Cardiovascular research owes much to Ed Sonnenblick. Few would dispute that his understanding of the cellular basis of cardiac function have ushered in a new era of heart research, the era of the heart muscle cell.1,2 In the course of his work Ed had more than a passing interest in cardiac energetics, although he never ventured in the complexities of intermediary metabolism.3,4 If he had done so he would have enjoyed the logic of metabolic regulation as much as he enjoyed the logic of the organization of the sarcomere.5,6,7, In the following we have made an attempt to review metabolic aspects of energy transfer in the heart with a focus on the regulation of energy substrate metabolism in response to stress- the metabolic reserve of the heart.
A few principal comments are in order. The heart converts chemical energy present in the form of substrates and oxygen to mechanical energy (measured as cardiac work) and heat (measured as calories).8 An abrupt increase in cardiac work results in the recruitment of reserves in contractility and coronary flow.9,10 In clinical practice the coronary flow reserve is used to quantitate the capacity of the coronary circulation,11 while contractile reserve is used to quantitate the response of the heart muscle to inotropic stimulation.12 Missing in this equation is the heart’s metabolic reserve which cannot be assessed as readily as coronary flow reserve and contractile reserve. However we argue that increases in contractility are coupled to increases in cardiac metabolism and form an integral part of cardiac function. In contrast to contractile function, the metabolic function is, however, very difficult to assess.
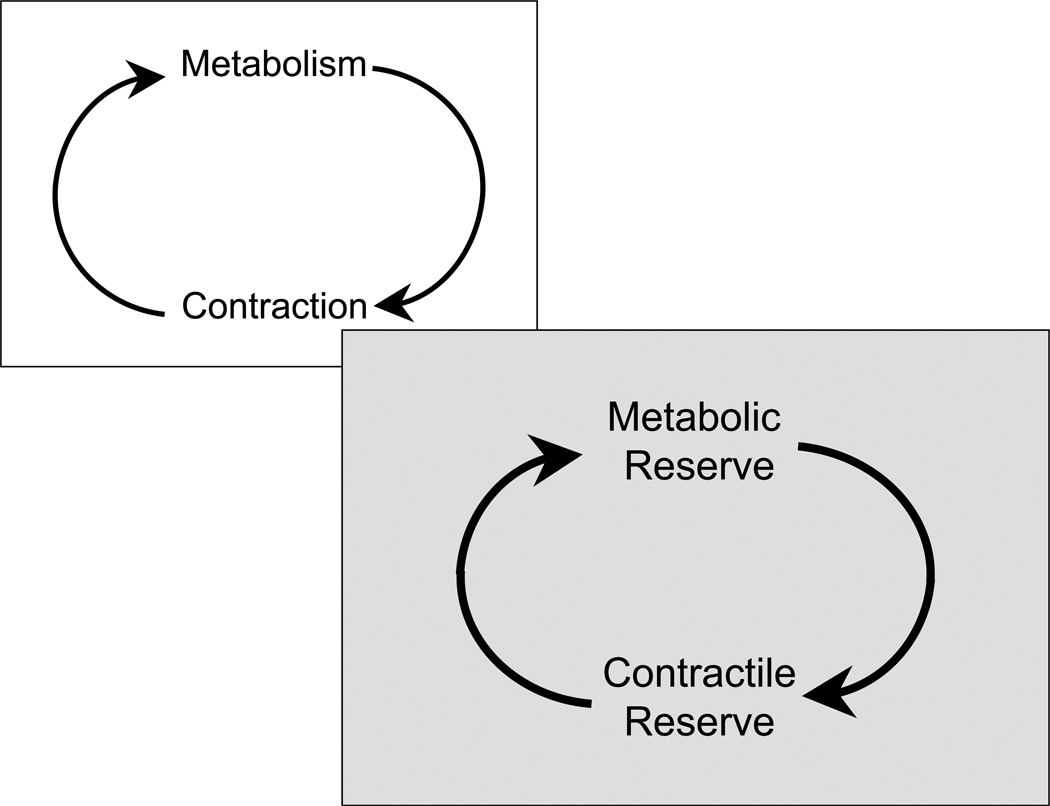
We have previously proposed that metabolism (in the form of metabolic signals) provides the link between contraction and gene expression.13 We now propose that metabolism forms a link between contractile reserve and coronary flow reserve (Figure 1). The metabolic reserve, called up by an acute increase in work load of the heart, involves the mobilization of glycogen, a switch of substrate oxidation from fat to carbohydrates and the capacity to increase oxidative metabolism of energy providing substrates. We will explore the notion that impairment of the metabolic reserve results in contractile dysfunction of the stressed heart, while restoration of the metabolic reserve restores contractile function.
Figure 1.
A single schematic showing the tight link between metabolism and contractile function of the heart. Increased contractile performance requires increased rates of energy substrate metabolism. See text for further discussion.
Muscle as a model for contractile and metabolic adaptation and maladaptation
Contractile Adaptation: Resting and Running
In order to understand the concept of metabolic reserve in heart muscle it helps to examine analogies provided by the physiology of skeletal muscle. Here we follow Ed Sonnenblick’s reasoning and experimental strategy: His dictum was that “a muscle is a muscle”. In the transition from rest to exercise the skeletal muscle mobilizes its energy stores in order to meet increased demands. There are no reserves of ATP in muscle. Instead, energy is stored in muscle in three forms (Table 1). The first is phosphocreatine which can rapidly donate its high energy phosphates to produce ATP from ADP. Energy available from phosphocreatine (PCr) is relatively modest, although it has been estimated that PCr can contribute about half of the amount of energy expended during a 100 meter sprint race.14,15 However, when energy requirements are sustained, PCr is used only during very rapid bursts of exercise16 and the muscles have to rely on aerobic metabolism of the other two major stores. The second endogenous form of energy is glycogen. Glycogen provides 16 kilojoules (KJ) of energy per gram (g) oxidized (Table 1). 17
Table 1.
Energy Stores in the Muscle
| Compound | Rate of Mobilization/ Rate of Restoration |
Energy yield (Kj/g wet weight) |
|---|---|---|
| Phosphocreatine | Very fast | 0.204 |
| Glycogen | Fast | 16 |
| Triglycerides | Slow | 35 |
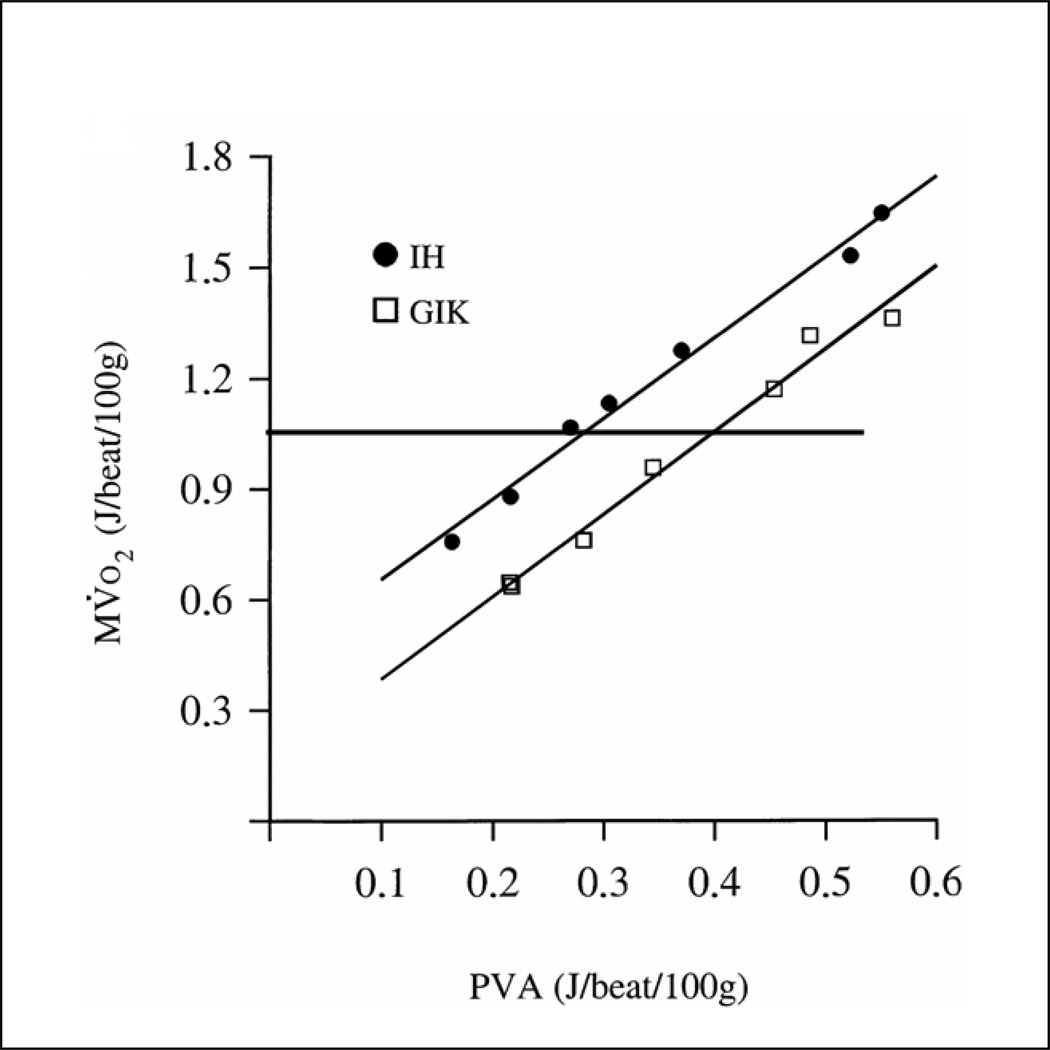
Because glycogen is stored along with hydration water, the muscle’s storage capacity for glycogen is limited. However, glycogen has the advantage that it consumes much less oxygen compared to fatty acids for the same amount of energy converted to mechanical work. In addition, the rapid activation of phosphorylase18 assures that glycogen stores are readily available for use as fuel in muscle. The third form of stored energy is represented by triglycerides. Triglycerides are slowly mobilized and provide 35 KJ of energy per gram oxidized 17. Most of the body’s energy stores consist of triglycerides in adipose tissue. Their oxidation is less efficient compared to glycogen, both in terms of the amount of oxygen required and in terms of the rate of energy conversion (power output). (Figure 2).19 In short in the absence of a “universal fuel” the body behaves like a hybrid engine and switches between fuels depending on fuel supply, hormone levels and the nature as well as the duration of stress (exercise). For instance athletes running long distances rely first on the aerobic metabolism of glycogen. After a short while glycogen stores are depleted and muscle oxidizes triglycerides.20,21 It has been shown that in marathon runners 10–50% of the energy required is provided by fatty acid oxidation.14 Elite marathon runners use triglycerides at the lower end of this range because triglycerides and fatty acids are a less efficient source compared to glycogen. In other words one of the training goals in marathon runners is the increase of glycogen stores22 in their skeletal muscles along with up-regulation of the enzymes of the metabolic pathways which contribute to glycogen oxidation.23 After the exercise, oxygen consumption remains high (excess post exercise oxygen consumption, EPOC) in order to repair the “damages” incurred by exercise. At this time the rate of fatty acid oxidation increases and the glycogen stores of the muscles are replenished with the use of carbohydrates.24,25 As it will be shown below, these principles of muscle metabolism also apply to the heart.
Figure 2.
Relationships between MV. O2 and PVA from one representative experiment. Modified with permission from Korvald et.al.19
Metabolic Adaptation of the Heart
Response to Increased Energy Demand
To reiterate upon increased energy demand the muscles initially rely on carbohydrate oxidation. What are the mechanisms for enhanced carbohydrate oxidation with exercise? Observations first made in skeletal muscle of animals provide a conceptual framework for mechanisms of enhanced carbohydrate metabolism.
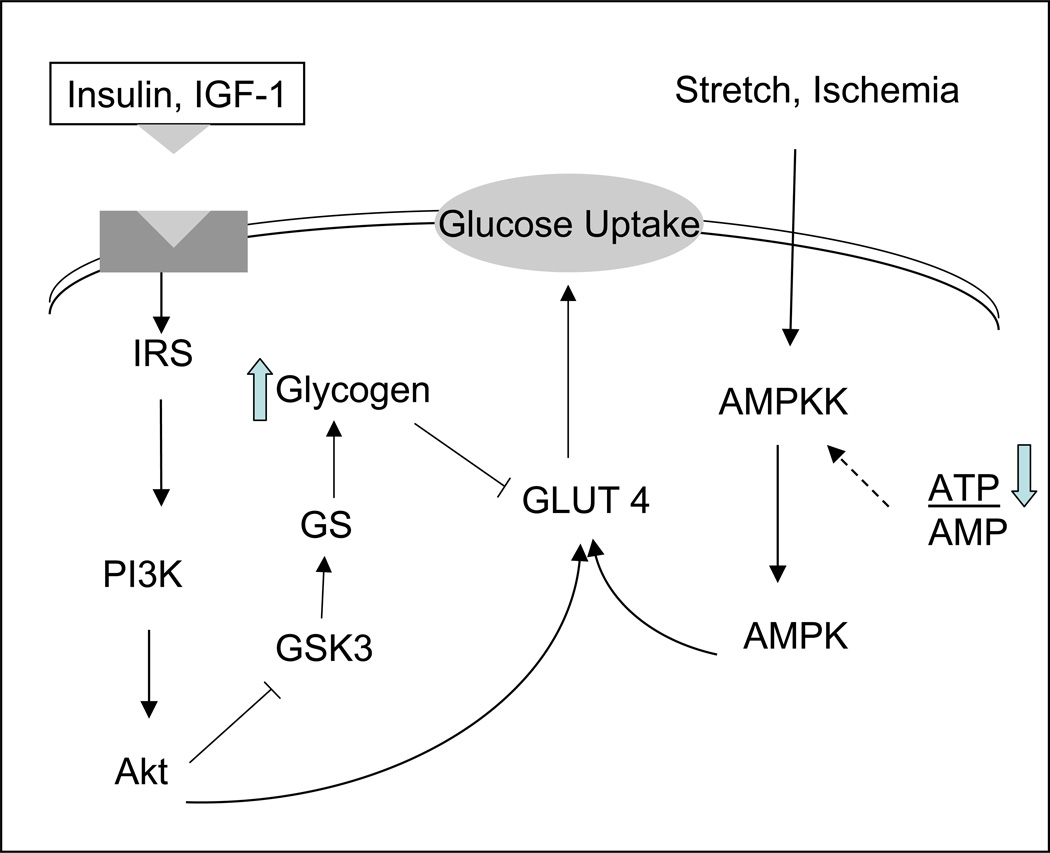
The transport of glucose in the muscle is enhanced. This is related to increased levels in the glucose transporter GLUT4 mRNA and GLUT4 protein, for instance in exercising rats.26 As shown in Figure 3 this can happen through receptor dependent and receptor independent mechanisms. Besides contractile activity glucose transport activity is stimulated by insulin or hypoxia and ischemia and is increased roughly in proportion to the adaptive increase in GLUT4 protein in epitrochlearis muscles. When epitrochlearis muscle is incubated in a medium containing glucose and insulin, glycogen accumulation over 3 h is twice as great in muscles of rats that exercised as in control muscles,27 suggesting that a rapid increase in GLUT4 expression is an early adaptive response of skeletal muscle to exercise and that the physiological role of this adaptation is to enhance the replenishment of muscle glycogen stores. From the same group came evidence that glycogen supercompensation masks the effect of a training induced increase in GLUT-4 on muscle glucose transport28 and that prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise.29 It is tempting to speculate that this adaptive mechanism helps restore glycogen in the “fight and flight” response. At the same time, a reversal of the exercise-induced increase in GLUT4 in response to carbohydrate feeding and glycogen supercompensation, probably prevents excessive glycogen accumulation, as observed in certain inborn errors of metabolism (Pompe’s disease, von Gierke’s disease). Glycogen content in muscle does not affect GLUT 4 alone. The transcriptional activation of metabolic genes in response to exercise is blunted when pre-exercise muscle glycogen content is high, whereas transcriptional activation is enhanced when pre-exercise muscle glycogen content is low. This raises the possibility that signalling mechanisms regulating exercise-responsive genes may be sensitive to muscle glycogen content.30 When glycogen content is high, muscle preferentially uses glycogen as a source of energy due to its higher efficiency as a fuel. Conversely when glycogen stores are low, skeletal muscle fatty acid oxidation and mitochondrial biogenesis are induced.31 This induction can be prevented by a high carbohydrate diet during recovery.32 The findings support the hypothesis that factors associated with substrate availability and/or cellular metabolic recovery (muscle glycogen restoration) influence the transcriptional regulation of metabolic genes in skeletal muscle of humans during recovery from exercise. The control of metabolism in recovery by glycogen levels underlines its importance as the metabolic reserve of the muscles.
Figure 3.
Receptor dependent and receptor independent translocation of GLUT4. See text for further discussion.
Response to Altered Metabolic Environment (Exercise, Starvation, Diabetes)
Exercise, starvation and diabetes are three physiological states characterized by increased blood levels of free fatty acids (FFA).33 The surplus of FFAs leads to activation of the enzymes of beta oxidation.34. This is coordinated by the increase of peroxisome proliferator activated receptor alpha (PPAR-α).35 In this context skeletal muscle uses the substrate that is available in the specific environment. This adaptation offers two significant advantages. First it supports the muscle’s metabolic needs and second avoids the accumulation of metabolic byproducts that could be harmful to the muscle.
Metabolic Maladaptation
We will use the example of diabetes and heart failure to assess metabolic maladaptation of skeletal muscle as it also applies to the heart. Overexpression and overactivity of Glycogen Synthase Kinase 3 (GSK-3) in skeletal muscle of rodent models of obesity and obese type 2 diabetic humans are associated with an impaired ability of insulin to activate glucose disposal and glycogen synthase (GS).36 In skeletal muscle of prediabetic obese Zucker rats chronic selective GSK-3 inhibition significantly improves insulin receptor substrate -1 (IRS-1) signaling and enhances insulin stimulated glucose transport.37 Increased cell surface GLUT 4 protein has also been documented in Zucker diabetic fatty rats.38 The overexpression of carnitine palmitoyltransferase I (CPT I) (a key enzyme of fatty acid oxidation) normalizes insulin-stimulated glucose metabolism in muscle cells pretreated with palmitate. Those cells also seem to be protected against the palmitate induced accumulation of diacylglycerol and ceramide, and PKC theta and zeta activation, all of which are signaling molecules of insulin resistance.39 Overexpression of liver CPT I also increases insulin-stimulated incorporation of glucose into glycogen and deoxyglucose uptake in palmitate-treated cells. In the latter study, however, the insulin sensitizing effect was independent of changes in intracellular lipid content.40 Another pathway that seems to be involved in this process is that of AMP kinase (AMPK). AMPK activity in skeletal and cardiac muscle results in increased rates of glucose transport and may play a role in enhancing muscle and whole body insulin sensitivity for glucose transport under conditions such as exercise.41 The mechanisms for AMPK-mediated glucose transport involve proximal signals that are distinct from that of insulin (Figure 3).
In this context it is of interest that patients with congestive heart failure (CHF) develop impaired insulin-stimulated whole-body glucose uptake, despite normal insulin signalling in skeletal muscle at the level of IRS-1, PI-3 kinase or Akt. Thus, insulin signalling defects are not a primary cause of impaired whole-body insulin-mediated glucose uptake in CHF. In the same vein exercise training is beneficial to treat the metabolic abnormalities associated with CHF and improves insulin action on whole-body glucose uptake independent of enhanced insulin signal transduction. Thus, altered insulin action on glucose uptake in CHF patients occurs at a distal component of the insulin signal transduction pathway regulating glucose uptake.42 Patients with heart failure due to coronary artery disease are insulin resistant; this insulin resistance affects both the myocardium and the skeletal muscle and is independent of blood flow.43 Type 2 diabetes mellitus confers a greater degree of myocardial insulin resistance to those patients.44 Reduced suppression of circulating free fatty acids (rather than further reduction of myocardial GLUT 4 levels) seems to limit myocardial glucose utilization in the diabetic patients with CHF due to coronary artery disease.44 It is important to note that a direct relation between myocardial glucose uptake (measure of insulin resistance) and left ventricular ejection fraction (measure of contractile reserve) has been documented in the literature.45
Contractile and Metabolic Reserve of the Heart
Glucose, Lactate, Glycogen
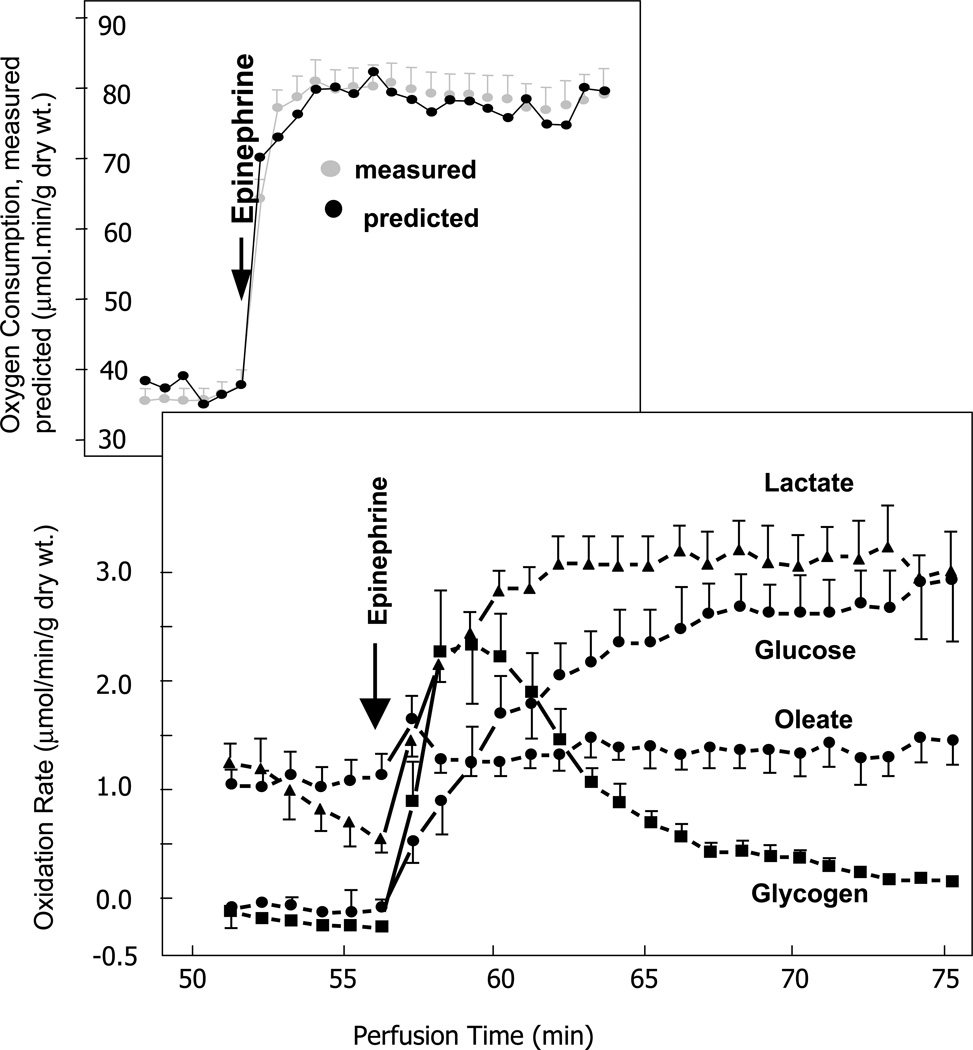
Nature has endowed the heart with a huge potential for ATP generation. Nature also prevents the heart from generating ATP unless it needs ATP and uses it! 46 During an acute increase in heart work, induced by stimulation with epinephrine and an increase in afterload, rates of oleate and triglyceride oxidation remain unchanged (Figure 4). Instead, the rate of glycogen oxidation increases instantly and then transiently. At the same time the oxidation of glucose and lactate increases more gradually and remains elevated throughout the stimulation.47 With prolonged inotropic stimulation, glycogen levels are restored. Glycogen synthesis results from activation of GS, which in turn is regulated by a series of complex reaction sequences.48 The importance of glycogen as the energy reserve of the heart can be demonstrated by another experiment. During an acute low to high work transition of the rat heart improved homeostasis is demonstrated at the metabolic milieu of exercise (increased lactate due to increased skeletal muscle glycolysis and increased free fatty acids due to adrenergic stimulation of lipolysis in adipose tissue). The improved homeostasis is manifested by attenuation of net glycogenolysis.27 The glycogen sparing is partly explained by de novo glycogen synthesis during or immediately following glycogen breakdown. In the cardiomyocyte, the allosteric activator of glycogen synthase, glucose-6-phospate, is elevated in the metabolic milieu of exercise. In a practical setting it has been shown that marathon and ultramarathon runners start replenishing their skeletal muscle glycogen stores even during the time of the race.14,22
Figure 4.
Rates of substrate oxidation. Rates during the chase period of the protocol are depicted. ●, 14CO2 from exogenous [U-14C]glucose; ■ 14CO2 from [14C]glycogen; ▲, 14CO2 from exogenous [U-14C]lactate; ○, 3H2O from exogenous [9,10-3H]oleate. Values for glycogen were corrected for incomplete labeling assuming uniform isotopic dilution at an enrichment of 54.7%. Values are the mean 6 S.E. for five perfusions in each treatment group. Adapted from reference 47. See text for further details.
GSK-3 which phosphorylates and inactivates GS, has been implicated in the regulation of multiple physiologic processes in the heart and skeletal muscle, including glycogen turnover and cell growth (Figure 3). In metabolic terms inhibition of GSK-3 results in activation of GS as well as basal and insulin stimulated increase in glucose uptake of skeletal muscle without increasing the total protein content of glucose transporters GLUT1 and GLUT4.49 Bouts of submaximal and maximal exercise activate the serine- threonine kinase Akt and decrease GSK-3a activity, both in humans and rats.50 Contractile activity is also partly responsible for the activation of Akt.51 In Akt over-expressing transgenic mice left ventricular contractile function is increased at baseline compared to wild type mice.52 With increasing concentrations of dobutamine contractility became the same in both groups. Because transgenic mice started from a hypercontractile state the relative increase in contractile performance was less in Akt over-expressing hearts than in wild types. [39] Akt increases cardiac size and contractility under resting conditions, while it phosphorylates and deactivates GSK -3. When phosphorylated, GSK-3 inhibits GS phosphorylation. When dephosphorylated, GS promotes glycogen synthesis (Figure 3). The essence of this complex signaling sequence is the build-up of metabolic reserve that is readily mobilized in times of need.
Fatty Acids, Ketone Bodies, Triglycerides
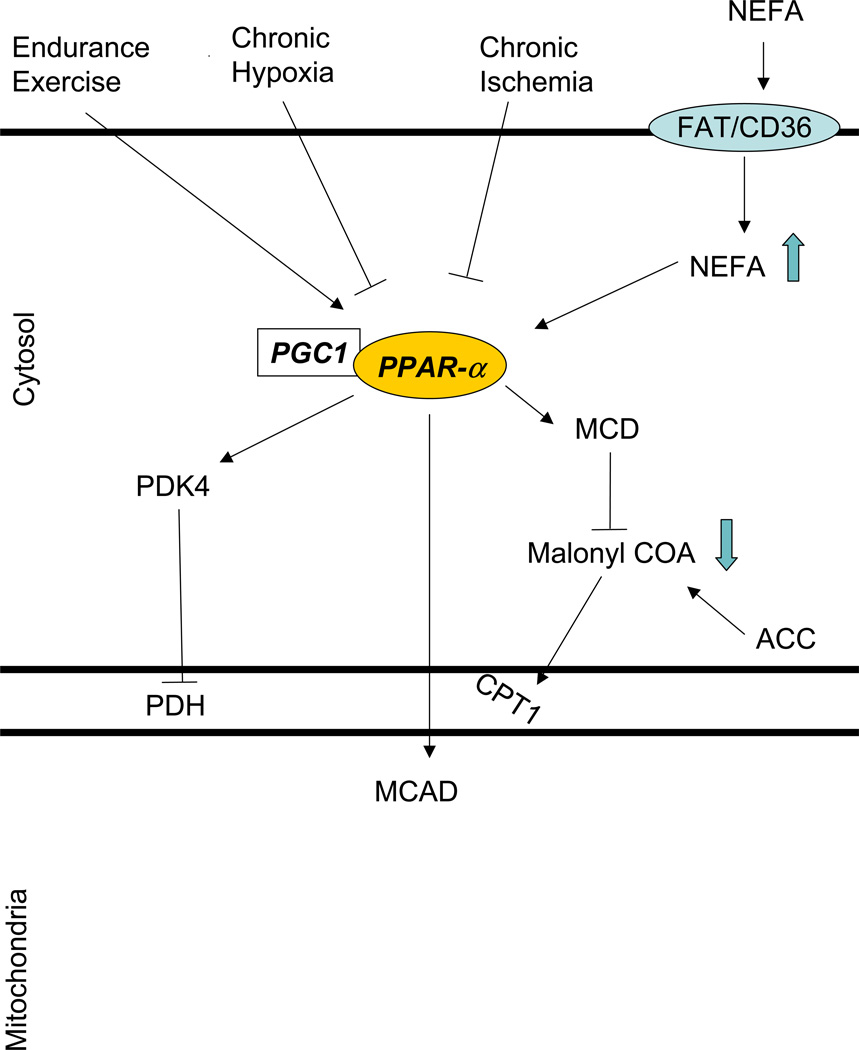
The regulation of long chain fatty acid metabolism in response to acute increase in cardiac work is equally complex (Figure 5). In contrast to glucose metabolism fatty acid metabolism is regulated predominantly at the transcriptional level and through feed-forward mechanisms. For example exercise raises free fatty acid levels in the blood.33 Most likely as a result of ligand activation the content of PPAR-α and its family of target proteins that regulate fatty acid oxidation [medium-chain and very long chain acyl-CoA dehydrogenase (MCAD and VLCAD)] are doubled with endurance training in skeletal muscle.53 Indeed, exercise increases transcription and/or mRNA content of pyruvate dehydrogenase kinase 4 (PDK4), uncoupling protein 3, lipoprotein lipase, CPT I, hexokinase II, peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC-1α), and PPAR-α.31 Endurance exercise also increases PGC-1α and the nuclear respiratory factors 1 and 2 54,55 which have been shown to promote mitochondrial biogenesis.56
Figure 5.
Fatty acid metabolism is regulated at the transcriptional level. See text for further details.
Coupled Metabolic and Contractile Reserve of the Heart in the Perinatal Period
The fetal heart is an excellent example for the concept of a cardio-metabolic reserve. It operates in a hypoxic environment and oxidizes lactate and glucose 57, while its ability to oxidize long chain fatty acids is limited.58 Commensurate with augmented carbohydrate metabolism is the prominence of glycogen in the fetal heart. Glycogen occupies more than 30% of the cell volume in fetal cardiomyocyte59, and fetal mitochondria are incompletely developed.60 In spite of the hypoxic environment cardiac output is normal in the fetal heart.61 Collectively, the metabolic features of the fetal heart prepare the organism with the stress of birth. Birth is the ultimate challenge for the cardio-matabolic reserve of the heart. The accumulation of glycogen (metabolic reserve) in the fetal heart is a mechanism for the heart to deal with the increased energy demands. Abnormal cardiac development and function can be demonstrated in mice which lack heart glycogen due to disruption of the GYS1 gene which encodes GS.62
Immediately after birth glycolysis remains the predominant source of energy.63 Shortly afterwards, however, there is a shift from glucose and lactate oxidation to fatty acid oxidation. This energy metabolic switch is paralleled by changes in the expression of the enzymes involved in the respective metabolic pathways.64 Recent evidence indicates that this postnatal gene regulatory effect involves the actions of the nuclear receptor PPARα and its coactivator (PGC-1α).56 PGC-1α promotes mitochondrial biogenesis and the activation of PPAR-α leads to the increase of muscle CPT I. The latter is also an effect of the reduction in malonyl CoA in the newborn heart.65 This switch to free fatty acid oxidation ultimately serves better the energy needs in the postnatal heart. With hypertrophy, atrophy and heart failure66–68 the heart reverts to predominant glucose metabolism and re-expresses the fetal gene pattern. The DNA binding activities and nuclear expression of repressors like Sp1/3 and the chicken ovalbumin upstream promoter transcription factor (COUP-TF) in normal fetal mouse heart are similar to those in the hypertrophied adult heart.66 These results identify a transcriptional regulatory mechanism involved in the re-induction of a fetal metabolic program during different disease states of the myocardium. This may be teleologically related to the better energy efficiency of glucose oxidation that preserves contractile function during those disease states, as we will see below.
Impaired Contractile and Metabolic Reserve of the Diseased Heart
Hypertrophy - Atrophy
Pressure overload (i.e. consequent to hypertension, or aortic stenosis) leads to left ventricular hypertrophy. The structural changes are preceded by changes in the metabolic machinery of the heart.69 Even before there is any evidence of cardiac hypertrophy in hearts from hypertensive rabbits the rates of glucose utilization are increased and rates of ketone body utilization are decreased. Activities of key enzymes of carbohydrate metabolism (phosphorylase, hexokinase, phosphofructokinase, and lactate dehydrogenase) are increased, while those of ketone body metabolism (3-oxoacid-CoA transferase, acetoacetyl-CoA synthase) are decreased and those of the citric acid cycle (citrate synthase, 2-oxoglutarate dehydrogenase) are not different between groups. In an experimental model of right ventricular pressure overload the expression of genes involved in the thioesterification, mitochondrial import, and beta-oxidation of fatty acids are coordinately down-regulated.66 Studies of the gene encoding human MCAD, which catalyzes a rate-limiting step in the FAO cycle, confirm that repression of MCAD gene expression in the hypertrophied ventricle occurs at the transcriptional level.66 The master-switch in all of those changes is the PPAR-α 70 together with the coactivator PGC-1a.71,72 During cardiac hypertrophy the activity and gene expression of PPAR-α are both downregulated. The rapid decline in PPAR-α activity is thought to be due to its phosphorylation by extracellular signal-regulated kinase (ERK),73 a member of the mitogen activated protein kinases (MAPK) family which is involved in the cardiac growth signaling. At the same time transcriptional repressors, like the (COUP-TF)/erbA-related protein 3 and Sp1 and Sp3 proteins, interact with the pressure overload responsive unit.66 COUP-TF is known to repress the transcriptional activity of the MCAD promoter leading in decreased fatty acid oxidation.74
Another signaling pathway of cardiac growth is the pathway of serine-threonine kinase Akt.75 Specifically Fas ligand induces Akt and GSK3β phosphorylation in cardiomyocytes.76 Phosphorylation leads to inactivation of GSK3β (see above). GSK3β is considered a negative regulator of cardiac hypertrophy.77,78 In this way induction of Akt leads to cardiac hypertrophy. It has also been shown that induction of Akt leads to prevention of apoptosis and regulation of glucose metabolism.79 As already discussed, the inactivation of GSK3β also leads to increased glycogen synthesis.80 Thus the GSK3β pathway regulates both cardiac glycogen metabolism and cardiac growth. The expression of GSK 3β in the postnatal cardiomyocyte leads to impairment in growth and contractile function due to down-regulation of the sarcoplasmic reticulum calcium ATPase (SERCA2α) by GSK-3β. 78
The hypertrophy and subsequent metabolic switch to glucose oxidation may be an adaptive mechanism. In a model of PPAR-α deficient hearts, which mimics the hypertrophied heart, the substrate switch from fatty acid to glucose and lactate is sufficient to sustain normal energy metabolism and contractile function at rest.81. As already mentioned Akt induction leads to hypertrophy with preserved contractile function and cardioprotection after ischemia and reperfusion.82 Also the myosin isoform MHC-β is upregulated during cardiac hypertrophy. The decreased contractile velocity of MHC-β is associated with greater economy in force generation, improving myocardial efficiency.83 The switch in sarcomeric proteins may be induced by glucose regulated transcription factors like Sp1 providing another evidence of the tight link of metabolic and contractile reserve in the heart.84,85
Decreased ATP synthesis (measured by 31P magnetization transfer) during high workload in the PPAR-α deficient hearts which mimic hypertrophy though results in progressive depletion of the metabolic reserve (i.e., inability to increase glucose and lactate oxidation from baseline) and failure to sustain high contractile performance. Interestingly, the metabolic and functional defects in PPAR-α (−/−) hearts can be corrected by over-expressing the insulin-independent glucose transporter GLUT1, which increased the capacity for glucose utilization beyond the intrinsic response to PPAR-α deficiency. It does so partly by increasing the glycogen content of the heart.81 Finally PPAR-α reactivation in hypertrophied hearts blocks skeletal α-actin induction, reverses the down-regulation of measured PPAR-α regulated genes, and prevents substrate switching. This PPAR-α reactivation concomitantly results in severe depression of cardiac power and efficiency in the hypertrophied heart measured ex vivo.86 Thus, PPAR-α down-regulation and a metabolic switch are essential for the maintenance of contractile function of the hypertrophied heart, at least during rest. Furthermore pharmacologically induced regression of pressure-overload cardiac hypertrophy normalizes glucose metabolism as well as left ventricular function during reperfusion.87 In the atrophied heart a similar down-regulation of PPAR-α and PPAR-α regulated genes along with induction of the fetal gene program has been documented.88,89
Ischemia
A decrease in oxygen delivery to the myocardium results in down-regulation of oxidative metabolism and reduced contractile function. It seems that the acute switch from aerobic to anaerobic metabolism is necessary for immediate cell survival. This switch is achieved by a change-over from fatty acid to glucose and lactate oxidation. Over the long-term hypoxia inducible factor 1a (HIF-1a) is responsible for the transcriptional up-regulation of glucose transporter 1 and various glycolytic enzymes.90 In cultures of myocardial cells hypoxia reduces PPAR-α /RXR binding activity at the promoter region (fatty acid responsive element 1 FARE-1) of the muscle CPT-I gene which encodes the enzyme responsible for the rate limiting step in fatty acid oxidation.91 It does so by diminishing the availability of retinoid X receptor (RXR) which forms a heterodimer with PPAR- α, necessary for the PPAR-α’s metabolic actions. In vivo hypoxia decreases transcript levels of PPAR-α and PPAR-α regulated genes (PDK4, mCPT-I, and malonyl-CoA decarboxylase (MCD) mRNA levels) resulting in decreased fatty acid oxidation and increased reliance of the heart on glucose.92 Hypoxia induces changes in myosin heavy chains isoforms in myocardial cells in vivo and in vitro93 Specifically a shift from MHCα to the more oxygen efficient MHCβ is noted. This transcriptional adaptation may be part of a program that preserves cardiac function during decreased oxygen supply.
In perfused rat hearts, preconditioning increases phosphorylation of GSK-3 β, a downstream target of PI3-kinase and protein kinase B (PKB). This leads to inactivation of GSK-3 β. Inhibition of GSK-3 β results in improved post-ischemic function and reduced infarct size after preconditioning.94 These findings indicate that inhibition of GSK-3 β is protective and that this PI3-kinase-dependent signaling pathway may play an important role in ischemic preconditioning. Another study shows that erythropoetin and preconditioning lead to additive infarct size-limiting effects by additive phosphorylation of GSK-3 β at the time of reperfusion by Akt-dependent and -independent mechanisms.95 Akt over-expression has been shown to decrease infarct size after ischemia.82 It is important to notice that GSK-3 β inhibition also leads to increased glycogen synthase activity. It may be that preservation of metabolic reserve (i.e., glycogen) results in improved post-ischemic function.
Persistent stunning can be induced regionally by repetitive episodes of ischemia and full reperfusion. After repeated episodes of ischemia and reperfusion myocardial function does not decrease further despite a similar reduction in coronary blood flow as with the first episode. So repetitive ischemia- reperfusion leads to a stable reduced contractile function.96 At the same time an increase in glucose uptake and decrease in free fatty acid uptake is noted. The mechanism behind it may be a reduction in PPAR-α regulated genes.97 Studies reveal enhanced glycogen deposition in those areas, which can be explained by decreased GSK-3 protein levels and activity. These results indicate that persistent stunning, even in the absence of chronic ischemia, can mimic the phenotype of myocardial hibernation (glycogen deposition and stable reduced regional contractile function).96 In this way a decrease in the flow does not lead in a decrease in function and this is due to the switch in metabolic substrates and the accumulation of glycogen (increased metabolic reserve which permits some preservation of function). The down regulation of PPAR-α and PPAR-α regulated genes in this condition is partly controlled by reactive oxygen species (ROS).97 It is postulated that the utilization of fatty acids in the post-ischemic myocardium leads to reactive oxygen species accumulation, which may be detrimental for the heart. The down-regulation of PPAR-α by ROS is therefore a protective mechanism which allows the preservation of metabolism and function. Another detrimental effect of a high concentration of fatty acids after reperfusion is the fact that they abolish the cardioprotective effect of insulin and its ability to inhibit AMPK.98 This is probably related to the fact that AMPK activation is associated with acceleration of fatty acid oxidation in the reperfused heart99 which leads to decreased glucose oxidation (Randle hypothesis).100 At the same time, however, increased AMPK activation increases glycolysis.101 Its product, pyruvate, which cannot be oxidized leads to lactate and hydrogen ion accumulation. The result is acidocis which causes impairment of contractile function102, but also an inhibition of 5’ nucleotidase.103
Furthermore reactivation of PPAR-α in mice exposed to repetitive ischemia reperfusion worsened contractile function, induced micro-infarctions, and increased intramyocardial triglyceride deposition, features suggestive of cardiac lipotoxicity.97 Similarly another study shows that the chronic activation of PPAR-α in the heart is detrimental to the recovery of cardiac power after ischemia and reperfusion.104
In pigs with hibernating myocardium graded epinephrine infusion results in increased segment shortening and myocardial oxygen consumption in the absence of increase in blood flow.105 The preserved function is associated with increased lactate uptake by the myocardium and no lactate production suggesting an adaptive mechanism that prevents supply-demand mismatch in this case. A classic example for cardio-metabolic reserve.
To summarize, the above data suggest that in hypoxia, preconditioning, and persistent stunning resulting in hibernating myocardium and in hibernating myocardium itself the relationship between contractile and metabolic reserve is preserved. What those conditions have in common is a switch to glucose as an energy substrate (acutely in hypoxia and preconditioning and long-term in hibernation) along with down-regulation of PPAR-α transcript levels reflecting decreased fatty acid oxidation.
Diabetes
The diabetic heart functions in an environment rich in FFAs and glucose.106 Those two substrates compete with each other. The increased supply of FFA leads to increased intracellular levels of fatty acids. Fatty acid ligands activate the PPAR-α expression.107 The PPAR-α then increases the expression of genes in three sites of the fatty acid utilization: a) transport in the cell,108 b) import in the mitochondrium,34 and c) fatty acid oxidation.109 The PPAR-α overexpression in mouse hearts leads in increased fatty acid oxidation and repression of glucose uptake and utilization similar to the one noted in diabetic hearts.110 The glucose utilization is repressed in three levels: a) fatty acyl-CoAs inhibit insulin mediated glucose transport by inhibiting insulin receptor substrates111 and protein kinase B,112 b) fatty acyl-CoAs inhibit hexokinase, which phosphorylates glucose upon its entrance to the cell,113 and c) the increase in PPAR-α expression and activity leads to increased PDK4 expression and increased acetyl CoA/CoA ratio both of which inhibit the pyruvate dehydrogenase complex.114 Despite the decreased insulin-mediated glucose transport, the glucose uptake of the diabetic heart is comparable to the normal heart due to the hyperglycemia.115 Due to the inhibition of glucose utilization glycolytic intermediates accumulate in the cardiomyocyte.116 Initially all those changes which are accompanied by changes in the cardiomyocyte contractile proteins do not lead in major changes in contractile function.117 Thus, this is still a state of adaptation of metabolism to preserve function.106
However, when diabetes progresses, or when additional stresses are posed on the heart, metabolic mal-adaptation occurs. The expression of PPAR-α decreases118 with concomitant reduction in fatty acid oxidation. Decreased expression of PPAR-α in obese Zucker diabetic fatty rats119 and PPAR-α regulated genes (MCAD, m-CPT I) in the obese Zucker rat heart120 has been demonstrated. The mismatch between the increased fatty acid delivery and decreased oxidation leads to intramyocardial lipid accumulation. Indeed among patients with heart failure the higher levels of intramyocardial lipid deposition are noted in those with diabetes and obesity.121 Increased lipid accumulation in the heart can lead to insulin resistance as it has been shown for the skeletal muscle111,122 further impairing metabolism in the heart. Increased triglyceride deposition in the heart leads to elevated ceramide and inducible nitric oxide synthase levels both of which result in increased apoptosis of the cardiomyocyte and contractile dysfunction.119 Contractile dysfunction related to increased lipid accumulation in the cardiomyocyte has been demonstrated in several other studies of obesity and diabetes both in humans and animals.120,121 In addition to impairing contractile function elevated plasma free fatty acids can impair left ventricular diastolic function in severely obese patients.123 Diastolic dysfunction may be related to the alteration of proteins of the sarcoendoplasmic reticulum like SERCA2α. The down-regulation of myocyte enhancing factor 2C (MEF2C) and MEF2C–regulated genes (GLUT4, SERCA2a, and MHCα) in the failing hearts of patients with diabetes suggests a transcriptional mechanism that might contribute to the pathogenesis and contractile dysfunction of heart failure patients with diabetes. Reversal of the diastolic dysfunction can be demonstrated after weight reduction in obese patients.
A second mismatch exists in the diabetic heart. This is between the maintained glucose uptake and the decreased glucose oxidation. Failure to control the glucose levels can lead to the development of insulin resistance.124 Also the accumulated glycolytic products are shifted to the pentose phosphate and hexosamine biosynthetic pathways. Both result in activation of glucose sensing transcription factors like Sp1. As mentioned previously Sp1 can decrease the expression of MCAD which controls one of the rate limiting steps in fatty acid oxidation.66 It has recently been shown that glucose decreases the expression of PPAR-α and PPAR-α regulated genes in islet cells.125 Whether this is true for the heart as well is not yet known. This could though be another mechanism, which contributes to excessive lipid accumulation and contractile dysfunction in the diabetic heart, which functions in a high fatty acid and glucose milieu. Glucose sensing transcription factors have also been implicated in the transcription of MHC isoforms and skeletal α-actin126,127 providing another link between metabolism and function.
As shown above insulin resistance and metabolic maladaptation can cause contractile dysfunction of the heart. Recently we have shown that by changing the metabolic profile, reducing myocardial lipid accumulation, and promoting the down-regulation of PPAR-α regulated genes, PPAR-γ activation leads to an increased capacity of the myocardium to oxidize glucose and to a tighter coupling of oxidative metabolism and contraction in the setting of insulin resistance and type 2 diabetes.128 Similarly, Zhou, et al. showed that troglitazone (PPAR-γ agonist) lowered myocardial triglycerides and ceramide while completely reversing evidence of apoptosis and loss of cardiac function in obese rats.119
In summary, obesity and diabetes induce metabolic, histologic and functional changes in the heart. The first stage is that of adaptation where metabolism is altered so that cardiac function is maintained. In the second stage of maladaptation the metabolic mismatch leads to contractile dysfunction. Correction of metabolic dysregulation can reverse contractile dysfunction. However, the cardio-metabolic reserve is lost in the mal-adapted “lipotoxic” heart.
Heart Failure
The enzymes of fatty acid oxidation are reduced in the human failing heart.88,129 In addition to the reduction in fatty acid oxidation an increase in glucose oxidation has been demonstrated in the failing human heart.130 Earlier studies performed in human heart by Bing and coworkers131 came to the conclusion that energy substrate metabolism was not altered in failing heart but that the heart was deficient to utilize energy for effective muscular contraction. The question whether metabolic changes are the cause or consequence of impaired contractile function in non-ischemic heart is still open today. The failing adult heart reverts to a fetal metabolic gene profile by downregulating adult gene transcripts.68 Specifically, the levels of m CPT I, MHCα and GLUT 4 decrease while MHCβ increases 67. Those changes where similar to the ones induced by hypertrophy. It was proposed that similar changes during both hypertrophy and unloading may reflect an adaptation to the heart in the energy requirements while preserving function. Indeed those changes in the unloaded heart may contribute to the improvement in left ventricular function of some patients after the implantation of left ventricular assist devices.
Loss of this adaptation may lead to advanced stages of heart failure. In a recent study down-regulation of PPAR-α, mCPT1 and MCAD was noted in the failing compared to the non-failing hearts.121 In failing hearts though with significant lipid accumulation upregulation of PPAR-α regulated genes was noted (MCAD, mCPT1) similar to the lipotoxic ZDF rat heart. We proposed that when the failing heart (which adapts with down-regulation of the FAO pathways) faces an increased fatty acid load (as in diabetes or obesity), intramyocardial lipid accumulation occurs. This is associated with an up-regulation in PPARα activity and in MHC-β transcript levels.121 Both lead in further contractile dysfunction. Similarly, reactivation of PPARα was shown to have detrimental effects during a) repetitive ischemia and reperfusion leading to cardiac lipotoxicity,97 b) in recovery of cardiac power after ischemia and reperfusion,104 and c) in a pressure-overload model of hypertrophied rat heart.86
Lastly, in a model of experimental mitral regurgitation (MR) it was shown that chronic MR leads to intramyocyte myocardial lipid accumulation and contractile dysfunction. As in the cases of diabetes and obesity mentioned before, the administration of the PPAR-γ agonist rosiglitazone ameliorated MR-induced LV dysfunction accompanied by a decline in lipid content.132 This occurs most likely by restoring the faulty energy substrate utilization, another proof of the tight link among metabolism and function.
Conclusion
The heart responds to inotropic stimuli by increasing its contractile function, which is accompanied by an increase of coronary flow. This response can only be accomplished by an appropriate metabolic response. The match between contractile and metabolic reserve is characteristic of metabolic adaptation. Breech of this balance results in maladaptation with detrimental consequences for myocardial function.
Acknowledgements
We thank Roxy A. Tate for editorial assistance. Work in the authors’ laboratory has, in part, been supported by grants from the National Heart Lung and Blood Institute of the US Public Health Service (R01 HL/AG 61483).
Grant funding: National Heart, Lung and Blood Institute R01 HL/AG 61483.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sonnenblick EH, Stam AC., Jr Cardiac muscle: activation and contraction. Annu Rev Physiol. 1969;31:647–674. doi: 10.1146/annurev.ph.31.030169.003243. [DOI] [PubMed] [Google Scholar]
- 2.Stam AC, Jr, Weglicki WB, Feldman JC, et al. Canine myocardial sarcolemmer: Its preparation and enzymic activity. J Mol Cell Cardiol. 1970;1:117–130. [Google Scholar]
- 3.Sonnenblick EH, Ross J, Jr, Braunwald E. Oxygen consumption of the heart. Newer concepts of its multifactoral determination. Am J Cardiol. 1968;22:328–336. doi: 10.1016/0002-9149(68)90117-3. [DOI] [PubMed] [Google Scholar]
- 4.Soonpaa M, Koh G, Klug M, et al. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenblick EH. Force-velocity relations in mammalian heart muscle. Am J Physiol. 1962;202:931–939. doi: 10.1152/ajplegacy.1962.202.5.931. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Jr, Covell JW, Sonnenblick EH, et al. Contractile state of the heart characterized by force-velocity relations in variably afterloaded and isovolumic beats. Circ Res. 1965;18:149–163. [Google Scholar]
- 7.Spann JF, Jr, Buccino RA, Sonnenblick EH, et al. Contractile state of cardiac muscle obtained from cats with experimentally produced ventricular hypertrophy and heart failure. Circ Res. 1967;21:341–354. doi: 10.1161/01.res.21.3.341. [DOI] [PubMed] [Google Scholar]
- 8.Taegtmeyer H. Energy metabolism of the heart from basic concepts to clinical applications. Curr Prob Cardiol. 1994;19:57–116. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 9.Cohn PF. Evaluation of inotropic contractile reserve in ischemic heart disease using postextrasystolic potentiation. Circulation. 1980;61:1071–1075. doi: 10.1161/01.cir.61.6.1071. [DOI] [PubMed] [Google Scholar]
- 10.Bonow RO. Contractile reserve and coronary blood flow reserve in collateral-dependent myocardium. J Am Coll Cardiol. 1999;33:705–707. doi: 10.1016/s0735-1097(98)00650-0. [DOI] [PubMed] [Google Scholar]
- 11.Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459–474. doi: 10.1016/s0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 12.Galatro K, Chaudhry FA. Prognostic implications of myocardial contractile reserve in patients with ischemic cardiomyopathy. Echocardiography. 2000;17:61–67. doi: 10.1111/j.1540-8175.2000.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 13.Taegtmeyer H, Golfman L, Sharma S, et al. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 14.Newsholme EA, Leech L, Duester G. Fuel Management, in Keep on Running: The Science of Training and Performance. West Sussex, UK: John Wiley & Sons, Ltd; 1994. pp. 95–112. pp. 95–11. [Google Scholar]
- 15.Duffield R, Dawson B, Goodman C. Energy system contribution to 100-m and 200-m track running events. J Sci Med Sport. 2004;7:302–313. doi: 10.1016/s1440-2440(04)80025-2. [DOI] [PubMed] [Google Scholar]
- 16.Johansen L, Quistorff B. 31P-MRS characterization of sprint and endurance trained athletes. Int J Sports Med. 2003;24:183–189. doi: 10.1055/s-2003-39085. [DOI] [PubMed] [Google Scholar]
- 17.Newsholme E, Leech L, Duester G. Filling the Fuel Tanks, in Keep On Running: The Science of Training and Performance. West Sussex, UK: John Wiley & Sons, Ltd.; 1994. pp. 70–94. pp 70–94. [Google Scholar]
- 18.Krebs EG, Fischer EH. The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochim Biophys Acta. 1956;20:150–157. doi: 10.1016/0006-3002(56)90273-6. [DOI] [PubMed] [Google Scholar]
- 19.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–H1351. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 20.Callow M, Morton A, Guppy M. Marathon fatigue: the role of plasma fatty acids, muscle glycogen and blood glucose. Eur J Appl Physiol Occup Physiol. 1986;55:654–661. doi: 10.1007/BF00423212. [DOI] [PubMed] [Google Scholar]
- 21.Sjodin B, Svedenhag J. Applied physiology of marathon running. Sports Med. 1985;2:83–99. doi: 10.2165/00007256-198502020-00002. [DOI] [PubMed] [Google Scholar]
- 22.van der Vusse GJ, Janssen GM, Coumans WA, et al. Effect of training and 15-, 25-, and 42-km contests on the skeletal muscle content of adenine and guanine nucleotides, creatine phosphate, and glycogen. Int J Sports Med. 1989;10(Suppl 3):S146–S152. doi: 10.1055/s-2007-1024963. [DOI] [PubMed] [Google Scholar]
- 23.Sherman WM, Costill DL, Fink WJ, et al. Effect of a 42.2-km footrace and subsequent rest or exercise on muscle glycogen and enzymes. J Appl Physiol. 1983;55:1219–1224. doi: 10.1152/jappl.1983.55.4.1219. [DOI] [PubMed] [Google Scholar]
- 24.Borsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003;33:1037–1060. doi: 10.2165/00007256-200333140-00002. [DOI] [PubMed] [Google Scholar]
- 25.Maehlum S, Grandmontagne M, Newsholme EA, et al. Magnitude and duration of excess postexercise oxygen consumption in healthy young subjects. Metabolism. 1986;35:425–429. doi: 10.1016/0026-0495(86)90132-0. [DOI] [PubMed] [Google Scholar]
- 26.Ren JM, Semenkovich CF, Gulve EA, et al. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem. 1994;269:14396–14401. [PubMed] [Google Scholar]
- 27.Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1490–H1501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- 28.Host HH, Hansen PA, Nolte LA, et al. Glycogen supercompensation masks the effect of a training induced increase in GLUT-4 on muscle glucose transport. J Appl Physiol. 1998;85:133–138. doi: 10.1152/jappl.1998.85.1.133. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Roves PM, Han DH, Song Z, et al. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol Endocrinol Metab. 2003;285:E729–E736. doi: 10.1152/ajpendo.00216.2003. [DOI] [PubMed] [Google Scholar]
- 30.Pilegaard H, Keller C, Steensberg A, et al. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol. 2002;541:261–271. doi: 10.1113/jphysiol.2002.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arany Z, He H, Lin J, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Pilegaard H, Osada T, Andersen LT, et al. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism. 2005;54:1048–1055. doi: 10.1016/j.metabol.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Johnson R, Walton J, Krebs H, et al. Metabolic fuels during and after severe exercise in athletes and non-athletes. Lancet. 1969;2:452–455. doi: 10.1016/s0140-6736(69)90164-0. [DOI] [PubMed] [Google Scholar]
- 34.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 35.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 36.Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr Drug Targets. 2006;7:1435–1441. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- 37.Dokken BB, Henriksen EJ. Chronic selective glycogen synthase kinase-3 inhibition enhances glucose disposal and muscle insulin action in prediabetic obese Zucker rats. Am J Physiol Endocrinol Metab. 2006;291:E207–E213. doi: 10.1152/ajpendo.00628.2005. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen EJ, Kinnick TR, Teachey MK, et al. Modulation of muscle insulin resistance by selective inhibition of GSK-3 in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2003;284:E892–E900. doi: 10.1152/ajpendo.00346.2002. [DOI] [PubMed] [Google Scholar]
- 39.Sebastian D, Herrero L, Serra D, et al. Carnitine Palmitoyltransferase I (Cpt I) Overexpression Protects L6e9 Muscle Cells From Fatty Acid-Induced Insulin Resistance. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00360.2006. [DOI] [PubMed] [Google Scholar]
- 40.Perdomo G, Commerford SR, Richard AM, et al. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem. 2004;279:27177–27186. doi: 10.1074/jbc.M403566200. [DOI] [PubMed] [Google Scholar]
- 41.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867–E877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 42.Kemppainen J, Tsuchida H, Stolen K, et al. Insulin signalling and resistance in patients with chronic heart failure. J Physiol. 2003;550:305–315. doi: 10.1113/jphysiol.2003.042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paternostro G, Camici PG, Lammertsma AA, et al. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996;98:2094–2099. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutka DP, Pitt M, Pagano D, et al. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol. 2006;48:2225–2231. doi: 10.1016/j.jacc.2006.06.078. [DOI] [PubMed] [Google Scholar]
- 45.Iozzo P, Chareonthaitawee P, Dutka D, et al. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. doi: 10.2337/diabetes.51.10.3020. [DOI] [PubMed] [Google Scholar]
- 46.Racker E. Energy cycles in health and disease. Curr Top Cell Regul. 1981;18:361–375. doi: 10.1016/b978-0-12-152818-8.50027-x. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 48.Taegtmeyer H. Glycogen in the heart--an expanded view. J Mol Cell Cardiol. 2004;37:7–10. doi: 10.1016/j.yjmcc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Nikoulina SE, Ciaraldi TP, Mudaliar S, et al. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51:2190–2198. doi: 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto K, Arnolds DE, Ekberg I, et al. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem Biophys Res Commun. 2004;319:419–425. doi: 10.1016/j.bbrc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto K, Aschenbach WG, Hirshman MF, et al. Akt signaling in skeletal muscle regulation by exercise and passive stretch. Am J Physiol Endocrinol Metab. 2003;285:E1081–E1088. doi: 10.1152/ajpendo.00228.2003. [DOI] [PubMed] [Google Scholar]
- 52.Condorelli G, Drusco A, Stassi G, et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horowitz JF, Leone TC, Feng W, et al. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279:E348–E355. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- 54.Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 55.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher DJ, Heymann MA, Rudolph AM. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol. 1980;238:H399–H405. doi: 10.1152/ajpheart.1980.238.3.H399. [DOI] [PubMed] [Google Scholar]
- 58.Bartelds B, Knoester H, Smid GB, et al. Perinatal changes in myocardial metabolism in lambs. Circulation. 2000;102:926–931. doi: 10.1161/01.cir.102.8.926. [DOI] [PubMed] [Google Scholar]
- 59.Navaratnam V. Heart Muscle: Ultrastructural Studies. New York: Cambridge University Press; 1987. [Google Scholar]
- 60.Rolph T, Jones C, Parry D. Ultrastructural and enzymatic development of fetal guinea pig heart. Am J Physiol. 1982;243:H87–H93. doi: 10.1152/ajpheart.1982.243.1.H87. [DOI] [PubMed] [Google Scholar]
- 61.Fisher D, Heymann M, Rudolph A. Myocardial consumption of oxygen and carbohydrates in newborn sheep. Pediatr Res. 1981;15:843–846. doi: 10.1203/00006450-198105000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Pederson BA, Chen H, Schroeder JM, et al. Abnormal cardiac development in the absence of heart glycogen. Mol Cell Biol. 2004;24:7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of ATP production immediately after birth. Am J Physiol. 1991;261:H1698–H1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 64.Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clinical & Experimental Pharmacology & Physiology. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 65.Onay-Besikci A, Campbell FM, Hopkins TA, et al. Relative importance of malonyl CoA and carnitine in maturation of fatty acid oxidation in newborn rabbit heart. Am J Physiol Heart Circ Physiol. 2003;284:H283–H289. doi: 10.1152/ajpheart.00461.2002. [DOI] [PubMed] [Google Scholar]
- 66.Sack MN, Disch DL, Rockman HA, et al. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci U S A. 1997;94:6438–6443. doi: 10.1073/pnas.94.12.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Depre C, Shipley GL, Chen W, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 68.Razeghi P, Young ME, Alcorn JL, et al. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 69.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 70.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 71.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor alpha (PPARalpha) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol. 2002;34:1249–1257. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- 72.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barger PM, Brandt JM, Leone TC, et al. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Disch DL, Rader TA, Cresci S, et al. Transcriptional control of a nuclear gene encoding a mitochondrial fatty acid oxidation enzyme in transgenic mice: role for nuclear receptors in cardiac and brown adipose expression. Mol Cell Biol. 1996;16:4043–4051. doi: 10.1128/mcb.16.8.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Latronico MV, Costinean S, Lavitrano ML, et al. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 76.Badorff C, Ruetten H, Mueller S, et al. Fas receptor signaling inhibits glycogen synthase kinase 3 beta and induces cardiac hypertrophy following pressure overload. J Clin Invest. 2002;109:373–381. doi: 10.1172/JCI13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haq S, Choukroun G, Kang ZB, et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michael A, Haq S, Chen X, et al. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- 79.Matsui T, Tao J, del Monte F, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 80.Lawrence JC, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- 81.Luptak I, Balschi JA, Xing Y, et al. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 82.Matsui T, Li L, Wu J, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 83.Holubarsch C, Goulette RP, Litten RZ, et al. The economy of isometric force development, myosin isoenzyme pattern and myofibrillar ATPase activity in normal and hypothyroid rat myocardium. Circ Res. 1985;56:78–86. doi: 10.1161/01.res.56.1.78. [DOI] [PubMed] [Google Scholar]
- 84.Young ME, McNulty P, Taegtmeyer H. Adaptation, maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 85.Young ME, Yan J, Razeghi P, et al. Proposed regulation of gene expression by glucose in rodent heart. Gene Reg Sys Biol. 2007 doi: 10.4137/grsb.s222. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young ME, Laws FA, Goodwin GW, et al. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 87.Wambolt RB, Henning SL, English DR, et al. Regression of cardiac hypertrophy normalizes glucose metabolism and left ventricular function during reperfusion. J Mol Cell Cardiol. 1997;29:939–948. doi: 10.1006/jmcc.1996.0336. [DOI] [PubMed] [Google Scholar]
- 88.Razeghi P, Young ME, Ying J, et al. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–209. doi: 10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 89.Sharma S, Ying J, Razeghi P, et al. Atrophic remodeling of the transplanted rat heart. Cardiology. 2006;105:128–136. doi: 10.1159/000090550. [DOI] [PubMed] [Google Scholar]
- 90.Semenza GL, Agani F, Iyer N, et al. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann N Y Acad Sci. 1999;30:262–268. doi: 10.1111/j.1749-6632.1999.tb09241.x. [DOI] [PubMed] [Google Scholar]
- 91.Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the PPAR{alpha}/ RXR gene regulatory pathway in cardiac myocytes. A mechanisms for O{sub2}-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem. 2001;276:27605–27612. doi: 10.1074/jbc.M100277200. [DOI] [PubMed] [Google Scholar]
- 92.Razeghi P, Young ME, Abbasi S, et al. Hypoxia in vivo decreases peroxisome proliferator-activated receptor alpha-regulated gene expression in rat heart. Biochem Biophys Res Commun. 2001;287:5–10. doi: 10.1006/bbrc.2001.5541. [DOI] [PubMed] [Google Scholar]
- 93.Razeghi P, Essop MF, Huss JM, et al. Hypoxia-induced switches of myosin heavy chain iso-gene expression in rat heart. Biochem Biophys Res Commun. 2003;303:1024–1027. doi: 10.1016/s0006-291x(03)00478-9. [DOI] [PubMed] [Google Scholar]
- 94.Tong H, Imahashi K, Steenbergen C, et al. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 95.Nishihara M, Miura T, Miki T, et al. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- 96.Kim SJ, Peppas A, Hong SK, et al. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92:1233–1239. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 97.Dewald O, Sharma S, Adrogue J, et al. Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation. 2005;112:407–415. doi: 10.1161/CIRCULATIONAHA.105.536318. [DOI] [PubMed] [Google Scholar]
- 98.Folmes CD, Clanachan AS, Lopaschuk GD. Fatty acids attenuate insulin regulation of 5'-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ Res. 2006;99:61–68. doi: 10.1161/01.RES.0000229656.05244.11. [DOI] [PubMed] [Google Scholar]
- 99.Kantor PF, Dyck JR, Lopaschuk GD. Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci. 1999;318:3–14. doi: 10.1097/00000441-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 101.Marsin AS, Bertrand L, Rider MH, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 102.Orchard CH. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J Physiol. 1987;384:431–449. doi: 10.1113/jphysiol.1987.sp016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bak M, Ingwall J. Acidosis during ischemia promotes adenosine triphosphate resynthesis in post-ischemic rat heart. J Clin Invest. 1994;93:40–49. doi: 10.1172/JCI116974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sambandam N, Morabito D, Wagg C, et al. Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol. 2006;290:H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 105.Fallavollita JA, Malm BJ, Canty JMJ. Hibernating myocardiam retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res. 2003;92:48–55. doi: 10.1161/01.res.0000049104.57549.03. [DOI] [PubMed] [Google Scholar]
- 106.Taegtmeyer H, McNulty P, Young ME. Adaptation, maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 107.Kliewer SA, Umesono K, Noonan DJ, et al. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Motojima K, Passilly P, Peters JM, et al. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 109.Gulick T, Cresci S, Caira T, et al. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci USA. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmitz-Peiffer C. Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal. 2000;12:583–594. doi: 10.1016/s0898-6568(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 112.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 113.Thompson AL, Cooney GJ. Acyl-CoA inhibition of hexokinase in rat and human skeletal muscle is a potential mechanism of lipid-induced insulin resistance. Diabetes. 2000;49:1761–1765. doi: 10.2337/diabetes.49.11.1761. [DOI] [PubMed] [Google Scholar]
- 114.Wu P, Inskeep K, Bowker-Kinley MM, et al. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48:1593–1599. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]
- 115.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 116.Chen V, Ianuzzo C, Fong B, et al. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984;33:1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- 117.Depre C, Young ME, Ying J, et al. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 118.Young ME, Patil S, Ying J, et al. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 2001;15:833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 119.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Young ME, Guthrie PH, Razeghi P, et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 121.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 122.Itani SI, Zhou Q, Pories WJ, et al. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–1358. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 123.Leichman JG, Aguilar D, King TM, et al. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am J Clin Nutr. 2006;84:336–341. doi: 10.1093/ajcn/84.1.336. [DOI] [PubMed] [Google Scholar]
- 124.Rossetti L, Smith D, Shulman GI, et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roduit R, Morin J, Masse F, et al. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-alpha gene in the pancreatic beta -cell. J Biol Chem. 2000;275:35799–35806. doi: 10.1074/jbc.M006001200. [DOI] [PubMed] [Google Scholar]
- 126.Gulick J, Subramaniam A, Neumann J, et al. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- 127.Ojamaa K, Samarel AM, Klein I. Identification of a contractile-responsive element in the cardiac alpha-myosin heavy chain gene. J Biol Chem. 1995;270:31276–31281. doi: 10.1074/jbc.270.52.31276. [DOI] [PubMed] [Google Scholar]
- 128.Golfman LS, Wilson CR, Sharma S, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–E336. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- 129.Sack MN, Rader TA, Park S, et al. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 130.Davila-Roman VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 131.Blain JM, Schafer H, Siegel AL, et al. Studies on myocardial metabolism. VI. Myocardial metabolism in congestive failure. Am J Med. 1956;20:820–833. doi: 10.1016/0002-9343(56)90203-0. [DOI] [PubMed] [Google Scholar]
- 132.Nemoto S, Razeghi P, Ishiyama M, et al. PPAR-gamma agonist rosiglitazone ameliorates ventricular dysfunction in experimental chronic mitral regurgitation. Am J Physiol Heart Circ Physiol. 2005;288:H77–H82. doi: 10.1152/ajpheart.01246.2003. [DOI] [PubMed] [Google Scholar]