Abstract
Aberrations in the phosphatidylinositide-3-kinase (PI3K) signaling pathway play a key role in the pathogenesis of numerous cancers by altering cellular growth, metabolism, proliferation, and apoptosis (1). Mutations in the catalytic domain of PI3K that generate a dominantly active kinase are commonly found in human colorectal cancers and have been thought to drive tumor progression, but not initiation (2). However, the effects of constitutively activated PI3K upon the intestinal mucosa have not been previously studied in animal models. Here, we demonstrate that the expression of a dominantly active form of the PI3K protein in the mouse intestine results in hyperplasia and advanced neoplasia. Mice expressing constitutively active PI3K in the epithelial cells of the distal small bowel and colon rapidly developed invasive adenocarcinomas in the colon that spread into the mesentery and adjacent organs. The histological characteristics of these tumors were strikingly similar to invasive mucinous colon cancers in humans. Interestingly, these tumors formed without a benign polypoid intermediary, consistent with the lack of aberrant WNT signaling observed. Together, our findings indicate a non-canonical mechanism of colon tumor initiation that is mediated through activation of PI3K. This unique model has the potential to further our understanding of human disease and facilitate the development of therapeutics through pharmacologic screening and biomarker identification.
Introduction
Colorectal cancer remains a leading cause of cancer-related death, despite significant advances in treatment options. Targeting oncogenic pathways has been and continues to be a significant interest of many investigators. The PI3K/AKT signaling cascade has been identified as promising target for drug development. Many new inhibitors of PI3K and the downstream signaling molecules are currently in clinical development; however, their role in the clinical setting has yet to be well defined.
The PI3K/AKT pathway transmits signals from various transmembrane growth factor receptors through a kinase cascade to nuclear transcription factors (1). PI3K initiates this signaling pathway through the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 then activates the serine/threonine kinase AKT, which phosphorylates multiple downstream targets responsible for a wide variety of vital cellular functions. One of the prominent targets is the mammalian target of rapamycin (mTOR), a serine/threonine kinase that is an important regulator of cell growth and metabolism. This kinase then mediates activation of the eukaryotic translation initiation factor 4E binding protein (4E-BP1) and the p70S6 ribosomal kinase (S6) that are involved in protein synthesis.
Mutations of the PIK3CA gene, encoding the p110 catalytic subunit of the PI3K kinase, are present in 20 to 30% of human colon cancers (3). Three mutations are common: E542K, E545K, and H1047R (4) and result in a dominantly active form of the PI3K protein. PIK3CA mutations have been investigated in numerous cancer cell lines; however the effect of a dominant active PI3K has not previously been investigated in the mammalian intestine. We describe a novel mouse model designed to provide insight into the biological effects of a dominant active form of PI3K in the colon and once characterized to further test new therapeutic agents and identify biomarkers.
Materials and Methods
Mouse Husbandry
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of Wisconsin in Madison, following the guidelines of the American Association for the Assessment and Accreditation of Laboratory Animal Care. FC+ mice [FVB/N-Tg(Fabp1-Cre)1Jig; NCI Mouse Repository; Strain number - 01XD8] were maintained by backcrossing to FVB mice (FVB/J; The Jackson Laboratory; Stock number - 001800). PIK3ca*+ mice [C57BL/6-Gt(ROSA)26Sortm7(Pik3ca*,EGFP)Rsky/J; The Jackson Laboratory; Stock Number – 012343] were maintained by crossing siblings for fewer than five generations. FC+ PIK3ca*+ and FC0 PIK3ca*+ littermates were generated by crossing FC + females with PIK3ca*+ males (herein, + denotes carrier and 0 denotes non-carrier). For comparison, F1 ApcMin/+ hybrids were generated by crossing SWR females (SWR/J; The Jackson Laboratory; Stock Number - 00689) to C57BL/6 ApcMin/+ males (C57BL/6J ApcMin/J; The Jackson Laboratory; Stock Number - 002020). Offspring carrying ApcMin/+ were treated with drinking water containing 4% dextran sodium sulfate as described previously (5). This treatment increases the multiplicity of tumors in the colon.
Genotyping
Mice were genotyped for FC, PIK3ca*, and the Min allele of Apc as described previously (6–8).
Imaging
Animals were fasted for 6 hours prior to injection of either 18F-FDG (160 μCi; IBA Molecular, Romeoville, IL) or 18F-FLT (140 μCi; University of Wisconsin Cyclotron, Madison, WI). After injection, the animals were kept under anesthesia for 60 minutes and then prepared for dual hybrid microPET/CT colonography as described previously (9). A 10-minute PET acquisition was performed and CT scan followed immediately. A similar approach was used to acquire images after injection of 124I-CLR1404 (120 μCi; Novelos, Madison, WI) except that the animals were not fasted prior to injection of the imaging agent and PET scanning was performed 98 hours after injection. In this case, the duration of the PET scan was set based on coincidence detection, which was 30 million counts or approximately 15 minutes. Maximum intensity projections were created in Siemens Inveon Research Workplace (Knoxville, TN). The PET images were reconstructed using OSEM3D/MAP (OSEM3D, 2 iterations; MAP 18, iterations 16 subsets). Attenuation correction was performed using the CT data. The CT images were reconstructed using standard conebeam reconstruction. Movies were created from 3D representations of the PET and CT data using AMIRA Version 5.2 software package (Visage Imaging Inc., San Diego, CA).
Histology
Mice were euthanized by CO2 asphyxiation. Small bowel and colon were removed, flushed with PBS, cut open lengthwise, splayed out, and fixed in 10% buffered formalin for 24 to 48 hours. Tissues were then stored in 70% ethanol. The duodenum, ileum, and colon were rolled, processed, embedded in paraffin, and cut into 5 μm sections. In some cases, tumors were isolated prior to rolling and then handled in the same manner. Sections were stained with either Hematoxylin and Eosin (H&E) for histological review or Periodic Acid-Schiff (PAS) stain to assess goblet cells.
Immunohistochemistry
Immunohistochemistry was carried out using the Histomouse™ Max Broad Spectrum (DAB) kit as instructed by the manufacturer (Invitrogen, Carlsbad, CA) except for the following modification: antigen unmasking was performed by boiling the samples for 20 min in citrate buffer (pH 6.0) or treating samples with proteinase K for 5 minutes. The primary antibodies included rabbit anti-pAKT (Ser473, 1:100, Cell Signaling Technology, Beverly, MA), mouse anti-β-catenin (1:400, BD Biosciences – Clone 14, San Diego, CA), rabbit anti-cytokeratin 7 (clone SP52, Ventana, Tucson, AR), rabbit anti-cytokeratin 20 (clone SP33, Ventana), rabbit anti-lysozyme (1:500, DAKO, Carpinteria, CA), rabbit anti-PCNA (1:1000, Cell Signaling Technology), and rabbit anti-synaptophysin (1:500, ABCAM, Cambridge, MA).
Western Blot Analysis
Tissue samples were collected and flash frozen. After 24 hours, the samples were sonicated in T-PER tissue protein extraction reagent (Thermo Scientific, Pittsburg, PA), proteasome inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), and PMSF (Sigma-Aldrich). Extracted protein (30μg) from experimental mice and controls was loaded onto pre-cast 10% polyacrylamide gels (BioRad, Hercules, CA). The gels were run at 200 volts for 30 minutes and transferred to PVDF Immobilon-P membranes (Millipore, Bedford, MA) at 100 volts for 45 minutes. The membranes were blocked with 5% non-fat dry milk for one hour and then probed with primary antibodies against p110α, pAKT (Ser473), pS6 (Ser235/236), p4E-BP1 (Thr37/46), or pMAPK (Thr202/Tyr 204) (Cell Signaling Technology) in bovine serum albumin (BSA) (Sigma-Aldrich) at a 1:1000 ratio for 16 hours. Following this incubation, membranes were washed in TBS-T wash buffer (Tris buffered saline, 0.05% Tween 20) before being probed with goat anti-rabbit horseradish peroxidase labeled antibody (Millipore) in 5% non-fat dry milk or BSA at ratio of 1:10,000 for 1 to 2 hours. The membranes were then washed again with TBS-T. SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Pittsburg, PA) was added according to the manufacturer’s instructions and incubated for five minutes. Following removal of the substrate, the membranes were placed in plastic sleeves and exposed to film. Anti-GAPDH antibody (Cell Signaling) was utilized as a loading control at a ratio of 1:5000.
Real-Time PCR
Tissue from mice was isolated and stored in RNAlater (Qiagen, Valencia, CA). Samples were disrupted in RLT buffer with a Kontes Pellet Grinder (Kimple & Chase, Vineland, NJ). RNA was prepared using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. The yield was quantified with a Nanodrop DU-800 (Thermo Scientific). cDNA was generated using the ImProm II Reverse Transcription System (Promega, Madison, WI) from 500ng RNA following the manufacturer’s instructions. qPCR amplifications were prepared with 100ng cDNA, Sso Advanced SYBR Green Supermix (Bio-Rad), and primers for mouse phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PI3Kca; NM008839) Forward 5′-ATCATGCAAATCCAGTGCAA-3′ and Reverse 5′-CAGCTGTCCGTCATCTTTCA-3′ and ribosomal protein L13A (RPL13A; NM_009438) forward 5′-TTCGGCTGAAGCCTACCAGAAAGT-3′ and reverse 5′-TCTTCCGATAGTGCATCTTGGCCT-3′. Real-time PCR was performed with a CFX96 Real-Time PCR machine (Bio-Rad). Melt-curve analysis and serial dilution of cDNA confirmed amplification of a single product with high efficiency. Gene expression was normalized to RPL13A and reactions were performed in triplicate.
Results and Discussion
We sought to determine whether activation of the PI3K/AKT cascade affects homeostasis in the mammalian intestine. Mice carrying a transgene in which the fatty acid binding protein promoter is fused to Cre recombinase (FC) were crossed to mice carrying a transgene encoding a chimeric protein with the iSH2 domain of the p85 regulatory subunit fused to the N-terminus of p110 catalytic subunit (PIK3ca*; Supplementary Fig. S1A; refs 10,11). (FVB/J x C57Bl/6J)F1 progeny carrying both transgenes (FC+ PIK3ca*+) express this dominantly active form of p110 in epithelial cells of the distal small bowel and colon because the Cre recombinase excises a stop sequence upstream of PIK3ca*.
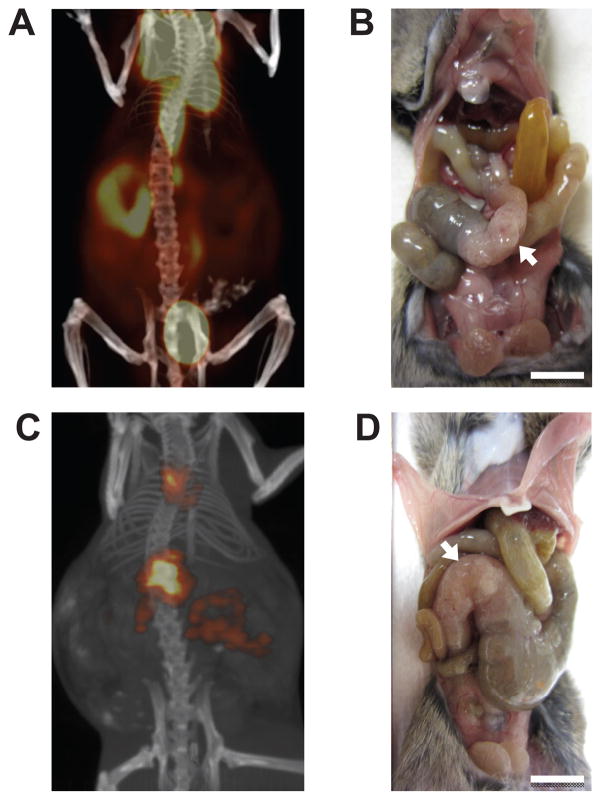
The majority of FC+ PIK3ca*+ mice became moribund between 40 and 60 days of age (Supplementary Fig. S1B). These mice were imaged with a Siemens Dual Hybrid PET/CT scanner, revealing high avidity for fluorodeoxyglucose (18F; FDG), CLR1404 (124I; a phospholipid ether analogue) (12) and fluoro-L-thymidine (18F; FLT) in the proximal colon and low avidity for FDG in the distal small bowel and the remainder of the colon (Figs. 1A, 1C, and Supplementary Movie S1). Mice were sacrificed and tissues were imaged ex vivo (data not shown). The high avidity in the proximal colon was due to the uptake of imaging agents by large tumors (Figs. 1B and 1D). No signal was detected in any FC0 PIK3ca*+ littermates.
Figure 1. FC+ PIK3ca*+ mice develop intestinal obstruction due to large proximal colon tumors.
The tumors exhibit a high avidity for FDG (A) and CLR1404 (C). The mouse was injected with the imaging agent, scanned, and the data was reconstructed using standard algorithms. Following scan acquisition, each mouse was sacrificed and the abdominal wall was dissected revealing tumors in the proximal colon (B and D, arrows). Scale bars: 1 cm.
At necropsy, most FC+ PIK3ca*+ mice (15/17) were found to have severe obstruction of the proximal colon, resulting in marked dilation of the small bowel and cecum (Fig. 2A). Obstructive enteropathy was always caused by the presence of a massive tumor in the proximal colon. On gross examination, these colon tumors penetrated through the serosa and had an impressive enlargement of blood vessels and mesenteric lymphatic tissue (Fig. 2A). In a group of five FC+ PIK3ca*+ mice, we sectioned en bloc the tumor and mesenteric lymphatic tissue. The lymphatic tissue was hyperplastic in all mice; tumor deposits were identified within the mesenteric adipose tissue in 3 of 5 mice. In other FC+ PIK3ca*+ mice, the intestines were removed, split lengthwise, and splayed open, revealing large flat thickened rugal folds and plaque-like tumors in the proximal colon without a significant luminal exophytic component (Fig. 2A).
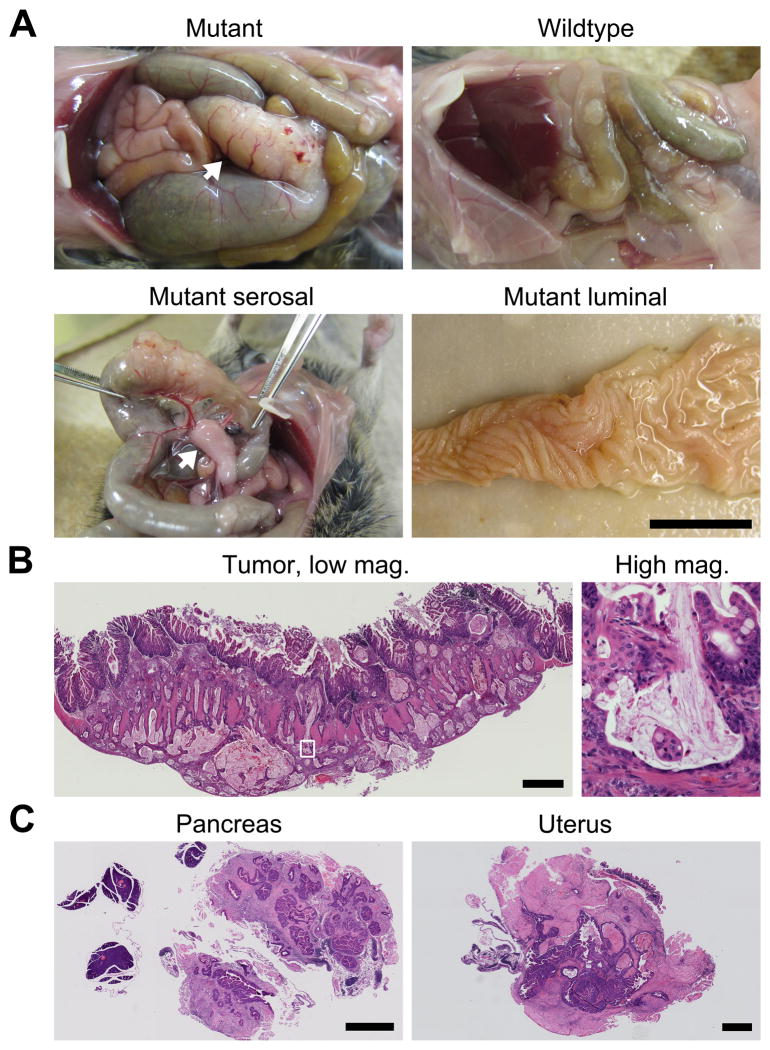
Figure 2. The large tumors in the proximal colon are invasive mucinous adenocarcinomas.
A FC+ PIK3ca*+ mouse and its wild-type littermate were sacrificed at 56 days of age and dissected (A upper left and right, respectively). In the mutant, severe dilation of the small bowel and cecum was due to obstruction by a massive tumor (A upper left, arrow). The tumor was associated with large penetrating blood vessels and thickened mesentery (A lower left, arrow). The intestine was removed, split lengthwise, and splayed out. The epithelium was hyperplastic but no obvious polypoid intermediary was observed (A lower right). The tumor was removed, embedded in paraffin, and cut. Sections were stained with H&E (B). Islands of malignant glands in mucin lakes were evident at higher magnification (B right). One cecal tumor appeared to directly extend to surrounding organs including the pancreas and in the uterus (C). Scale bars: 1 cm (A) and 1 mm (B, and C).
Histological examination of the proximal colon confirmed the presence of moderately differentiated, diffusely invasive mucinous adenocarcinomas with extension through the muscularis propria and serosa into the pericolonic adipose tissue (Figs. 2B and 3A). These tumors exhibited mucinous differentiation with islands of malignant glands within mucin lakes (Fig. 2B) as well as budding at the leading edge of invasion fronts. The masses elicited a desmoplastic reaction as well as inflammatory infiltration (Fig. 2B). The neoplastic cells exhibited high-grade nuclear atypia, phosphorylation of AKT, and an increase in cellular proliferation, as compared to the normal colonic epithelium from FC+ PIK3ca*+ or FC0 PIK3ca*+ mice and adenomas from ApcMin/+ mice (Fig. 3B and Supplementary Figs. S2 and S3). In addition, neoplastic cells were weakly positive for cytokeratin 20 (IHC 1+) and negative for cytokeratin 7 (Supplementary Fig. S4), indicating that the transformed cells originated from the intestinal epithelium (13). Areas of invasion were covered by hyperplastic crypts with focal crypt dilatation and branching or at times a denuded epithelium.
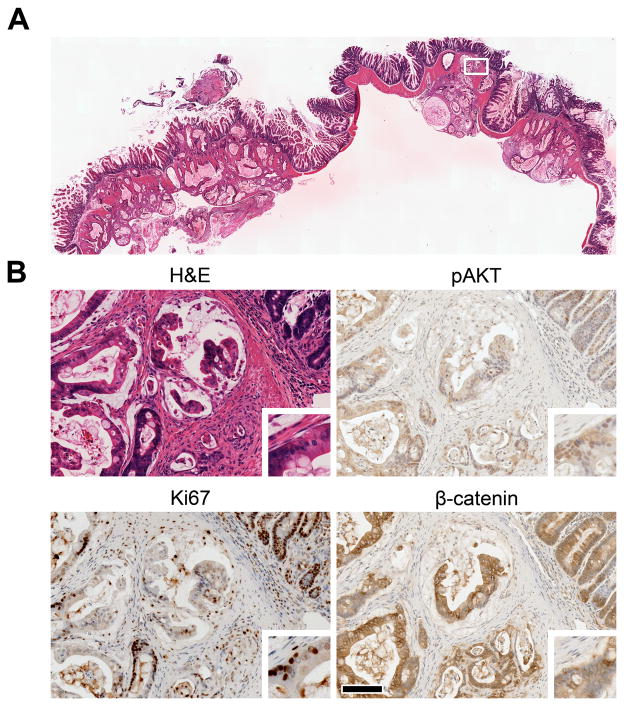
Figure 3. PIK3ca*inducescellular proliferation.
A section of a tumor from a 61-day-old FC+ PIK3ca*+ mouse was stained with H&E (A). The tumor invaded through the musculature into the adipose tissue of the colonic mesenteries. The boxed region in A is shown at higher magnification (B). The activation of AKT, a downstream target of PI3K, is revealed by immunohistochemistry (IHC) using antibodies specific for pAKT (B upper right). The majority of nuclei are positive (brown). This change was coupled with an increase in cellular proliferation as measured by IHC using antibodies against Ki67 (B lower left). The invasive adenocarcinomas that develop in this model do not appear to rely on aberrant WNT signaling, as β-catenin is membrane-bound rather than nuclear, as shown by IHC (B lower right). This localization of β-catenin was observed in 10 out of 10 tumors. Insets were taken from the left edge of each panel and magnified two-fold (B). Scale bar: 100 μm.
About half of the FC+ PIK3ca*+ mice (6/17) also developed cecal tumors. Direct extension was observed of one tumor into the lymphatic, ovary, uterus, and pancreatic tissue (Fig. 2C), which would be consistent with stage T4 invasion of human cancers. No gross evidence of liver or lung metastasis was identified.
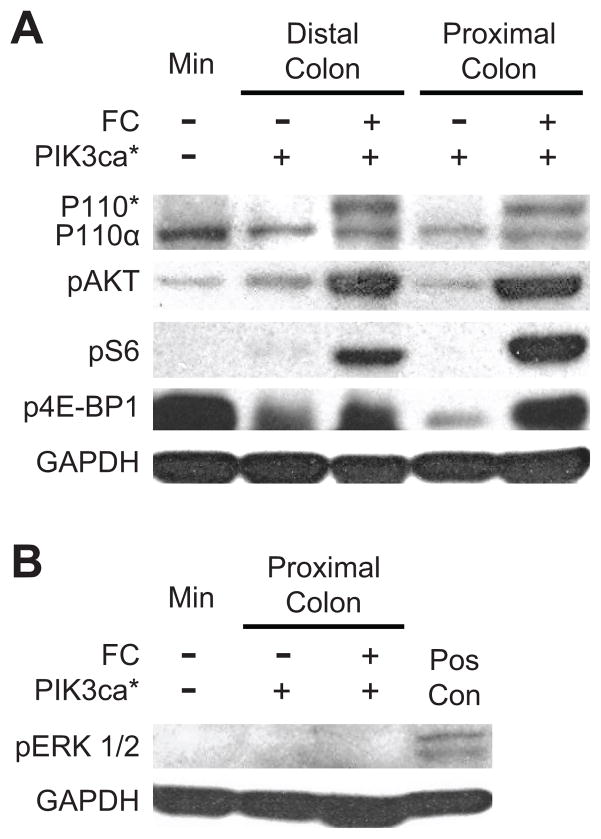
The development of advanced cancers in this model is mediated by the expression of the dominant active form of PIK3ca* and consequently activation of several of its targets (Figs. 3B and 4A and Supplementary Fig. S5). The presence of the PIK3ca* protein was confirmed in the mucosa of the proximal and distal colon (Fig. 4A). In addition, downstream activation of AKT and subsequent phosphorylation of S6 and 4E-BP1 were observed (Fig. 4A). These observations confirm that the transgene was transcribed and translated in the colon of FC+ PIK3ca*+ mice and that its expression resulted in increased downstream activation of the PI3K/AKT/mTOR pathway. By contrast, phosphorylation of ERK 1/2 was not noted above baseline, indicating that activation of the Raf/MEK/ERK cascade is not involved in tumorigenesis in this model (Fig. 4B).
Figure 4. Activation of the PI3K/AKT/mTOR pathway occurs in the colonic mucosa and tumors of FC+ PIK3ca*+ mice without increasing pERK 1/2.
Protein extracts were prepared from scrapings of the mucosa that were taken from either the proximal or distal colon of experimental and control mice. Note that the scrapings from the proximal colon of FC+ PIK3ca*+ mice contained tumor tissue. Protein extracts were also prepared from a colon tumor that was taken from a Min (ApcMin/+) mouse for comparison. p110* is identifiable by western blot in the proximal and distal colon of FC+ PIK3ca*+ mice (A). The level of p110α is similar in all tissues tested. Up-regulation of the PI3K/AKT/mTOR pathway is noted by increased phosphorylation in AKT, S6, and 4E-BP1. Phosphorylation of ERK 1/2 was undetectable in protein extracts from proximal colon tumors of four FC+ PIK3ca*+ mice, a representative is shown (B). DLD-1, a human colorectal cancer cell line that express mutant KRAS, was used as a positive control (Pos Con); GAPDH was used as a loading control.
Most human colorectal adenomas carry truncating mutations in APC and the loss of this gene is thought to be the tumor-initiating event (14). These mutations result in translocation of β-catenin to the nucleus (Supplementary Fig. S3), and consequently change the pattern of gene expression. β-catenin was examined in the invasive adenocarcinomas of FC+ PIK3ca*+ mice and found to be localized to the cell membrane and cytoplasm (Fig. 3B). This pattern of localization indicates that tumor initiation in FC+ PIK3ca*+ mice is not mediated through aberrant WNT signaling. The lack of aberrant WNT signaling also correlates with the lack of an exophytic or polyp-like morphology in this model, as polyp formation would be expected in Apc mutant tumors. Given the lack of other induced genetic abnormalities and the short time frame in which these tumors develop, the initiating event in FC+ PIK3ca*+ cancers appear to be PI3K mediated.
With the goal of identifying precursor lesions or early tumors, FC+ PIK3ca*+ mice were sacrificed at fixed points in time. Cohorts of at least four mice were evaluated at 20, 30, 40, and 50 days of age. On histological examination, the distal small bowel and colon were hyperplastic in all mice examined and lesions similar to serrated sessile adenomas that are seen in humans were identified (Supplementary Fig. S6). Invasive mucinous adenocarcinomas were first observed in the 40-day cohort.
To examine whether intestinal cell fate is altered prior to tumorigenesis, we assessed the four major epithelial cell lineages in the colon (Supplementary Fig. S7). The relative number of absorptive cells, Paneth cells, and enteroendocrine cells in samples from FC+ PIK3ca*+ mice were similar to that observed in samples from controls. By contrast, goblet cells were slightly more abundant in the samples from experimental mice than in samples from controls.
Animal models have led to significant advances in our understanding of the biology of many cancer types, including colorectal cancer. This study is the first to describe highly invasive mucinous adenocarcinomas in the mammalian intestine resulting from the expression of a dominantly active form of PI3K. These tumors are quite comparable to cancers in humans, especially those on the right side of the colon. Shared histological characteristics include high-grade nuclear atypia, budding at the leading edge of the invasion fronts, and mucin lakes (15).
Recently, a model lacking the expression of PTEN in the intestine was described (16). This model has a drastically different phenotype with only 19% of mice developing tumors in the small intestine by 12 months of age. Despite having activated AKT signaling, only one invasive adenocarcinoma was identified. We hypothesize that the primary difference between these two models relates to the roles of PTEN and PI3K in the AKT signaling pathway (i.e. tumor suppressor gene versus an oncogene). These models also have different genetic backgrounds and Cre expression was controlled by different promoters.
Interestingly, in FC+ PIK3ca*+ mice, tumor initiation appears to be independent of WNT signaling and invasive cancers develop rapidly without a benign polypoid or exophytic precursor lesion. These tumors appear to develop through a novel non-canonical pathway to tumor initiation mediated by PI3K. Human tumor cell lines including the commonly investigated RKO human colon cancer cell line have been described that possess activating mutations of PIK3CA but lack mutations of APC and CTNNB1 (16). In addition, human colorectal tumors carrying activating mutations in PIK3CA often express normal levels of β-catenin (17). Thus, non-polypoid tumors that arise quickly as a consequence of mutations in PIK3CA might explain the development of interval cancers that occur between screening colonoscopies. Further investigations need to examine this novel pathway to tumorigenesis described in this study.
This model will also aid in the development and testing of pharmacologic agents. Targeting oncogenic pathways has led to recent advances in the treatment of multiple cancers, including the use of vemurafenib to treat melanomas harbouring BRAF mutations, erlotinib for lung cancers possessing EGFR mutations, and crizotinib to treat lung cancers harbouring the EML4-ALK translocation. PIK3CA has been identified as an important oncogene in multiple cancers and thus modelling this mutation in the mammalian colon is important. This murine model with rapidly developing invasive colorectal cancers is an exciting model of human colon cancer that has the potential to be instrumental in the development of targeted therapeutics and biomarker identification.
Supplementary Material
Acknowledgments
We thank Ella Ward and Jane Weeks in Experimental Pathology at the UW Carbone Cancer Center for technical assistance and Drs. Jeff Bacher, William F. Dove, Norman Drinkwater, Greg Kennedy, Paul Lambert, Mark Reichelderfer, H. Ian Robins, and William Schelman for critical review of this manuscript. The project was supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology through a Young Investigator Award (D.A.D.); the National Cancer Institute of the U.S. National Institutes of Health through P01 AI084853 (J.R.T.), P30 CA014520 (Core Grant, University of Wisconsin Carbone Cancer Center), P50 CA095103 (Gastrointestinal Specialized Program of Research Excellence Grant, Vanderbilt Ingram Cancer Center), R01 CA123438 (R.B.H), T32 CA009614 (D.A.D.), T32 CA009135 (J.N.H. and C.D.Z.), and T32 CA090217 (T.J.P.O.); and start-up funds (R.B.H.) from the UW Division of Gastroenterology and Hepatology, the UW Department of Medicine, and the UW School of Medicine and Public Health. This paper is dedicated to the memory of Joseph E. Hoeger.
Footnotes
Author Contributions A.A.L., D.A.D., and R.B.H. designed, performed and analyzed experiments, and wrote the manuscript. C.D.Z., M.F., T.J.P.O., J.N.H., L.A.N., D.M.A., L.C., and J.R.T. performed and analyzed experiments. R.S., M.K.W., and J.P.W. analyzed experiments. A.A.L. and D.A.D. contributed equally to this work; all authors discussed results and edited the manuscript.
Author Information The authors declare competing financial interests: Dr. Jamey Weichert is the founder of Cellectar, Inc. (Madison, WI), which holds the licensing rights to the CLR1404 technology, and therefore has a financial interest in this agent.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Huang CH, Mandelker D, Gabelli SB, Amzel LM. Insights into the oncogenic effects of PIK3CA mutations from the structure of p110alpha/p85alpha. Cell Cycle. 2008;7:1151–1156. doi: 10.4161/cc.7.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, et al. Hot-spot mutations in p110α of phosphatidylinositol 3-kinase (PI3K) Cell Cycle. 2010;9:596–600. doi: 10.4161/cc.9.3.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 6.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 9.Durkee BY, et al. Reproducibility of tumor volume measurement at microCT colonography in living mice. Acad Radiol. 2008;15:334–341. doi: 10.1016/j.acra.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klippel A, et al. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinchuk AN, et al. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J Med Chem. 2006;49:2155–2165. doi: 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]
- 13.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 14.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 15.Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103–107. doi: 10.1002/1096-9098(200010)75:2<103::aid-jso6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.The data was obtained from the Wellcome Trust Sanger Institute Cancer Genome Project web site, http://www.sanger.ac.uk/genetics/CGP
- 17.Nosho K, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.