The patient was further treated with extracorporeal membrane oxygenation, but the pulmonary hypertension persisted.
Outcome and follow-up
The report describes a case of Eisenmenger syndrome, which is defined as a patent ductus arteriosus with right-to-left shunt. The patient received extracorporeal membrane oxygenation, but the high pulmonary hypertension persisted and the patient died from multiple organ failure after 1 month of treatment. Patient with this syndrome have a perinatal death rate of approximately 25% and a maternal death rate exceeding 50%.
Discussion
A patent ductus arteriosus is a neonatal congenital heart disease in which the ductus arteriosus fails to close after birth. If left untreated in a patient, the ductus may eventually progress from left and right shunt to right and left shunt, a medical condition known as Eisenmenger syndrome.
The syndrome has a high death rate and is associated with a risk of sudden death.1–3 Maternal mortality in patients with Eisenmenger syndrome is reportedly 30–50%.4 5 The rate of fetal mortality in patients with the syndrome is approximately 25%, while the maternal mortality rate exceeds 50%. Several cases of sudden death have been reported in women at 4–6 weeks postpartum.4 Although the exact pathophysiological cause of postpartum sudden death is unclear, it may involve pregnancy hormones acting in concert with the progression of Eisenmenger syndrome.
One study of Eisenmenger syndrome5 revealed clinical deterioration in all postpartum survivors analysed and concluded that physicians should advise patients diagnosed with the syndrome to terminate pregnancy. No currently available treatment has proven effective for the clinical treatment and management of Eisenmenger syndrome.5 Patients with end-stage Eisenmenger syndrome eventually require heart and lung transplantation. As the disease progresses to end-stage, patients with severely damaged lungs and heart require cardiac and respiratory support by extracorporeal membrane oxygenation. These patients are likely to die while awaiting heart and lung transplantation, whereas surviving patients can still receive heart and lung transplantation. However, mortality from heart and lung transplantation remains high.5
In patients with this congenital heart disease, those without complications usually have a fairly healthy childhood and gradually become overtly cyanotic during their second or third decade. However, good-to-excellent functional capacity continuing through the third decade has been reported.6 Fortunately, the 25-year-old woman reported in this case had a successful pregnancy and spontaneously delivered safely and naturally without complications. In this case, the good functional capacity of the patient despite her history of congenital heart disease during second to third decade was a major factor in her safe and successful pregnancy and delivery. During pregnancy, patients with congenital heart disease usually require a specialised care team that includes an anaesthetist, a cardiologist and an obstetrician starting at 25 weeks.7 Although the patient reported here was known to have an unspecified congenital heart disease, the patient and her family were not overly concerned because of the absence of symptoms such as dyspnoea, DOE and orthopnoea. She also declined further evaluation of the congenital heart disease before pregnancy. Owing to her symptoms, the patient was suggested for a left-to-right shunt in the patent ductus arteriosus before pregnancy. In the postpartum period, the left-to-right shunt progressed to a right-to-left shunt owing to severe dyspnoea, orthopnoea and desaturation.8 Generally, the blood volume of a healthy woman increases by 50% during 20–32 weeks of gestation.9 Heart rate and stroke volume also increased, which results in a high cardiac output. However, systemic and pulmonary vascular resistances decrease. In contrast, pulmonary pressure remains high in pulmonary hypertensive patients during this period because the pulmonary vascular resistance has already been elevated.10 The risks of hypercoagulation and thromboembolic events have also increased by a combination of factors associated with pregnancy including increased circulating levels of clotting factors, decreased protein S levels, increased resistance to activated protein C and increased risk of progesterone-mediated venous stasis.11
Studies of Gleicher et al4 and Stoddart and O'Sullivan12 show that thromboembolic events are a major cause of death during pregnancy in patients with Eisenmenger syndrome. Smedstad et al7 recommended anticoagulation therapy with heparin or warfarin during both the antepartum and postpartum periods, in patients with Eisenmenger syndrome, because pregnancy can easily cause venous stasis and clot formation associated with hypercoagulation and chronic hypoxia. High haematocrit levels also worsen coagulation and blood conductivity during pregnancy. Abnormally high pulmonary vascular resistance increases the risks of pulmonary embolism and shunt reversal. In postpartum patients with Eisenmenger syndrome, symptoms such as sudden onset of tachycardia, dyspnoea, DOE, orthopnoea and intermittent chest pain should raise a strong suspicion of acute pulmonary embolism, and ECG, arterial blood gas, D-dimer and CXR should be checked immediately. Notably, a blood testing showing a high D-dimer level (>500 ng/ml) cannot rule out pulmonary embolism. Haemodynamically unstable patients in critical condition should undergo transthoracic or transoesophageal echocardiography instead of CT to confirm a diagnosis of pulmonary embolism.13 If acute pulmonary embolism is confirmed, the patient should be treated with thrombolysis and heparin. The transthoracic echocardiographic window obtained in the patient reported here was insufficient for an accurate evaluation. A case report had mentioned that the sensitivity of transthoracic echocardiography for diagnosis of patent ductus arteriosus with Eisenmenger syndrome has been estimated as low as 12%. Although transoesophageal echocardiography is superior to transthoracic echocardiography for the diagnosis of patent ductus arteriosus with Eisenmenger syndrome, but has some serious complications. 3D reconstruction CT is a safe and good tool to distinguish patent ductus arteriosus with Eisenmenger syndrome from pulmonary hypertension.14 Therefore, multidetector CT with 3D reconstruction was performed.
The patient reported in this study had a history of congenital heart disease and was treated for symptoms of dyspnoea, orthopnoea and chest pain at 1 week after delivery. Blood tests, CXR and transthoracic echocardiography revealed a high D-dimer, an enlarged pulmonary trunk with cardiomegaly, a hypertrophic right ventricle and a high peak systolic pressure gradient across the tricuspid valve. She was diagnosed with pulmonary embolism based on the outstanding features of pulmonary hypertension revealed by CXR and transthoracic echocardiography. CT revealed that her congenital heart disease contributed to her symptoms but no obvious thrombus was noted over the pulmonary main trunk. Transoesophageal echocardiography and 3D reconstruction CT angiography was performed for further evaluation. Based on a giant patent ductus arteriosus revealed by angiography, the patient was diagnosed with Eisenmenger syndrome.
Learning points.
In postpartum patients with congenital heart disease, three-dimensional reconstruction CT angiography over the chest or transoesophageal echocardiography is recommended for diagnosing pulmonary embolism.
Postpartum patients with patent ductus arteriosus complicated by Eisenmenger syndrome may present with dyspnoea, and chest x-ray and transthoracic echocardiography may reveal the clinical features resembling those of pulmonary embolism.
Eisenmenger syndrome has a high death rate, and misdiagnosis of pulmonary embolism is common in postpartum patients with high pulmonary artery pressure.
Footnotes
Funding: This work was supported by the NSYSU-KMU Joint Research Project (NSYSUKMU 2012-026).
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Huyghe de Mahence A, Andre-Fouet X, Bernet D, et al. Syndrome d'Eisenmenger et grossesse. Ann Cardiol Angeiol (Paris) 1985;2013:547–9 [PubMed] [Google Scholar]
- 2.Heytens L, Alexancer JP. Maternal and neonatal death associated with Eisenmenger's syndrome. Acta Anaesthesiol Belg 1986;2013:45–51 [PubMed] [Google Scholar]
- 3.Lieber S, Dewilde P, Huyghens L, et al. Eisenmenger's syndrome and pregnancy. Acta Cardiol 1985;2013:421–4 [PubMed] [Google Scholar]
- 4.Gleicher N, Midwall J, Hochberger D, et al. Eisenmenger's syndrome and pregnancy. Obstet Gynecol Surg 1979;2013:721–41 [DOI] [PubMed] [Google Scholar]
- 5.Daliento L, Somerville J, Presbitero P, et al. Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J 1998;2013:1845–55 [DOI] [PubMed] [Google Scholar]
- 6.Bonow RO, Mann PL, Zipes DP, et al. Braunwald's heart disease, a textbook of cardiovascular medicine. 9th edn. Philadelphia: Elsevier Science 2011:1418–9 [Google Scholar]
- 7.Smedstad KG, Cramb R, Morison DH. Pulmonary hypertension and pregnancy: a series of eight cases. Can J Anaesth 1994;2013:502–12 [DOI] [PubMed] [Google Scholar]
- 8.Bassily-Marcus AM, Yuan C, Oropello J, et al. Pulmonary hypertension in pregnancy: critical care management. Pulm Med 2012;2013:709407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J 1992;2013:540–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madden BP. Pulmonary hypertension and pregnancy. Int J Obstet Anesth 2009;2013:156–64 [DOI] [PubMed] [Google Scholar]
- 11.Stone SE, Morris TA. Pulmonary embolism during and after pregnancy. Crit Care Med 2005;2013:S294–300 [DOI] [PubMed] [Google Scholar]
- 12.Stoddart P, O'Sullivan G. Eisenmenger syndrome in pregnancy: a case report and review. Int J Obstet Anesth 1993;2013:159–68 [DOI] [PubMed] [Google Scholar]
- 13.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;2013:266–74 [DOI] [PubMed] [Google Scholar]
- 14.Ahmetoqlu A, Kosucu P, Gokce M, et al. Case report: 3 D CT angiography in a patent ductus arteriosus associated with Eisenmenger syndrome. Tani Girisim Radyol 2003;2013:84–6 [PubMed] [Google Scholar]


