Abstract
Many pro-inflammatory molecules, such as interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha (TNF-alpha) are somnogenic, while many anti-inflammatory molecules inhibit sleep. Sleep loss increases the production/release of these sleep regulatory pro-inflammatory molecules. Further, sleep changes occurring during various pathologies are mediated by these inflammatory substances in response to pathogen recognition and subsequent inflammatory cellular pathways. This review summarizes information and concepts regarding inflammatory mechanisms of the innate immune system that mediate sleep. Further, we discuss sleep-immune interactions in regards to sleep in general, pathologies, and sleep as a local phenomenon including the central role that extracellular ATP plays in the initiation of sleep.
Keywords: Sleep, Innate Immunity, Inflammation, Cytokines, ATP, glia, Review
2. INTRODUCTION
Sleep is vital to many biological activities including host responses to pathogens (1). Inflammation is an immunological process triggered by a variety of self or non-self stimuli, including pathogens, irritants, cellular damage or dysregulation, local brain use, and waking activity (2). Sleep and the immune system are highly conserved between animals from flies to humans. In fact, homologous genes for many sleep and immune-associated genes are found in mice and even invertebrates such as D. Melanogaster and C. Elegans (3). Inflammation modulates sleep and the effects of sleep loss and conversely sleep or sleep loss alters inflammatory responses including the production of inflammation regulatory substances activated by neuronal/glial use and immunologic pathogen recognition (1).
3. INFLAMMATION
Inflammatory mechanisms operate over various time scales from seconds to days. These mechanisms alter many physiological and pathological functions including blood flow, cellular mediators, cell migration, and sleep (2). Primary sleep disorders, such as insomnia and obstructive sleep apnea, are associated with dysregulated inflammatory mechanisms (4). Persistent inflammation is also associated with many diseases, such as Type 2 diabetes (5), cardiovascular disease (6), colorectal cancer (7), inflammatory bowel disease (8), and asthma (9). Interestingly, chronic short sleep duration is also associated with inflammatory diseases including cardiovascular disease (10), Type 2 diabetes, and cancer (11). Moreover, pathogens including influenza, bacteria, and human immunodeficiency virus (HIV) alter sleep, in part, through inflammatory mechanisms (12).
In 1909, Kuniomi Ishimori published his findings that cerebral spinal fluid transferred from sleep deprived dogs into control dogs increased sleep in the recipients (13). This finding was confirmed independently by Henri Pieron soon after (14). These findings suggested that sleep regulatory substances are produced as a consequence of waking activity. Subsequently, most characterized sleep regulatory substances have been shown to directly or indirectly modulate inflammation (1). There are criteria that a molecule must meet to be considered a sleep regulatory substance (Table 1). Many molecules and their associated receptors, metabolites, micro RNAs, transcription factors, etc, have the capacity to alter sleep. Yet, only a few sleep regulatory substances have met all the criteria. They include interleukin-1beta (IL-1 beta), tumor necrosis-alpha (TNF-alpha), and growth hormone-releasing hormone (GHRH) for non-rapid eye movement (NREM) sleep, and prolactin and nitric oxide (NO) for rapid-eye movement (REM).
Table 1.
Criterion for sleep regulatory substance.
| Criterion For Sleep Regulatory Substance |
|---|
| In the brain, these sleep regulatory substances proclivity. and/or their receptors oscillate with sleep/wake In the brain, these sleep regulatory substances |
| Sleep increases or decreases when sleep regulatory substances are injected locally into the central nervous system or systemically. |
| The inhibition or removal of sleep regulatory substances changes sleep. |
| Sleep is altered by sleep regulatory substances in response to pathogens. |
4. GLIA
There is much evidence that glia play a significant role in sleep. Glia comprise approximately 90% of brain cells. Glia produce many humoral regulators of inflammation and sleep. Glia interaction with neurons is quite extraordinary, as they serve to clear neurotransmitters from synaptic clefts with membrane transporters, release many molecules such as cytokines, adenosine triphosphate (ATP), and adenosine that modulate inflammation and neuronal function, release neurotransmitters, and alter the local ionic environment (15). Glia including microglia, astrocytes, oligodendrocytes, and Schwann cells contain many of the same ligand-gated ion channels as neurons including GABA and glutamate receptors. Under cell culture conditions certain glia, such as astrocytes and oligodendrocytes, produce action potentials mediated by voltage-gated outward potassium currents (16, 17). However, it is debatable if this occurs in vivo. Thus, glia, through autocrine and paracrine mechanisms modulate inflammation, and as a consequence of their electrical actions likely are a key component in the genesis of sleep and the electroencephalogram.
5. CYTOKINES
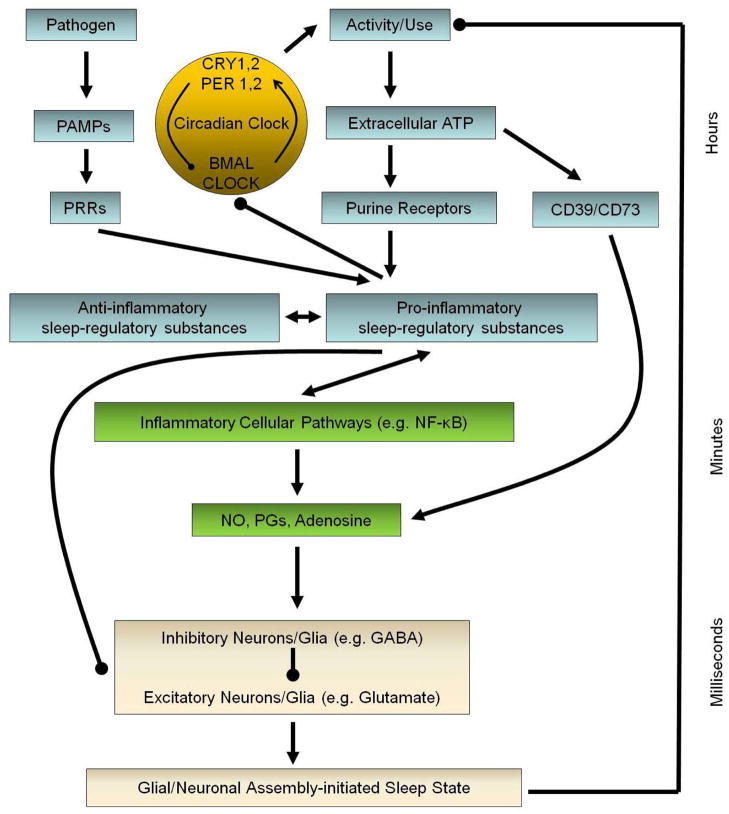
Protein, peptide, or glycoprotein molecules that signal inflammatory pathways through autocrine, paracrine, and/or endocrine mechanisms are termed cytokines (2). Neurons, glia, and a wide diversity of other cell types produce cytokines. Cytokines induce their effect at very low levels, such as pico or femtomolar concentrations. They induce pro- or anti-inflammatory responses. Cytokines, such as IL-1 beta or TNF-alpha, also modulate memory (18), performance, appetite (19) cognition (20), pain (21), fatigue (22), sleepiness (1), and sleep. IL-1 beta, TNF-alpha, and interleukin-6 (IL-6) are well-characterized pro-inflammatory somnogenic cytokines (1). However, many other cytokines are somnogenic or anti-somnogenic. Further, cytokines, such as IL-1 beta and TNF-alpha, alter expression of circadian genes including altering E-box regulatory elements activation through CLOCK-BMAL (23, 24). Cytokines are integral to sleep homeostat regulation (Figure 1).
Figure 1.
Sleep homeostat.
5.1. Interluekin-1
In 1984, IL-1beta was the first cytokine implicated in sleep regulation (25). IL-1beta is one of 11 family members with respective receptors termed the interleukin-1 family (IL1F) (26). The pro-inflammatory IL1F members, IL-1 beta, interleukin-1alpha (IL-1alpha), and interleukin-18 (IL-18) promote NREM sleep, while the anti-inflammatory IL1F member interleukin-1 receptor antagonist (IL-1RA) reduces NREM sleep (1). IL-1beta was first identified as an endogenous pyrogen (27), although it also functions to regulate immunity, inflammation, hyperalgesia, hematopoiesis, and central nervous system (CNS) processes such as sleep (26). IL-1beta and its receptors (interleukin-1 Type 1 and interleukin-1 Type 2) are propagated by a variety of cells within the CNS including neurons, astrocytes, macrophages, and microglia. The interleukin-1 Type 1 receptor is linked to pro-inflammatory actions. The interleukin-1 Type 2 receptor because it lacks an intracellular signaling domain competitively inhibits IL-1beta binding to the interleukin Type 1 receptor, thus inhibiting inflammation. Further, the IL-1RA competitively inhibits IL-1beta from binding to interleukin-1 Type 1 and Type 2 receptors resulting in anti-inflammatory reactions. Upon receptor activation, IL-1beta triggers many inflammatory cellular pathways including nuclear factor-kappa B (NF-kappaB), cyclooxygenase (COX), prostaglandin E2 (PGE2), mitogen-activated pathogen kinase (MAPK), and inducible nitric oxide synthase (iNOS).
Circadian fluctuations in IL-1beta and IL-18 expression occur in various brain areas coinciding with the sleep/wake cycle and sleep propensity (1). Systemic or central administration of IL-1beta enhances NREM sleep for hours. The IL-1RA inhibits IL-1beta induced NREM sleep in rats and rabbits when applied intraperitoneally or intraventricularly. IL-18 also enhances NREM sleep duration following systemic administration. Somnogenic doses of IL-1beta and IL-18 administration can attenuate REM sleep. Lower somnogenic doses of IL-1beta promote NREM sleep duration without affecting REM sleep. In contrast, high doses of IL-1beta inhibit both NREM and REM sleep. The IL-1RA does not alter REM sleep.
Increased EEG delta power occurring during NREM sleep is a classic measure of sleep intensity; e.g. EEG delta power during NREM sleep is high at the threshold of sleep induction (1). Both systemic and central IL-1beta administration enhances NREM sleep EEG delta power. Inhibiting IL-1beta with antibodies or pharmaceuticals reduces spontaneous NREM sleep and EEG delta power. Moreover, the enhanced NREM sleep and EEG delta power following sleep deprivation are attenuated with IL-1beta antibodies. Compared to control mice, mice lacking the interleukin Type 1 receptors have reduced NREM sleep and REM sleep during the active dark cycle but have normal sleep durations during most of the light cycle. Further, IL-1beta levels in response to certain pathologies, such as influenza, are associated with increases in NREM sleep duration and EEG delta power (12). IL-1beta effects on sleep duration and EEG delta power are found in multiple species suggesting an ancient link between inflammation and sleep regulation.
5.2. Tumor Necrosis Factor
The somnogenic actions of TNF-alpha were first published in 1987 (28). The TNF family is quite large with more than 40 ligand-receptor pairs (29). However, TNF-alpha is the most stringently investigated member of this family. TNF-alpha was discovered as an endotoxin-induced serum factor causing the necrosis of tumors and later as an endogenous pyrogen. Thereafter, TNF-alpha was shown to mediate inflammation, apoptosis, septic shock, viral replication, neuroprotection/neurotoxicisty, appetite, and sleep. TNF-alpha and its receptor tumor necrosis factor receptor 1 (TNFR1: 55 kD receptor) are produced by various CNS cell types including neurons, glia, and macrophages, while the other receptor for TNF-alpha, tumor necrosis factor receptor 2 (TNFR2), is found primarily on immunocytes. TNF-alpha is involved in activating many inflammatory cellular pathways, including NF-kappaB.
Wakefulness enhances TNF-alpha protein levels and expression in the brain (1). Thus, following sleep deprivation TNF-alpha expression is elevated. Further, the highest brain levels in rats occur at the onset of light, a period of maximal sleep propensity. Systemic or central administration of TNF- alpha enhances NREM sleep duration and EEG delta power during NREM sleep. This effect occurs in all species that have been investigated. REM sleep is suppressed following TNF-alpha administration but only at high doses. Further, NREM sleep duration in response to muramyl dipeptide (which increases NREM sleep duration) is inhibited when rabbits are given a TNF-alpha inhibitor (a biologically active fragment of the soluble tumor necrosis factor 55 kDa receptor) (12). Mice lacking the TNFR1 have lower spontaneous NREM and REM sleep durations during the dark to light transition period compared to mice with intact receptors (1). Moreover, mice lacking the TNFR1 do not exhibit enhanced NREM sleep after TNF-alpha administration, although they remain responsive to IL-1beta. Mice that lack TNFR1 and TNFR2 have attenuated NREM sleep in the active dark cycle and more REM sleep in the inactive light cycle (30). Pathogen-induced NREM sleep, such as with bacteria or influenza virus, is associated with increased TNF-alpha production (12).
A large literature indicates that circulating levels of TNF-alpha mediate sleep and sleepiness. Circulating TNF-alpha levels increase with sleep propensity (31). In humans, TNF-alpha levels are also increased in certain medical conditions associated with altered sleep including sleep apnea (32), fibromyalgia (33), insomnia (34), and obesity (35). Moreover, elevated circulating levels of TNF-alpha also occur with certain pathologies including influenza virus and trypanosome infections (36, 37). In rodents, elevated plasma TNF-alpha levels are associated with enhanced sleep following sleep deprivation (38). While the blood-brain barrier prevents the passage of many large molecules from the circulation to the brain, there are regions where molecules including TNF-alpha are transported across the barrier (39). Therefore, circulating pro-inflammatory cytokines, such as TNF-alpha, may be able to modulate brain inflammation and sleep by transversing this barrier. Systemic cytokines also signal the brain via vagal afferents (40, 41, 42).
6. INFLAMMATORY CELLULAR PATHWAYS
Sleep regulatory substances activate a number of inflammatory cellular pathways (1). These pathways in turn modulate sleep regulatory substances and sleep. In particular, IL-1beta and TNF-alpha are part of these inflammatory cellular pathways; they upregulate themselves as well as multiple other cytokines. NF-kappaB, NO, and COX inflammatory cellular pathways are implicated in sleep regulation; other cellular pathways exist that also alter sleep.
NF-kappaB is a transcription factor involved in cellular responses to cytokines, pathogens, neurotransmitters, and ATP (43). NF-kappaB is present in all nucleated cells including neurons and glia. Pro-inflammatory sleep regulatory substances, such as IL-1beta and TNF-alpha, are transcribed following NF-kappaB activation and nuclear localization (1). In contrast, anti-inflammatory anti-somnogenic sleep regulatory substances, such as IL-4 and IL-10, inhibit NF-kappaB activation. NF-kappaB exhibits a diurnal rhythm in the cortex and sleep deprivation activates cortical NF-kappaB (44). Further, inhibiting NF-kappaB activation reduces spontaneous NREM sleep. Nevertheless, mice lacking the NF-kappaB p50 subunit have enhanced spontaneous NREM and REM sleep (45). These mice respond to LPS with increased NREM and attenuated REM sleep. NF-kappaB activation is regulated by a number of molecules (i.e. p50, p65, relA, etc.) and it acts as an enhancer element for transcription of many pro-and anti-inflammatory molecules, which have the capacity to regulate sleep. Thus, NF-kappaB seems to be a key regulatory node in sleep regulation. However, the inflammatory-related interactions of this pathway with sleep have yet to be completely characterized.
Nitric oxide regulates physiological and immunological processes including vasodilatation, inflammation and sleep (46). Nitric oxide synthase (NOS) catalyzes the conversion of the nitrogen of arginine in the presence of nicotinamide adenine dinucleotide phosphate and dioxygen to NO. Many brain cells including macrophages, neutrophils, and microglia, produce inducible NOS (iNOS) and the brain specific neuronal NOS (nNOS). Brain iNOS has a circadian variation and increases with sleep propensity (47). In rats, iNOS increases during sleep deprivation. NREM and REM sleep are enhanced after administration of NO precursors, such as L-arginine, and other NO donors such as morpholinosydnonimine or SIN-1. Conversely, NREM and REM sleep are inhibited with iNOS inhibitors, such as N-nitro-L-arginine-methylester and 7-nitro-indazole. iNOS knockout mice have reduced NREM sleep and enhanced REM sleep compared to wild type mice. This change is similar to that observed in TNF double receptor knockout mice; TNF-alpha mediates many of its actions via iNOS. In addition, mice lacking iNOS or nNOS have reduced NREM sleep in response to influenza challenge compared to mice possessing those genes (48). The sleep regulatory effect of NO is complex because many sleep regulatory substances including IL-1 beta, TNF-alpha alter NO, iNOS, and nNOS production.
The enzyme COX converts arachidonic acid to prostaglandin H2; this is the rate limiting step in prostaglandin production (49). There are two separate COXs. COX-1 is constitutively expressed, while COX-2 is inducible. COX-2 modulates inflammation and sleep (1), and is found in most mammalian cells including macrophages, and microglia. Alternatively, inflammatory sleep regulatory substances including IL-1beta and TNF-alpha induce COX-2 expression (49). Inhibiting COX-2 attenuates spontaneous NREM and TNF-alpha-induced sleep (50, 51). Additionally, sleep is inhibited with the COX-1 and COX-2 inhibitor acetaminophen. Moreover, two downstream prostanoids, prostaglandin D2 and prostaglandin E2 are posited to be involved in sleep and wake regulation (52, 53), respectively.
7. PATHOGENS
Many pathogens, including bacteria influenza virus, and HIV are associated with altered sleep (12). The sleep regulatory substance Factor S isolated from human urine and rabbit brain was identified as muramyl peptide (54, 55). The structure of Factor S is similar to the monomeric muramyl peptides found in bacterial peptidoglycan. Subsequent research led to investigations of how pathogen-associated molecular patterns (PAMPs) and their respective pattern recognition receptors (PRRs) alter sleep (12). Intriguingly, pathogens altering sleep affect many of the same inflammatory cellular pathways, cytokines, and other sleep regulatory substances that waking activity does. It thus appears that the excessive sleep or sleepiness occurring with many infections is the consequence of amplification of physiological sleep regulator mechanisms.
The first description of the effects of a pathogen on sleep over the course of the induced acute phase response involved infecting rabbits with Staphylococcus aureus (56). Staphylococcus aureus infected rabbits exhibit enhanced NREM sleep and less REM sleep. Similar sleep patterns were found with other pathogens including Escherichia coli and Pasteurella multocida (12). However, microbial-mediated sleep effects vary depending upon the species, route of infectious agent entry, time of day of exposure, and prior sleep history. In addition, bacterial cell wall components, such as the endotoxin lipopolysaccharide—a component of the outer membrane of gram-negative bacteria, enhance NREM sleep when injected systemically or centrally. Finally, high doses of heat-killed bacteria also have the capacity to induce sleep and other facets of the acute phase response, thereby suggesting that bacterial replication per se is not required for bacterial PAMP recognition by PRRs. These effects, like muramyl peptides, are also mediated by cytokines. Sleep alterations induced by these pathogens on their components are quite profound, similar to those sleep responses induced by central or systemic application of IL-1beta or TNF-alpha. Indeed, the IL-1RA and anti-IL-18 attenuate the enhanced NREM sleep induced by muramyl dipeptides indicating that pro-inflammatory cytokines are responsible for bacterial pathogens alteration of sleep.
The host’s acute phase response to influenza viral infection involves many of the same responses induced by bacteria including increased sleep, fever (although mice exposed to influenza have reduced body temperature), and reduced activity (12). In humans, low titers of influenza only appear to alter sleep and not other acute phase responses. In mice and rabbits, the aforementioned acute phase response characteristics are observed with live but not killed influenza virus. However, the mechanisms for sleep-induced effects of influenza are not fully understood.
Influenza viral challenge is associated with IL-1beta, TNF-alpha, IL-6, and interferon production in the lung and brain and in mice sleep is enhanced for days during the infection (12). Mice lacking both TNF receptors have attenuated NREM sleep responses to influenza infection compared to mice that have intact receptors suggesting that TNF-signaling is required for the manifestation of the full acute phase response (30). Further, in response to influenza infection, mice lacking the iNOS gene (iNOS induction is downstream from TNF-alpha activation) have lower NREM sleep responses and greater REM sleep suppression compared to controls (48). Inflammatory sleep regulatory substances produced/regulated by glia mediate the acute phase sleep response to viral infection. For example, macrophages and microglia have the capacity to produce large amounts of IL-1beta and TNF-alpha in response to viral infection. Moreover, mice lacking macrophage inflammatory protein 1-alpha, which is involved with microglial regulation, do not have the typical dark phase enhanced sleep in response to influenza infection than wild types (57).
After intranasal influenza virus challenge, the virus is found in the olfactory bulb and is associated with increased pro-inflammatory cytokine production in the olfactory bulb (58). The virus is typically not detected in other brain regions such as the somatosensory cortex but has been reported in the hypothalamus (59). Nevertheless, in mice, the timing of the acute phase responses, including sleep alterations, correlates with virus localization to the lungs. Systemic inflammatory cytokines stimulate afferent vagal nerve inputs to promote sleep (40, 41, 42). Consequently, it is likely that the observed sleep effects following intranasal influenza inoculation involve inflammatory stimulation of vagal afferents.
One aspect of the immune response to viruses and its interaction with sleep involves antibody responses. For instance, in humans, acute sleep deprivation inhibits hepatitis A vaccine-generated antibodies (60). Further, a mild amount of sleep loss prior to immunization against influenza reduces immunoglobulin G antibodies to influenza for days, although this antibody titer difference disappears 3 weeks after immunization (61). Yet, in mice, mild amounts of sleep loss do not alter antibody development against influenza (62). Nonetheless, antibody protection and acute phase responses may interact to alter one another, although the mechanisms are mediated through different pathways. Thus, sleep alone is an insufficient measurement of influenza pathogenesis.
The effects of sleep loss on the host’s response to influenza remain to be clarified. Prior sleep loss before inoculation is described as being protective while sleep loss following inoculation being detrimental. (63, 64, 65). Sleep loss alters inflammatory substances and the magnitude and direction of the effects are likely dependent on the activation state of these substances. For instance, the length and type of sleep deprivation, and the time of day affect the ability of inflammatory substances to interact with the host’s response to the virus. In addition, the viral preparations used or the host’s immunological status are likely key elements. Sleep deprivation prior to infection may be protective as similarly found in mice with B16 melanoma or D. melanogaster with bacteria resistance (66, 67). Consequently, it is plausible that sleep deprivation under certain circumstances can prime the immune system by altering inflammatory agents or pathways to enhance the immune response to a pathogen.
Viruses, such as HIV, also alter sleep. Sleep complaints and excessive daytime sleepiness, insomnia, and night time awakenings are common in HIV infected individuals (68). Further, disturbed sleep is magnified in these individuals as the disease progresses. A reduction in EEG slow wave activity is often found in individuals with HIV (69). However, individuals with HIV that do not show other typical symptoms associated with AIDS have enhanced slow wave sleep before acquired immune deficiency syndrome develops (70, 71). HIV infects microglia, and individuals with HIV have impaired macrophage and microglial functioning (72). Thus, impairment in these particular cells is likely to alter sleep regulatory substances which further alter sleep. Indeed, HIV is associated with impaired TNF-alpha release (73). Consequently, it is likely that individual with HIV have impaired abilities to mount the necessary daily sleep regulatory cytokine responses via microglia leading to their sleep disturbances and reduced EEG slow wave activity.
7.1. Pattern Recognition Receptors
Pathogens, including bacteria, flagellum, viruses, and their components including muramyl dipeptides, LPS, RNA, and DNA are pathogen-associated molecular patterns (PAMPs) (74). PAMPs are highly conserved molecular components of pathogens. Pathogenic components are recognized by pattern recognition receptors (PRRs) on or within certain cells. PAMPs play a vital role to the identification and processing of pathogens. Enhanced NREM sleep following muramyl dipeptide, LPS, gram negative and gram positive bacteria, viral RNA, and many other pathogens occur through the activation of PAMP receptors (12). PAMP receptors on the cell surface, endosome or within the cytosol include toll-like receptors (TLRs), and nucleotide binding oligomerization domain-like receptors (74). Upon PRR activation inflammatory pathways, including NF-kappaB, COX and NOS, activate inflammatory molecules, sleep regulatory substances, and cytokines directly or indirectly. Indeed, increased brain IL-1beta and TNF-alpha occur after viral or bacterial infection; these changes are likely responsible for the sleep responses associated with PRR stimulation. TLRs also activate the inflammasome leading to the activation of IL-1beta and TNF-alpha (see below) (75).
As already mentioned bacterial cell wall products such as muramyl peptides or LPS are PAMPs, and they are recognized by PRRs such as TLR2 or TLR4 (74). In the case of influenza virus, double-stranded (ds) viral RNA is the PAMP. The TLR3 recognizes viral ds RNA. Mice lacking TLR3 infected with mouse adapted influenza virus have reduced NREM sleep, body weight, and hypothermic responses compared to mice that possess that gene (76). Thus, PRRs are vital to the pathogen-induced sleep response by modulating inflammatory sleep regulatory substances.
8. SLEEP IS A LOCAL INFLAMMATORY PHENOMENON
Sleep always occurs following experimental- or pathological-induced brain lesions (1). This suggests that no specific area of brain is necessary for sleep to occur and that sleep is a fundamental self-organizing process of neuronal/glial networks. Regardless, the sleep disturbances following lesions are likely due to the local destruction of neurons and glia and subsequent inflammation and dysregulation of a competent inflammatory response necessary for normal sleep.
Sleep is a neuronal/glial network use-dependent process (1, 77). Increased slow wave sleep occurs following cognitive tasks or afferent stimulation in humans and/or rodents. Further, prolonged wakefulness associated with higher levels of neuronal activity and subsequent slow wave sleep responses. Moreover, the stimulation of specific whiskers of rodents’ increases cytokine expression and the probability of a sleep-like state occurring in the corresponding cortical column that receives afferent input from the stimulated whisker (78).
Intriguingly, sleep is not only use-dependent but also occurs locally (1, 77). This is evident in animals including birds and marine mammals that have unilateral sleep. Further, the aforementioned whisker stimulation and reciprocating sleep are localized to the stimulated column(s) (78). Further, in rodents, the administration of TNF-alpha directly onto a cortical column induces a sleep-like state in that particular column (79). In addition, the application of TNF-alpha or IL-1beta onto the cortex unilaterally enhances the intensity of NREM sleep ipsilaterally, but not contralaterally, without altering REM sleep or wakefulness (80, 81). Collectively, these findings suggest that sleep occurs locally and is mediated by local inflammatory sleep regulatory substances. In fact, this observation is logical since sleep regulatory substances such as cytokines mostly act locally through paracrine and autocrine mechanisms. While sleep regulatory circuits are not the impetus for sleep, they likely mediate the coordination of local sleep for the emergence of whole animal sleep. However, the exact mechanisms governing the coordination of local sleep into whole animal sleep remains unclear.
9. EXTRACELLULAR ATP
Local sleep and inflammatory processes are mediated, in part, by extracellular ATP. ATP is found in glia and neuronal synaptic vesicles, with 10–50 times higher presence in the vesicles than in the cytosol (82). ATP is co-released into the extracellular space with neurotransmitters including glutamate and GABA.
Extracellular ATP is broken down to adenosine through enzymes within the brain (83). Ectonucleoside triphosphate diphosphohydrolase (i.e. CD39) converts ATP and adenosine diphosphate to 5′adenosine monophosphate (AMP). AMP is then converted to adenosine by the 70-kDa glycosylphosphatidylinositol anchored ectoenzyme, ecto-5′-nucleotidase (i.e. CD73). CD39 and CD73 are found in many types of CNS cells, including glia and neurons. Interestingly, extracellular adenosine acting via the purine type 1 receptor, adenosine A2A, is regarded as an anti-inflammatory molecule which may alter sleep-related inflammation (84). We recently found that mice lacking CD73 have increased spontaneous sleep (Zielinski et al., unpublished observations). Although the mechanisms governing this observation have not been determined, our finding implicates the somnogenic actions of ATP and the modulation of inflammation by CD73 enzymatic activity.
The energy depletion hypothesis of sleep suggests that neuronal activity depletes mitochondrial ATP to enhance sleep (85). However, oxidative phosphorylation increases following sleep deprivation suggesting that wakefulness increases neuronal/glial ATP production and extracellular ATP release rather than reducing it (86). Moreover, the most robust depletion of mitochondrial ATP via exercise has very modest effects on altering sleep (87). Consequently, an extracellular ATP hypothesis is more plausible (1). Extracellular ATP increases following sleep deprivation and local use, and it is recognized by purine type 2 receptors, including the P2X7 receptor (88). Purinergic receptors are found widely in glia. Extracellular ATP via purinergic receptor recognition on microglia affect many sleep regulatory substances including IL-1beta, TNF-alpha, BDNF, cyclicAMP, phospholipase C, arachidonic acid, and NO (15, 89). Indeed, we recently found that ATP agonists enhance affects sleep while ATP antagonists inhibit sleep, in part, through the P2X7 receptor.
An important mediator between extracellular ATP or pathogenic induction of cytokine activation and sleep is likely the inflammasome. Inflammasomes are present in brain cells including glia and neurons (75). Extracellular ATP, LPS, and bacteria induce activation of the inflammasome and subsequent cytokine production. Caspase-1 activity is regulated by the nacht domain-, leucine-rich repeat, and pyrind domains-containing protein 3 (NALP3) inflammasome. IL-1 beta and IL-18 are regulated by caspase-1, which cleaves their pro-forms (i.e. pro-IL-1beta and pro-IL-18) into their active forms. Indeed, evidence indicates that caspase-1 activation modulates sleep. For example, inhibiting caspase-1 reduces LPS-induced inflammation and enhanced sleep (90).
Extracellular ATP activation of purine receptors induces the release of intracellular potassium, an event that is dependent upon cell membrane pore formation (75). ATP-induced loss of intracellular potassium induces the activation of the NALP3 inflammasome. When potassium efflux is blocked by exogenous potassium, however, the ability of ATP to induce IL-1beta and IL-18 is also blocked. Membrane potassium trafficking is involved in sleep regulation. Knockout mice of Kv3.2 (a channel present in neurons and glia) have reduced EEG NREM delta power (91). The shaker and sleepless genes that regulate sleep duration are part of voltage-gated potassium ion channels (92, 93). Moreover, mice lacking Kcna2 (which codes for Kv1.2 the alpha subunit of shaker potassium channel) have less spontaneous NREM sleep than wild types, although these mice do not respond differently to 6 h of sleep deprivation (94). Thus, a plausible mechanism for the sleep regulatory actions of potassium is through the activation of the inflammasome.
10. CONCLUSION
Sleep modulates and is modulated by inflammation, and inflammation induced by pathogens alters sleep. The inflammatory process is complex and involves interactions between neurons and glia to regulate sleep. These actions are targeted to local areas. Many sleep regulatory substances and their respective receptors and cellular pathways mediate inflammation to alter sleep; thus, inflammation produced via local activity or pathogen recognition is the impetus for sleep.
Acknowledgments
This work was supported in part by the National Institutes of Health (USA), grant numbers (NS25378, NS31453, and HD36520).
References
- 1.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy KM, Travers P, Walport M. Immunobiology: The Immune System. 7. The Wellcom Trust; UK: 2009. Janeway’s Immunobiology. [Google Scholar]
- 3.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:R670–R679. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 6.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 8.Ye JH, Rajendran VM. Adenosine: an immune modulator of inflammatory bowel diseases. World J Gastroenterol. 2009;15:4491–4498. doi: 10.3748/wjg.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 10.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Verkasalo PK, Lillberg K, Stevens RG, Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595–9600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 12.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Ishimori K. True causes of sleep: A hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakki Zasshi. 1909;23:429–457. [Google Scholar]
- 14.Legendre R, Pieron H. Recherches sur le besoin de sommeil consécutive à une veille prolongée. Z allg Physiol. 1913;14:235–262. [Google Scholar]
- 15.Fields RD, Burnstock G. Purinergic signaling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields RD. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist. 2008;14:540–543. doi: 10.1177/1073858408320294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otis TS, Sofroniew MV. Glia get excited. Nat Neurosci. 2008;11:379–380. doi: 10.1038/nn0408-379. [DOI] [PubMed] [Google Scholar]
- 18.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 19.Kontruek SJ, Brzozowski T, Konturek PC, Schumbert ML, Pawlik WW, Padol S, Bayner J. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol. 2008;59:7–31. [PubMed] [Google Scholar]
- 20.Trompet S, de Craen AJ, Slagboom P, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Ford I, Gaw A, Macfarlane PW, Packard CJ, Stott DJ, Jukema JW, Westendorp RG PROSPER Group. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131:1069–1077. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- 21.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 22.Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis--a pilot study. Rheumatol Int. 2005;25:481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- 23.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Clock gene modulation by TNF-alpha depends on calcium and p38 MAP kinase signaling. PNAS. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrzilka S, Taraborrelli C, Cavadini G, Fontana A, Birchler T. Clock gene modulation by TNF-alpha depends on calcium and p38 MAP kinase signaling. J Biol Rhythms. 2009;24:283–294. doi: 10.1177/0748730409336579. [DOI] [PubMed] [Google Scholar]
- 25.Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am J Physiol. 1984;246:R994–999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 27.Atkins E, Wood WB., Jr Studies on the pathogenesis of fever. I. The presence of transferable pyrogen in the blood stream following the injection of typhoid vaccine. JEM. 1955;101:5519–5528. doi: 10.1084/jem.101.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal BB. Signaling pathways of the TNF superfamily: a double-edged sword. Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 30.Kapás L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. J Appl Physiol. 2008;105:1187–1198. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 32.Li NF, Yao XG, Zhu J, Yang J, Liu KJ, Wang YC, Wang XL, Zu FY. Higher levels of plasma TNF-alpha and neuropeptide Y in hypertensive patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2010;32:54–60. doi: 10.3109/10641960902993087. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Moser M, Schiltenwolf M, Buchner M. Circulating cytokine levels compared to pain in patients with fibromyalgia – a prospective longitudinal study over 6 months. J Rheumatol. 2008;35:1366–1370. [PubMed] [Google Scholar]
- 34.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 35.Panagiotakos DB, Pitsayos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser L, Fritz RS, Straus SE, Gubareya L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 37.Vincendeau P, Bouteille B. Immunology and immunopathology of African trypanosomiasis. An Acad Bras Cienc. 2006;78:645–665. doi: 10.1590/s0001-37652006000400004. [DOI] [PubMed] [Google Scholar]
- 38.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29:393–398. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, Moinuddin A, Morley JE. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging. 2001;22:671–676. doi: 10.1016/s0197-4580(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Fang J, Guan Z, Brown RA, Krueger JM. Vagotomy attenuates tumor necrosis factor-alpha-induced sleep and EEG delta-activity in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1213–1220. doi: 10.1152/ajpregu.2001.280.4.R1213. [DOI] [PubMed] [Google Scholar]
- 41.Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. Am J Physiol. 1997;273:R1246–1253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1beta. Physiol Behav. 1998;64:361–365. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 43.O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- 45.Jhaveri KA, Ramkumar V, Trammell RA, Toth LA. Spontaneous, homeostatic, and inflammation-induced sleep in NF-kappaB p50 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1516–1526. doi: 10.1152/ajpregu.00262.2006. [DOI] [PubMed] [Google Scholar]
- 46.Murphy S, Gibson CL. Nitric oxide, ischemia and brain inflammation. Biochem Soc Trans. 2007;35:1133–1137. doi: 10.1042/BST0351133. [DOI] [PubMed] [Google Scholar]
- 47.Gautier-Sauvigné S, Colas D, Parmantier P, Clement P, Gharib A, Sarda N, Cespuglio R. Nitric oxide and sleep. Sleep Med Rev. 2005;9:101–113. doi: 10.1016/j.smrv.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Duricka D, Nelson S, Mukherjee S, Bohnet SG, Taishi P, Majde JA, Krueger JM. Influenza virus-induced sleep responses in mice with targeted disruptions in neuronal or inducible nitric oxide synthases. J Appl Physiol. 2004;97:17–28. doi: 10.1152/japplphysiol.01355.2003. [DOI] [PubMed] [Google Scholar]
- 49.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terao A, Matsumura H, Yoneda H, Saito M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998;9:3791–3796. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 51.Terao A, Matsumura H, Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J Neurosci. 1998;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- 53.Masek K, Kadlecová O, Pöschlová N. Effect of intracisternal administration of prostaglandin E1 on waking and sleep in the rat. Neuropharmacology. 1976;15:491–494. doi: 10.1016/0028-3908(76)90060-5. [DOI] [PubMed] [Google Scholar]
- 54.Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 55.Krueger JM, Pappenheimer JR, Karnovsky ML. Sleep-promoting effects of muramyl peptides. Proc Natl Acad Sci U S A. 1982;79:6102–6106. doi: 10.1073/pnas.79.19.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun. 1988;56:1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toth LA, Hughes LF. Macrophage participation in influenza-induced sleep enhancement in C57BL/6J mice. Brain Behav Immun. 2004;18:375–389. doi: 10.1016/j.bbi.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Majde JA, Bohnet SG, Ellis GA, Chruchill L, Leyva-Grado V, Wu M, Szentirmai E, Rehman A, Krueger JM. Detection of mouse-adapted human influenza virus in the olfactory bulbs of mice within hours after intranasal infection. J Neurovirol. 2007;13:399–409. doi: 10.1080/13550280701427069. [DOI] [PubMed] [Google Scholar]
- 59.Alt JA, Bohnet S, Taishi P, Duricka D, Obal F, Jr, Traynor T, Majde JA, Krueger JM. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav Immun. 2007;21:60–67. doi: 10.1016/j.bbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis. A Psychosom Med. 2003;65:831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 61.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 62.Renegar KB, Floyd R, Krueger JM. Effect of sleep deprivation on serum influenza-specific IgG. Sleep. 1998;21:19–24. [PubMed] [Google Scholar]
- 63.Renegar KB, Floyd RA, Krueger JM. Effects of short-term sleep deprivation on murine immunity to influenza virus in young adult and senescent mice. Sleep. 1998;21:241–248. [PubMed] [Google Scholar]
- 64.Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 2001;23:859–863. [PubMed] [Google Scholar]
- 65.Toth LA, Rehg JE. Effects of sleep deprivation and other stressors on the immune and inflammatory responses of influenza-infected mice. Life Sci. 1998;63:701–709. doi: 10.1016/s0024-3205(98)00321-x. [DOI] [PubMed] [Google Scholar]
- 66.Zielinski MR, Davis JM, Wyatt WC, JR, Montagu DL, Youngstedt SD. Effects of chronic sleep restriction and exercise training on metastasis. Med Sci Sports Exerc. 2007;39:62. [Google Scholar]
- 67.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135:563–572. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 69.Darko DF, Mitler MM, White JL. Sleep disturbance in early HIV infection. Focus. 1995;10:5–6. [PubMed] [Google Scholar]
- 70.Norman SE, Chediak AD, Kiel M, Cohn MA. Sleep disturbances in HIV-infected homosexual men. AIDS. 1990;4:775–781. doi: 10.1097/00002030-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Norman SE, Chediak AD, Freeman C, Kiel M, Mendez A, Duncan R, Simoneau J, Nolan B. Sleep disturbances in men with asymptomatic human immunodeficiency (HIV) infection. Sleep. 1992;15:150–155. doi: 10.1093/sleep/15.2.150. [DOI] [PubMed] [Google Scholar]
- 72.Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–194. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 73.Herbein G, Khan KA. Is HIV infection a TNF receptor signaling-driven disease? Trends Immunol. 2008;29:61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 76.Majde JA, Kapás L, Bohnet SG, De A, Krueger JM. Attenuation of the influenza virus sickness behavior in mice deficient in Toll-like receptor 3. Brain Behav Immun. 2010;24:306–315. doi: 10.1016/j.bbi.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nature Reviews Neuroscience. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 82.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 83.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Hand Exp Pharmacol. 2009;193:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- 85.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 86.Nikonova EV, Naidoo N, Mackiewicz M, Zhang L, Romer M, Cater JR, Scharf MT, Galante RJ, Pack AI. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–65. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–72. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signaling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Imeri L, Bianchi S, Opp MR. Inhibition of caspase-1 in rat brain reduces spontaneous nonrapid eye movement sleep and nonrapid eye movement sleep enhancement induced by lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2006;291:R197–204. doi: 10.1152/ajpregu.00828.2005. [DOI] [PubMed] [Google Scholar]
- 91.Vyazovskiy VV, Deboer T, Rudy B, Lau D, Borbely AA, Tobler I. Sleep EEG in mice that are deficient in the potassium channel subunit K. v. 3. 2. Brain Res. 2002;947:204–211. doi: 10.1016/s0006-8993(02)02925-6. [DOI] [PubMed] [Google Scholar]
- 92.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 93.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;32:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in Kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]