Abstract
Neuropeptide Y is a co-transmitter that is synthesized by chromaffin cells in the adrenal medulla. During the fight-or-flight response these cells release NPY in addition to epinephrine and norepinephrine. Following the stress-induced reflex, the levels of NPY are increased as part of a homeostatic response that modulates catecholaminergic signaling. Here we examined the control of NPY expression in mice after brief exposure to the cold water forced swim test. This treatment led to a shift in NPY expression between two populations of chromaffin cells that reversed over the course of one week. When NPY(GFP) BAC transgenic animals were exposed to stress, there was an increase in cytoplasmic, nonsecretable GFP, indicating that stress increased NPY promoter activity. In vivo blockage of Y2 (but not Y1 or Y5) receptors increased basal adrenal NPY expression and so modulated the effects of stress. We conclude that release of NPY mediates a negative feedback loop that inhibits its own expression. Thus the levels of NPY are determined by a balance between the potentiating effects of stress and the tonic inhibitory actions of Y2 receptors. This may be an efficient way to ensure the levels of this modulator do not decline following intense sympathetic activity.
Keywords: Neuropeptide Y, Adrenal, Stress, Autonomic nervous system, Co-transmitter
Introduction
A characteristic feature of the response to stress is an increase in the activity of endocrine cells in both the adrenal cortex and medulla. Activation of the hypothalamic-pituitary-adrenal (HPA) axis induces the release of steroid hormones from the adrenal cortex while exposure to stress evokes secretion of norepinephrine and epinephrine from chromaffin cells in the adrenal medulla. This change in release is rapidly followed by a compensatory change in secretory capacity. Thus stressors such as restraint, cold stress and hypoglycemia all lead to an increase in the expression of tyrosine hydroxylase, the rate limiting enzyme for catecholamine synthesis (Chuang and Costa, 1974; Fluharty et al., 1983; Nankova et al., 1994; Vietor et al., 1996). Stress also regulates the cortical expression of P450 11β-hydroxylase and aldosterone synthetase which are required for corticosterone and aldosterone production, respectively (Aguilera et al., 1995; Engeland et al., 1997). These coordinated changes in catecholamine release and synthesis are coupled processes, giving rise to the term “stimulus-secretion-synthesis coupling” (Thoenen et al., 1969a, b; Eiden et al., 1984; Mahata et al., 2003). They presumably ensure that the adrenal response does not become refractory after exposure to an acute stressor.
Although this activity-dependent increase in release capacity appears to be a failsafe mechanism, positive feedback loops carry a potential risk because increased filling of the secretory pool of granules could lead to an overly vigorous stress response. For example repeated exposure to stress can lead to a facilitated release of catecholamines and glucocorticoids (Lilly et al., 1986; Figueiredo et al., 2003; Kuzmiski et al., 2010) suggesting that a change in secretory capacity can have physiological consequences. Chronic diseases including type II diabetes and hypertension are also associated with altered sympathoadrenal activity and catecholamine secretion (Guyenet, 2006; McCrimmon and Sherwin, 2010; Ramanathan and Cryer, 2011) but whether disregulated secretory capacity contributes to these conditions is not known. Under normal circumstances what prevents an acute stressor from leading to an unrestrained increase in secretory capacity? We have recently found that the adrenal expression of tyrosine hydroxylase is tonically suppressed by neuropeptide Y and that loss of NPY leads to an elevated secretory capacity (Wang et al, submitted). Because NPY is a co-transmitter with the catecholamines in chromaffin cells and can be co-released (Varndell et al., 1984; Henion and Landis, 1990; Whim, 2006) this suggests that the peptide could play the restraining role outlined above. Co-transmitters (in particular neuropeptides) may be an effective way to provide feedback regulation because their release generally requires high frequency bursts of neuronal activity (Bloom et al., 1988; Leng and Ludwig, 2008) - precisely those conditions under which feedback modulation occurs.
The idea that NPY might be involved in the adaptive response to stress is consistent with the finding that the adrenal levels of NPY mRNA and peptide are raised after exposure to an acute stressor. This transcriptionally-dependent response is prevented either by adrenal denervation or by blockade of nicotinic ACh receptors indicating that preganglionic activity plays an important role in determining NPY expression (Hiremagalur et al., 1994; Jahng et al., 1997). Because the adrenal medulla contains several NPY receptor subtypes (Cavadas et al., 2006) this raises the possibility that the expression of NPY itself could also be controlled by an autocrine feedback loop. Nevertheless there is disagreement about which cells in the adrenal medulla express NPY (Varndell et al., 1984; Lundberg et al., 1986; Henion and Landis, 1990) and the role of the different Y receptor subtypes in modulating NPY expression has not been examined. In this study we use a molecular and transgenic approach to track NPY promoter activity and peptide expression after exposure to an acute stressor and then determine whether Y receptors are involved in regulating the adrenal expression of NPY in vivo.
Materials and Methods
Animals and stress paradigm
Wild type C67BL/6J mice and NPY(GFP) animals (van den Pol et al., 2009) were obtained from the Jackson laboratory. Male animals were used in most experiments with the exception of some of the experimental groups in Figures 1, 2C and 5. Animals were individually housed before (3–5 d) and after stress exposure. The cold water forced swim test was performed as described (Saal et al., 2003). Briefly, mice were placed in an 18 cm diameter cylinder containing 3 L of cold water (4–5 °C) for five to six minutes. After recovery under an infra red heat lamp (15–25 minutes) mice were returned to their home cages. Control animals were left undisturbed. Mice were sacrificed by decapitation, 1 d or one week later, as noted in the text. Littermates were used in all experiments (P24–26). All experiments involving animals were approved by the Animal Care and Use Committees at Louisiana State University Health Sciences Center and Pennsylvania State University.
Figure 1. The cold FST induced an increase in NPY expression in adrenal medullain situ.
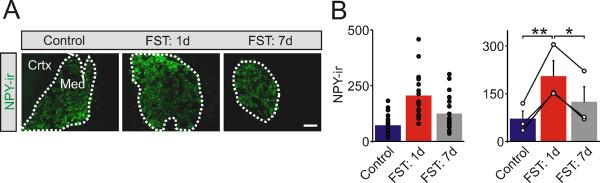
(A). Examples of NPY-ir in sections of adrenal medulla from control mice and 1 day and 1 week after exposure to the cold FST. NPY expression is restricted to the adrenal medulla (med) and absent from the cortex (crtx). (B). Group data shows that 1 day after exposure to the cold FST there is a significant increase in the levels of NPY-ir in the adrenal medulla, but that 1 week later the values have declined towards basal values. Left histogram shows NPY-ir in all cryosections (filled symbols). Right histogram indicates the mean ± SEM (n = 3). In this and subsequent figures open symbols indicate the average value from each animal and black lines link the matched control and stressed animals from each independent experiment. Scale bar 100 μm. *P < 0.05; **P < 0.01.
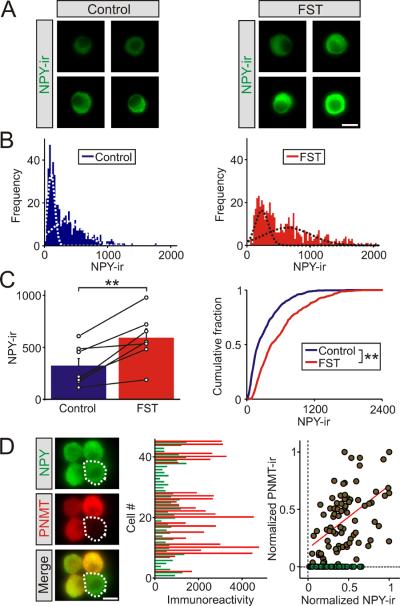
Figure 2. The cold FST induced an increase in NPY expression in adrenal chromaffin cellsin vitro.
(A). Examples of NPY-ir in chromaffin cells from a matched littermate pair of control mice and 1 day after the cold FST. (B). Frequency histograms of the levels of NPY-ir in chromaffin cells from matched control and stressed animals (≥477 cells for each distribution, n = 7 separate experiments). The histograms were each fitted with two Gaussian distributions (dashed lines, R2 = 0.93 and 0.80, respectively). (C). Group data (left panel) showing that the cold FST led to a significant increase in NPY-ir (mean ± SEM, n = 7 independent experiments). The open circles indicate the mean value from each animal (each was calculated from ≥60 chromaffin cells). The black lines link the matched animals from each independent experiment. Cumulative frequency distributions (right panel) confirm that the cold FST led to a significant increase in NPY-ir (≥ 477 cells for each distribution). (D). Examples of chromaffin cells co-stained for NPY and PNMT. Histogram (middle panel) of the levels of NPY- and PNMT-ir in individual cells from a typical experiment shows that NPY is expressed in both epinephrine and norepinephrine cells. Normalization of the levels of NPY and PNMT immunoreactivity reveals a population of NPY+/PNMT−cells (green symbols). A linear fit of the immunoreactive signal from the NPY+/PNMT+cells (red line) shows a very weak correlation between the two, consistent with the idea that the levels of NPY and PNMT expression are independent (R = 0.52, 125 cells from n = 3 independent experiments; 66% of cells were PNMT positive / epinephrine chromaffin cells). Scale bars 10 μm. **P < 0.01.
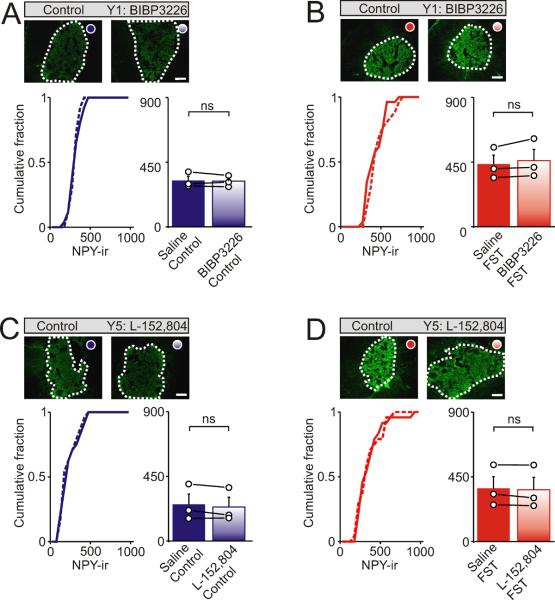
Figure 5. Y1 and Y5 receptors do not regulate NPY expression in the adrenal medulla.
Examples of NPY-ir in sections of adrenal medulla from control mice or after i.p. injection with Y1 and Y5 antagonists. (A). Under basal, non-stressed conditions BIBP3226 did not alter the levels of NPY-ir in the adrenal medulla. (B). BIBP3226 also did not prevent the increase in NPY-ir that occurred following exposure to the cold FST. (C, D). NPY-ir in the adrenal medulla from control mice or after injection with L-152,804 (a Y5 antagonist) showing that L-152,804 did not alter the levels of NPY-ir under non-stressed conditions or prevent the stress-induced increase in NPY-ir. Left cumulative distribution in each panel shows NPY-ir from all cryosections (bin width 50 intensity units). Right histograms indicate the mean ± SEM (n = 3 independent experiments; 8 - 10 cryosections per animal). Open circles indicate the average value from each animal. Black lines link the matched control and stressed animals from each independent experiment. Scale bars 100 μm. ns = not significant.
In vivo injection
Animals were injected (i.p.) with Y receptor antagonists; BIBP 3226 (1 mg / kg), BIIE 0246 (1 mg / kg) or L-152,804 (10 mg / kg) 15 mins before the forced swim test. Control animals were injected with the same volume (~200 μl) of sterile saline.
Cell culture and immunocytochemistry
Adrenal medullae, in most case from P24–26 mice, were dissociated and chromaffin cell cultures were prepared on poly-d-lysine coated coverslips as described (Whim, 2006). Two hrs after plating the cultures were fixed with 4% paraformaldehyde then stained (Mitchell et al., 2008). Primary antibodies were rabbit anti-NPY (1:40,000, Peninsula Labs, T-4070) and guinea pig anti-PNMT (1: 100 – 500, Acris, EUD7001). Secondary antibodies were donkey anti-rabbit Alexa 488 (1:200, Invitrogen) and donkey anti-guinea pig DyLight 549 (1:100, Jackson ImmunoResearch).
Immunohistochemistry
Adrenal glands were fixed in 4% paraformaldehyde in PBS overnight at room temperature then washed with PBS, rapidly frozen in 2-methylbutane on dry ice, embedded in cryomatrix medium and kept at −80°C until use. Frozen sections (30 μm) were washed with PBS, re-fixed with 4% paraformaldehyde for 20 mins then permeabilized in 0.3% Triton X-100 in PBS for 15 mins. Sections were then incubated in 3% H2O2 for 45–60 minutes to quench endogenous peroxidase activity and transferred to blocking reagent (in Tris buffered saline; TBS, Perkin Elmer) for 30 mins. Sections were incubated in primary antibody (rabbit anti-NPY; 1:10,000, Peninsula Labs, T-4070) at 4 °C overnight. Sections were washed with PBS containing 0.05% Tween-20 and incubated with a secondary antibody conjugated to peroxidase (1:500 donkey anti-rabbit; Jackson ImmunoResearch) for 30 mins at room temperature, washed with PBS containing 0.05% Tween-20, incubated in TSA-FITC (Perkin Elmer) for 10 mins, then sequentially washed with PBS containing 0.05% Tween-20, rinsed with distilled water and mounted in Vectashield.
Image analysis
Images were obtained with a Nikon TE2000U microscope and 10X and 60X oil immersion (1.4 numerical aperture) objectives and a Retiga 1300 monochrome camera. Image-Pro Plus 5.1 (Media Cybernetics), Origin Pro7, Excel and Minitab 15 were used for data analysis. To quantify the degree of staining in tissue sections, the medulla minus any cell-free areas was outlined and the mean pixel intensity was calculated (i.e. an area-independent measurement). Images were then background subtracted using AOI's from the adrenal cortex and 7 – 10 sections from each animal were measured. In each figure open circles indicate the mean value from each animal and black lines link the matched control and stressed animals from each independent experiment.
For analysis of cells in vitro, images of single chromaffin cells were taken at the level of the nucleus to maintain the same focal plane between cells. The mean pixel intensity was calculated as above, but background subtraction was not used except for the data shown in Figures 2D and 3D. In all staining experiments involving the quantification of the stress response, the slides were blinded until the analysis was complete.
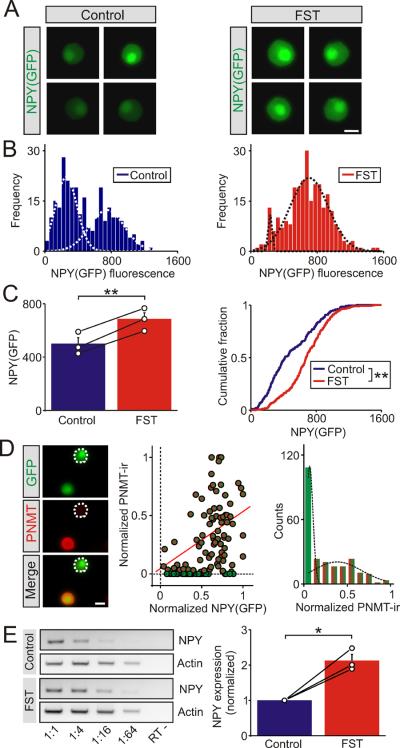
Figure 3. The cold FST increased the transcription of the NPY gene in the adrenal medulla.
(A). Examples of GFP expression in chromaffin cells from NPY(GFP) BAC mice under control conditions and 1 day after the cold FST. (B). Frequency histograms of GFP expression in chromaffin cells from matched control and stressed animals (270 cells for each distribution). The histograms were each fitted with two Gaussian distributions (dashed lines, R2 = 0.88 and 0.90, respectively).). (C). Group data (left panel) showing that the cold FST led to a significant increase in GFP expression (mean ± SEM, n = 3 independent experiments). The open circles indicate the mean value from each animal (each was calculated from 90 chromaffin cells). The black lines link the matched control and stressed animals from each independent experiment. Cumulative frequency distributions (right panel) confirm that the cold FST led to a significant increase in GFP expression (270 cells per distribution). (D). Examples of chromaffin cells acutely isolated from NPY(GFP) mice that were co-stained for PNMT. NPY(GFP) is expressed in both epinephrine and norepinephrine cells. Normalization of the levels of NPY(GFP) and PNMT-ir reveals a population of NPY(GFP)+/PNMT−cells (green symbols). A linear fit of the data (red line) shows a weak correlation between the two, consistent with the idea that NPY(GFP) and PNMT expression are independent (R = 0.41, 120 cells from n = 3 independent experiments; 70% of cells were PNMT positive / epinephrine chromaffin cells). Distribution of normalized PNMT-ir (right panel) is bimodal implying cells synthesize either epinephrine or norepinephrine (includes data from Figure 2D; 245 cells from n = 6 independent experiments, superimposed lines show two Gaussian distributions; R2 = 0.99). (E). Example of an RT-PCR experiment (left panel) measuring NPY and actin mRNA expression from a control and a stressed animal (3 hours after exposure to the cold FST). The negative controls lacked reverse transcriptase (“RT-“). The template cDNA was serially diluted from 1:1 – 1:64 as indicated. Group data (right panel) indicated that the level of NPY mRNA (1:16 dilution) was significantly higher in the stressed animals. Actin expression was used as an internal control and the results from the stressed animals were normalized to matched controls from a non-saturated portion of the dilution curve (mean ± SEM, n = 3). The open black circles indicate the value from each individual animal. The black lines link the matched control and stressed animals from each individual experiment. *P < 0.05; **P < 0.01. Scale bars 10 μm.
Reverse transcription PCR
mRNA was prepared from the adrenal medulla using TRIzol (Invitrogen) following the manufacturer's instructions. Samples were treated with DNase (Ramamoorthy and Whim, 2008), purified using RNeasy (Qiagen) and cDNA was obtained by reverse transcription. For semi-quantitative RT-PCR, sequential dilutions of cDNA's at 1:4, 1:16 and 1:64 were used for PCR amplification and the results were quantified only within the linear range. NPY expression was normalized by actin expression. Primers and PCR protocols were as follows; NPY; 5' CACGATGCTAGGTAACAAG 3' and 5' CACATGGAAGGGTCTTCAAG 3' (294 bp, annealing Tm 55 °C, 35 cycles); actin; 5' GCCAACCGTGAAAAGATGAC 3' and 5' CAACGTCACACTTCATGATG 3' (523 bp, annealing Tm 55 °C, 35 cycles). The latter two primers have been previously described (van den Pol et al., 2009).
Data analysis
Statistical significance was assessed using the Student's t test. Comparisons between three or more groups were made with a general linear model ANOVA (post-hoc Tukey's paired comparison). Population distributions were fit using OriginPro 7. Since the absolute level of staining varied between individual experiments comparisons were made between paired control and experimental groups (which were processed in parallel and generated from littermates in each independent experiment). In the figures individual data points are plotted as filled symbols. In histograms with error bars black lines connect the average values (open symbols) from paired control and experimental animals. The Kolmogorov–Smirnov test was used for analyzing statistical significance in the cumulative fraction data sets.
Results
Acute stress leads to a sustained increase in the adrenal expression of neuropeptide Y
Previous studies have shown that a variety of chronic stressors including restraint, cold and hypoxia lead to an increase in the adrenal expression of NPY (Hiremagalur et al., 1994; Han et al., 2005; Raghuraman et al., 2011). To determine whether the peptidergic synthetic capacity of the sympathoadrenal system is also responsive to an acute stress, we exposed mice to the cold water forced swim test (FST) for 5 minutes. Control and stressed mice were then sacrificed 1 day or 1 week later and the level of NPY-immunoreactivity (NPY-ir) was examined in adrenal cryosections. In all cases NPY-ir was restricted to the medulla as expected for a co-transmitter that is expressed by chromaffin cells (Figure 1A). Quantification of NPY-ir indicated that 1 day after the FST there was a significant increase in NPY expression compared to matched control animals (69.9 ± 25.6 vs 203.4 ± 50.6 arbitrary intensity units; mean ± SEM, n = 3, P < 0.01) and that the levels had returned towards baseline 1 week after the stress exposure (Figure 1B).
To examine the stress-induced change in NPY expression at the level of single cells, the effect of the FST was measured from individual chromaffin cells isolated in vitro (Figure 2A). In cells from control animals the NPY-ir intensity distribution was skewed and after stress the data was well fit with the sum of two Gaussian distributions. This suggested that the in vivo treatment had led to a shift in NPY expression between two populations of cells, with stress resulting in an increase in the proportion of cells expressing high levels of NPY and a corresponding decline in the number of low-expressing cells (Figure 2B). Group data confirmed that stress resulted in a significant increase in the level of NPY-ir (Figure 2C) and led to a large rightward shift in the cumulative distribution of intensities (Figure 2C).
The presence of two populations of NPY-ir cells raised the question of whether these corresponded to epinephrine and norepinephrine chromaffin cells. To address this issue, acutely isolated cells were co-stained for NPY and PNMT. The latter is a selective marker of epinephrine-synthesizing cells (Ebert et al., 2004). NPY-ir was observed in both PNMT positive and negative chromaffin cells (Figure 2D). This is in contrast to previous studies in which NPY was reported to be restricted to either epinephrine (Lundberg et al., 1986; Henion and Landis, 1990) or norepinephrine-synthesizing chromaffin cells (Varndell et al., 1984). When the levels of immunoreactivity were quantified no examples of PNMT positive / NPY negative cells were found (Figure 2D middle panel) consistent with the ubiquitous expression of NPY in chromaffin cells. Finally, the correlation coefficient between the levels of NPY- and PNMT-ir in single cells was very low (Figure 2D, right panel; R = 0.52). Thus the two populations of NPY-expressing chromaffin cells do not appear to be related to their ability to synthesize either epinephrine or norepinephrine.
The effect of acute stress involves an increase in NPY synthesis
Neuropeptide Y enters the regulated secretory pathway and is released via exocytosis. Thus unlike the stress-induced increase in the enzyme tyrosine hydroxylase (Thoenen, 1970; Nankova et al., 1994), the change in NPY-ir could be due to an increase in synthesis or a reduction in secretion (leading to elevated levels of intracellular NPY). To determine whether the change in NPY-immunoreactivity reflected a genuine increase in peptide synthesis, we examined the effects of stress on GFP expression in NPY(GFP) BAC mice. In these animals GFP is produced as a cytoplasmic protein whose expression is controlled by regulatory elements in the NPY gene (van den Pol et al., 2009). Therefore we reasoned that an increase in GFP expression would reflect a transcriptional activation of the NPY gene by stress. In the adrenals of NPY(GFP) mice, GFP expression was limited to tyrosine hydroxylase-expressing cells consistent with the restriction of NPY expression to chromaffin cells (Wang et al, submitted). When the levels of GFP were measured from chromaffin cells in vitro we found that GFP expression was significantly higher in cells isolated from stressed animals (Figure 3A). In the control animals the intensity distribution was well fit with the sum of two Gaussian distributions again indicating the presence of two populations of NPY-expressing chromaffin cells (Figure 3B). Stress led to a decrease in the proportion of low GFP-expressing cells and an increase in the number of cells expressing high levels of GFP (Figure 3B). Group data showed that stress resulted in a significant increase in the level of GFP (Figure 3C, left panel) and led to a large rightward shift in the cumulative distribution of intensities confirming the increase in GFP expression (Figure 3C, right panel). These results indicate that stress leads to an increase in NPY gene transcription. When acutely isolated cells were stained for PNMT (Figure 3D, left panel) we found that although all chromaffin cells expressed NPY(GFP), a fraction of chromaffin cells were PNMT negative consistent with NPY synthesis by both epinephrine and norepinephrine chromaffin cells. The correlation coefficient was again low (Figure 3D, middle panel; R = 0.41) implying that NPY expression is not related to the ability to synthesize epinephrine. The normalized PNMT-ir intensity distribution was clearly skewed and well fit by the sum of two Gaussian distributions (Figure 3D, right panel) consistent with the idea that chromaffin cells release either epinephrine or norepinephrine (Petrovic et al., 2010). Finally semi-quantitative RT-PCR experiments were used to confirm that the levels of NPY mRNA were indeed significantly higher in the stressed animals compared to matched controls (Figure 3E).
Y2, but not Y1 or Y5 receptors, regulate the expression of adrenal NPY in vivo
What is the activity-dependent signal that mediates the increase in NPY expression? Previous studies have shown that preventing splanchnic nerve activity and the activation of nicotinic receptors eliminates the stress-induced increase in adrenal NPY mRNA (Hiremagalur et al., 1994; Jahng et al., 1997). However the adrenal medulla contains mRNA encoding the Y1, Y2 and Y5 neuropeptide Y receptors (Cavadas et al., 2006). Because chromaffin cells can co-release the catecholamines and NPY (Cavadas et al., 2001; Whim, 2006) one possibility is that NPY could locally regulate its own expression - this would also have been prevented by splanchnic nerve blockade. To test the involvement of Y receptors we individually blocked the Y1, Y2 and Y5 receptors by i.p. injection of antagonists before exposure to the forced swim test. These experiments showed that BIIE 0246 (1 mg/ kg), a Y2 antagonist (Doods et al., 1999), led to a significant increase in the basal levels of NPY-ir (Figure 4A; 277.2 ± 25.9 vs 429.0 ± 50.2 arbitrary intensity units; control vs BIIE0246 injection; mean ± SEM, n = 3, P < 0.05). In the BIIE 0246-injected animals, stress did not lead to an additional elevation in NPY-ir perhaps because of the effect of BIIE0246 on basal NPY expression (Figure 4B; 491.5 ± 64.7 vs 484.6 ± 63.8 arbitrary intensity units; FST vs FST after BIIE0246 injection; mean ± SEM, n = 3, not significant).
Figure 4. Y2 receptors negatively regulate NPY expression in the adrenal medulla.
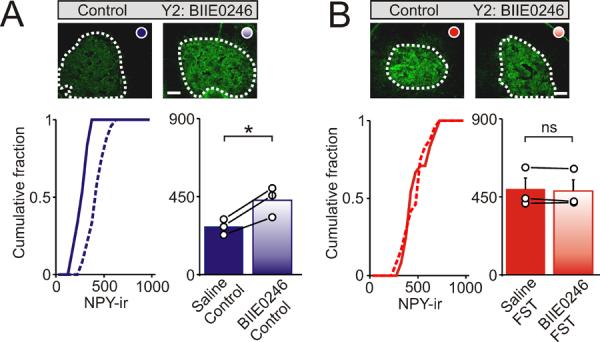
Examples of NPY-ir in sections of adrenal medulla from control mice and after i.p. injection with BIE0246 (a Y2 antagonist). (A). Under basal, non-stressed conditions BIIE0246 led to a significant increase in NPY-ir. (B). In contrast BIIE0246 did not alter the levels of NPY-ir following exposure to the cold FST. Cumulative distributions in each panel shows NPY-ir from all cryosections (24 sections, bin width 50 intensity units). Right histograms indicate the mean ± SEM (n = 3 independent experiments; 8 cryosections per animal). Open circles indicate the average value from each animal. Black lines link the matched control and stressed animals from each independent experiment. Scale bars 100 μm. *P < 0.05; ns = not significant.
In contrast although germline Y1 knockout mice have elevated levels of NPY (Cavadas et al., 2006) we did not detect an effect of acute Y1 receptor blockade on NPY-ir. Thus injection of BIBP3226 (1 mg / kg), a Y1 antagonist (Doods et al., 1996) did not alter either the basal expression of NPY (Figure 5A) or prevent the potentiating effects of acute stress (Figure 5B). Because we have found that this concentration of BIBP3226 leads to a significant increase in the adrenal expression of tyrosine hydroxylase (Wang et al, submitted) and is sufficient to block an NPY-induced increase in blood pressure in rats (Doods et al., 1996), the lack of effect on NPY expression is unlikely to be due to an ineffective concentration of antagonist. In similar experiments L-152,804 (10 mg / kg), a Y5 antagonist (Kanatani et al., 2000; Schroeder et al., 2005) also did not alter either the basal expression of NPY (Figure 5C) or prevent the potentiating effects of acute stress (Figure 5D). Mice with a germline deletion of the Y5 receptor also show no change in the whole brain levels of NPY mRNA (Marsh et al., 1998). From these experiments we conclude that rather than being responsible for the effects of stress, NPY actually negatively regulates its own expression via the Y2 receptor.
Discussion
A large and rapid increase in catecholamine secretion from sympathetic neurons is a canonical feature of the stress response. To compensate for this change in release, exposure to a stressor is usually followed by a sustained elevation in the expression of tyrosine hydroxylase, the rate limiting enzyme for catecholamine synthesis. When the stress-induced change in tyrosine hydroxylase expression is lost, the ability to adequately respond to a stressor is also altered, consistent with a functional role for stimulus-secretion-synthesis coupling (Hamelink et al., 2002). However while the cellular and molecular mechanisms that regulate the initiation of the sympathoadrenal stress response are well known (for review see Kvetnansky et al., 2009), the factors that limit its extent are less clear. Here we examined the effect of stress on the adrenal expression of neuropeptide Y which is co-released with the catecholamines and negatively regulates tyrosine hydroxylase expression (Cavadas et al., 2006).
Using a variety of approaches we find that an acute stressor that lasts only 5 minutes leads to a large increase in adrenal NPY expression. Thus both brief and extended exposure to stress can regulate the adrenal expression of NPY (Hiremagalur et al., 1994; Jahng et al., 1997; Han et al., 2005). Quantitative analysis of NPY expression using immunocytochemistry or transgenic expression of NPY(GFP) indicates that there are two populations of chromaffin cells that differ in their relative expression of NPY and that acute stress shifts the balance between the two. Unexpectedly these two populations do not correspond to epinephrine- and norepinephrine-containing chromaffin cells as assessed by PNMT-ir. Thus our findings differ from previous work in which NPY was localized either to epinephrine (Lundberg et al., 1986; Henion and Landis, 1990) or norepinephrine chromaffin cells (Varndell et al., 1984). It is now appreciated that epinephrine- and norepinephrine-synthesizing chromaffin cells are functionally distinct. Thus hypoglycemia leads preferentially to epinephrine secretion while a fall in blood pressure activates the norepinephrine-synthesizing chromaffin cells (Scheurink and Ritter, 1993; Morrison and Cao, 2000; Verberne and Sartor, 2010). The fact that acute stress does not lead to a uniform increase in NPY expression is consistent with the adrenal containing a heterogeneous mixture of chromaffin cells.
What is the functional significance of the rise in NPY expression? Because NPY inhibits the adrenal expression of tyrosine hydroxylase (Cavadas et al., 2006) it appears that NPY is an endogenous regulator of the stress response. By limiting tyrosine hydroxylase expression and thus the adrenal secretory capacity, NPY may act as a brake thus ensuring that the potentiating effect of stress on catecholamine secretion and synthetic capacity does not lead to a pathologically overactive stress response. Although Y receptors are widely distributed throughout the peripheral nervous system the NPY that is released from the adrenal medulla is thought to act locally because the plasma levels of NPY show little change following adrenalectomy (Castagne et al., 1987; Morris et al., 1987; Bernet et al., 1998) but see (Evequoz et al., 1995). Consistent with a restricted site of action, functional NPY receptors are present on chromaffin cells (Zheng et al., 1997; Zhang et al., 2000; Cavadas et al., 2001; Rosmaninho-Salgado et al., 2007). Stimulation of these receptors has been variously reported to increase (Cavadas et al., 2001; Rosmaninho-Salgado et al., 2007) or decrease catecholamine release (Higuchi et al., 1988; Hexum et al., 1994) suggesting that NPY could also have a short-term feed-forward role in the stress response. Because the forced swim test leads to activation of chromaffin cells as monitored by changes in c-fos-ir (Wang et al, submitted) it seems likely that adrenal NPY contributes to the modulation we have described. However we do not eliminate the possibility that other neuronal sources of NPY are also involved.
Using the in vivo injection of antagonists we find that Y2 receptors selectively regulate the adrenal expression of NPY. Thus injection of BIIE0246 increased the basal levels of NPY-ir. Y2 receptors are often found presynaptically (Qian et al., 1997; Sun et al., 2001; Fu et al., 2004; Stanic et al., 2006) but because a Y2-dependent increase in intracellular calcium has been reported for human chromaffin cells in vitro (Cavadas et al., 2001), it is possible that these receptors are also located post-synaptically in the adrenal gland. Nevertheless our results do not exclude the possibility that the relevant receptors are located outside the adrenal. This sub-type specificity is curious because the adrenal medulla contains at least 3 classes of Y receptors and these are generally thought to couple to the same downstream signaling pathways (see Michel et al., 1998). In conditional, but not germline, Y2 knockout mice there is an increase in the level of NPY mRNA (Naveilhan et al., 1999; Sainsbury et al., 2002) which is consistent with a negative feedback mechanism controlling NPY expression. Of particular interest is a study in which Y2 receptors were selectively deleted from NPY-expressing hypothalamic neurons. In these animals Y2 knockout led to an increase in the level of NPY mRNA in the arcuate nucleus (Shi et al., 2010). Our finding that acute inhibition of Y2 receptors leads to an elevation in adrenal NPY-ir suggests that a Y2-mediated autocrine regulation of NPY expression may be a widespread mechanism that modulates the synthesis of this peptide.
In conclusion we find that a feedback loop alters the level of NPY expression in the adrenal gland under both basal and stress-induced conditions. This appears to be an efficient system that ensures the levels of this peptidergic modulator are maintained even in the face of sustained sympathetic activity.
Acknowledgements
we thank Dr. June Liu for critically reading the manuscript and Dr. Ya-Ping Tang for the use of equipment. MDW current address; Department of Cell Biology and Anatomy, LSU Health Sciences Center, 1901 Perdido Street, New Orleans, LA 70112. QW current address; Division of Hypothalamic Research, University of Texas Southwestern Medical Center, Dallas, TX 75390-9077. This work was supported by a grant from the National Institutes of Health (RO1DK080441) to MDW.
Footnotes
The authors have no conflicts of interests to declare.
References
- Aguilera G, Kiss A, Sunar-Akbasak B. Hyperreninemic hypoaldosteronism after chronic stress in the rat. J Clin Invest. 1995;96:1512–1519. doi: 10.1172/JCI118189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet F, Dedieu JF, Laborie C, Montel V, Dupouy JP. Circulating neuropeptide Y (NPY) and catecholamines in rat under resting and stress conditions. Arguments for extra-adrenal origin of NPY, adrenal and extra-adrenal sources of catecholamines. Neurosci Lett. 1998;250:45–48. doi: 10.1016/s0304-3940(98)00454-6. [DOI] [PubMed] [Google Scholar]
- Bloom SR, Edwards AV, Jones CT. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Corder R, Gaillard R, Mormede P. Stress-induced changes of circulating neuropeptide Y in the rat: comparison with catecholamines. Regul Pept. 1987;19:55–63. doi: 10.1016/0167-0115(87)90074-7. [DOI] [PubMed] [Google Scholar]
- Cavadas C, Silva AP, Mosimann F, Cotrim MD, Ribeiro CA, Brunner HR, et al. NPY regulates catecholamine secretion from human adrenal chromaffin cells. J Clin Endocrinol Metab. 2001;86:5956–5963. doi: 10.1210/jcem.86.12.8091. [DOI] [PubMed] [Google Scholar]
- Cavadas C, Cefai D, Rosmaninho-Salgado J, Vieira-Coelho MA, Moura E, Busso N, et al. Deletion of the neuropeptide Y (NPY) Y1 receptor gene reveals a regulatory role of NPY on catecholamine synthesis and secretion. Proc Natl Acad Sci USA. 2006;103:10497–10502. doi: 10.1073/pnas.0600913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Costa E. Biosynthesis of tyrosine hydroxylase in rat adrenal medulla after exposure to cold. Proc Natl Acad Sci USA. 1974;71:4570–4574. doi: 10.1073/pnas.71.11.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, et al. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- Doods HN, Wieland HA, Engel W, Eberlein W, Willim KD, Entzeroth M, et al. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul Pept. 1996;65:71–77. doi: 10.1016/0167-0115(96)00074-2. [DOI] [PubMed] [Google Scholar]
- Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the Crerecombinase gene at the phenylethanolamine n-methyltransferase locus: a new model for studying the developmental distribution of adrenergic cells. Dev Dyn. 2004;231:849–858. doi: 10.1002/dvdy.20188. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Giraud P, Dave JR, Hotchkiss AJ, Affolter HU. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984;312:661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Engeland WC, Levay-Young BK, Rogers LM, Fitzgerald D. Differential gene expression of cytochrome P450 11beta-hydroxylase in rat adrenal cortex after in vivo activation. Endocrinology. 1997;138:2338–2346. doi: 10.1210/endo.138.6.5157. [DOI] [PubMed] [Google Scholar]
- Evequoz D, Grouzmann E, Nussberger J, Niederberger M, Brunner HR, Waeber B. Beta-adrenoceptor stimulation increases neuropeptide Y release from sympathetic nerves in intact rats. Regul Pept. 1995;58:163–166. doi: 10.1016/0167-0115(95)00065-j. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Fluharty SJ, Snyder GL, Stricker EM, Zigmond MJ. Short- and long-term changes in adrenal tyrosine hydroxylase activity during insulin-induced hypoglycemia and cold stress. Brain Res. 1983;267:384–387. doi: 10.1016/0006-8993(83)90895-8. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Chen X, Yang CL, Vickery L, Wu Y, Naes L, et al. Influence of cold stress on neuropeptide Y and sympathetic neurotransmission. Peptides. 2005;26:2603–2609. doi: 10.1016/j.peptides.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Henion PD, Landis SC. Asynchronous appearance and topographic segregation of neuropeptide-containing cells in the developing rat adrenal medulla. J Neurosci. 1990;10:2886–2896. doi: 10.1523/JNEUROSCI.10-09-02886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexum TD, Zheng J, Zhu J. Neuropeptide Y inhibition of nicotinic receptor-mediated chromaffin cell secretion. J Pharmacol Exp Ther. 1994;271:61–66. [PubMed] [Google Scholar]
- Higuchi H, Costa E, Yang HY. Neuropeptide Y inhibits the nicotine-mediated release of catecholamines from bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1988;244:468–474. [PubMed] [Google Scholar]
- Hiremagalur B, Kvetnansky R, Nankova B, Fleischer J, Geertman R, Fukuhara K, et al. Stress elicits trans-synaptic activation of adrenal neuropeptide Y gene expression. Brain Res Mol Brain Res. 1994;27:138–144. doi: 10.1016/0169-328x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Joh TH, Wessel TC. Expression of catecholamine-synthesizing enzymes, peptidylglycine alpha-amidating monooxygenase, and neuropeptide Y mRNA in the rat adrenal medulla after acute systemic nicotine. J Mol Neurosci. 1997;8:45–52. doi: 10.1007/BF02736862. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Ishihara A, Iwaasa H, Nakamura K, Okamoto O, Hidaka M, et al. L-152,804: orally active and selective neuropeptide Y Y5 receptor antagonist. Biochem Biophys Res Commun. 2000;272:169–173. doi: 10.1006/bbrc.2000.2696. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat Neurosci. 2010;13:1257–1264. doi: 10.1038/nn.2629. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly MP, Engeland WC, Gann DS. Adrenal medullary responses to repeated hemorrhage in conscious dogs. Am J Physiol. 1986;251:R1193–1199. doi: 10.1152/ajpregu.1986.251.6.R1193. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T, Hemsen A, Theodorsson-Norheim E, Pernow J, Hamberger B, et al. Neuropeptide Y-like immunoreactivity in adrenaline cells of adrenal medulla and in tumors and plasma of pheochromocytoma patients. Regul Pept. 1986;13:169–182. doi: 10.1016/0167-0115(86)90224-7. [DOI] [PubMed] [Google Scholar]
- Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, et al. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem. 2003;278:32058–32067. doi: 10.1074/jbc.M305545200. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59:2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Mitchell GC, Wang Q, Ramamoorthy P, Whim MD. A common single nucleotide polymorphism alters the synthesis and secretion of neuropeptide Y. J Neurosci. 2008;28:14428–14434. doi: 10.1523/JNEUROSCI.0343-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Kapoor V, Chalmers J. Plasma neuropeptide Y concentration is increased after hemorrhage in conscious rats: relative contributions of sympathetic nerves and the adrenal medulla. J Cardiovasc Pharmacol. 1987;9:541–545. doi: 10.1097/00005344-198705000-00006. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1763–1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- Nankova B, Kvetnansky R, McMahon A, Viskupic E, Hiremagalur B, Frankle G, et al. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci U S A. 1994;91:5937–5941. doi: 10.1073/pnas.91.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, et al. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- Petrovic J, Walsh PL, Thornley KT, Miller CE, Wightman RM. Real-time monitoring of chemical transmission in slices of the murine adrenal gland. Endocrinology. 2010;151:1773–1783. doi: 10.1210/en.2009-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Colmers WF, Saggau P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci. 1997;17:8169–8177. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman G, Kalari A, Dhingra R, Prabhakar NR, Kumar GK. Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing. Antioxid Redox Signal. 2011;14:1179–1190. doi: 10.1089/ars.2010.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy P, Whim MD. Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. J Neurosci. 2008;28:13815–13827. doi: 10.1523/JNEUROSCI.5361-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes. 2011;60:602–606. doi: 10.2337/db10-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J, Araujo IM, Alvaro AR, Duarte EP, Cavadas C. Intracellular signaling mechanisms mediating catecholamine release upon activation of NPY Y1 receptors in mouse chromaffin cells. J Neurochem. 2007;103:896–903. doi: 10.1111/j.1471-4159.2007.04899.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, et al. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci U S A. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurink A, Ritter S. Sympathoadrenal responses to glucoprivation and lipoprivation in rats. Physiol Behav. 1993;53:995–1000. doi: 10.1016/0031-9384(93)90279-o. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The neuropeptide-Y Y5 receptor antagonist L-152,804 decreases alcohol self-administration in inbred alcohol-preferring (iP) rats. Alcohol. 2005;36:179–186. doi: 10.1016/j.alcohol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Shi YC, Lin S, Wong IP, Baldock PA, Aljanova A, Enriquez RF, et al. NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS One. 2010;5:e11361. doi: 10.1371/journal.pone.0011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hokfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Akk G, Huguenard JR, Prince DA. Differential regulation of GABA release and neuronal excitability mediated by neuropeptide Y1 and Y2 receptors in rat thalamic neurons. J Physiol. 2001;531:81–94. doi: 10.1111/j.1469-7793.2001.0081j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970;228:861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Mueller RA, Axelrod J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1969a;169:249–254. [PubMed] [Google Scholar]
- Thoenen H, Mueller RA, Axelrod J. Increased tyrosine hydroxylase activity after drug-induced alteration of sympathetic transmission. Nature. 1969b;221:1264. doi: 10.1038/2211264a0. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varndell IM, Polak JM, Allen JM, Terenghi G, Bloom SR. Neuropeptide tyrosine (NPY) immunoreactivity in norepinephrine-containing cells and nerves of the mammalian adrenal gland. Endocrinology. 1984;114:1460–1462. doi: 10.1210/endo-114-4-1460. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Sartor DM. Rostroventrolateral medullary neurons modulate glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2010;299:E802–807. doi: 10.1152/ajpendo.00466.2010. [DOI] [PubMed] [Google Scholar]
- Vietor I, Rusnak M, Viskupic E, Blazicek P, Sabban EL, Kvetnansky R. Glucoprivation by insulin leads to trans-synaptic increase in rat adrenal tyrosine hydroxylase mRNA levels. Eur J Pharmacol. 1996;313:119–127. doi: 10.1016/0014-2999(96)00508-0. [DOI] [PubMed] [Google Scholar]
- Whim MD. Near simultaneous release of classical and peptide cotransmitters from chromaffin cells. J Neurosci. 2006;26:6637–6642. doi: 10.1523/JNEUROSCI.5100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zheng J, Vorce RL, Hexum TD. Identification of an NPY-Y1 receptor subtype in bovine chromaffin cells. Regul Pept. 2000;87:9–13. doi: 10.1016/s0167-0115(99)00093-2. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhang P, Hexum TD. Neuropeptide Y inhibits chromaffin cell nicotinic receptor-stimulated tyrosine hydroxylase activity through a receptor-linked G protein-mediated process. Mol Pharmacol. 1997;52:1027–1033. doi: 10.1124/mol.52.6.1027. [DOI] [PubMed] [Google Scholar]