Abstract
Vitamin D deficiency is a global health problem that has various adverse consequences. Vitamin D is mainly synthesized in the skin by sunlight (UV light) irradiation; therefore, the vitamin D status is influenced by geographic locations, seasonal changes and skin pigmentations. The kidney is involved in the biosynthesis of 1,25-dihydroxyvitamin D and the reuptake of filtered 25-hydroxyvitamin D from the proximal tubules, thus vitamin D-deficiency is highly prevalent in patients with kidney disease who suffer renal insufficiency. There is a growing body of epidemiological and clinical evidence in the literature that links vitamin D deficiency to cardiovascular disease. The discovery of the vitamin D hormone functioning as an endocrine inhibitor of the renin-angiotensin system provides an explanation for this association. This review will discuss the mechanism underlying the connection between vitamin D and cardiovascular disease and its physiological and therapeutic implications.
Introduction
Vitamin D deficiency is a global health problem that has various adverse consequences 1. Vitamin D is synthesized in the skin from 7-dehydrocholesterol, and this reaction requires sunlight or ultraviolet (UV) irradiation. Vitamin D is converted in the liver to 25-hydroxyvitamin D [25(OH)D], which is the main circulating vitamin D metabolite and commonly used as an indicator of vitamin D status. 1,25-dihydroxyvitamin D [1,25(OH)2D3], the hormonal form of vitamin D, is synthesized in the kidney. Therefore, vitamin D status is influenced by geographic locations, seasonal changes and skin pigmentations 1. In addition to 1,25(OH)2D3 biosynthesis, the kidney is also required for the reuptake of filtered 25(OH)D from the proximal tubules 2, thus vitamin D-deficiency is highly prevalent in patients with kidney disease who suffer renal insufficiency 3. There is a growing body of epidemiological and clinical evidence in the literature that links vitamin D deficiency to cardiovascular disease. For example, patients with chronic kidney disease have much higher cardiovascular disease mortality at advanced stage compared to the general population 4. The discovery of the vitamin D hormone functioning as an endocrine inhibitor of the renin-angiotensin system (RAS) provides an explanation for this association. Here I will discuss the potential mechanism underlying the connection between vitamin D and cardiovascular disease and its physiological and therapeutic implications, with a focus on the RAS.
Association of Vitamin D Deficiency with Cardiovascular Disease
UV irradiation is required for the cutaneous synthesis of vitamin D. UV irradiation decreases with the increase in latitude, and high latitudes are correlated with high prevalence of hypertension and stroke incidents 5, 6. Winter season, which has low UV irradiation, is associated with high incidence of myocardial infarction 7. Dark skin pigmentation in the black population, which blocks UV light penetration, is associated with higher blood pressure 8, 9. Data from the National Health and Nutrition Examination Surveys (NHANES) III showed an inverse relationship between serum 25(OH)D and blood pressure in the general population 10. The NHANES III database also revealed an inverse correlation between serum 25(OH)D levels and the prevalence of cardiovascular risk factors including hypertension, diabetes, obesity and hyperlipidemia 11. Low vitamin D status was thought to be a contributing factor for congestive heart failure 12. A recent meta-analysis of 18 published studies confirms the inverse relationship between blood 25(OH)D levels and hypertension 13.
Other epidemiological studies have confirmed the association between vitamin D-deficiency and increased risk of cardiovascular problems. Prospective studies with cohorts from the Health Professionals’ Follow-Up Study (HPFS) and the Nurses’ Health Study (NHS) showed that serum 25(OH)D levels are inversely associated with the risk of incident hypertension during 4 years of follow-up 14. A nested case-control prospective study using the HPFS database also demonstrated an association of low serum 25(OH)D levels with higher risk of myocardial infarction, even after adjusting for factors known to be associated with coronary artery disease 15. Low serum 25(OH)D is also associated with incident cardiovascular disease in Framingham Offspring Study participants without prior cardiovascular disease during a mean follow-up of 5.4 years, after adjustment for C-reactive protein, physical activity or vitamin use 16. Therefore, vitamin D-deficiency is a risk factor for cardiovascular disease.
The Renin-Angiotensin System as a Major Target of Vitamin D
The renin-angiotensin system (RAS) is a regulatory cascade that has profound impact on the cardiovascular system. Renin is the rate-limiting step of the RAS cascade that converts angiotensinogen (AGT) to angiotensin (Ang) I. Angiotensin-converting enzyme (ACE) then converts Ang I to Ang II, the biological effector of the RAS. Systemic Ang II is a central regulator of blood pressure through increasing vasoconstriction, extracellular volume and cardiac output, and over-activation of the RAS results can lead to hypertension 17, 18. In addition to blood pressure control, Ang II has diverse pathological activities that promote fibrogenesis, inflammation, cell hypertrophy and proliferation 19-21. Thus over-activation of the RAS is detrimental.
Recent studies have well established the RAS as a major target of vitamin D, which may in part serve the link between vitamin D-deficiency and cardiovascular disease. In fact, two early articles reported an inverse relationship between circulating 1,25(OH)2D3 levels and plasma renin activity in hypertensive subjects more than two decades ago 22, 23; however, the significance of these studies was hardly recognized until the discovery that 1,25(OH)2D3 is a negative endocrine regulator of renin production 24. This discovery stemmed from our initial observation that vitamin D receptor (VDR)-null mice, a complete vitamin D-deficient model 25, develop hyperreninemia due to dramatic up-regulation of renin expression in the kidney 24. Plasma renin activity, plasma Ang II and plasma and urinary aldosterone levels in VDR-null mice are markedly elevated, leading to development of high blood pressure, cardiac hypertrophy and polyuria 24, 26, 27. Interestingly, polyuria seen in VDR-null mice appears to result from overdrinking due to up-regulation of renin in the central nerve system and activation of the brain RAS 28.
The critical role of vitamin D in RAS regulation was confirmed in another genetic model of vitamin D-deficiency, the Cyp27b1-null mice. These mice lack 1α-hydroxylase that is required for the biosynthesis of 1,25(OH)2D3. Similar to VDR knockout mice, Cyp27b1 knockout mice also develop hyperreninemia, hypertension and cardiac hypertrophy as a result of renin up-regulation, but these abnormalities could be corrected by exogenous 1,25(OH)2D3 administration 29. Moreover, we have taken a transgenic approach to confirm the inhibitory role of 1,25(OH)2D3 in renin expression. We showed that in transgenic mice that overexpress the human VDR in the juxtaglomerular cells, renal renin mRNA levels and plasma renin activity are significantly suppressed while serum calcium and parathyroid hormone levels are normal. Furthermore, the VDR transgene is able to rescue the hyperreninemia phenotype when introduced into VDR-null mice through breeding 30. We have also reported that pharmacological dose of vitamin D analogs can effectively inhibit renin expression in mice 31. Together these data establish the vitamin D hormone as a crucial negative endocrine regulator of the RAS.
The relevance of vitamin D regulating the RAS in humans has been confirmed in a number of recent studies. Epidemiological data from a large cohort of patients (>3000) referred for coronary angiography in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study demonstrated that both serum 25(OH)D and 1,25(OH)2D3 levels are independently and inversely associated with plasma renin concentration and Ang II levels 32. Vitamin D analog therapy has been reported to significantly suppress plasma renin activity in patients with late stage chronic kidney disease 33.
We have shown that 1,25(OH)2D3 inhibits renin gene transcription by targeting the cyclic AMP signaling pathway 34, a signaling pathway that plays a critical role in renin biosynthesis and release in response to various physiological factors 35. CREB and/or CREM interact with the cyclic AMP response element (CRE) in the renin gene promoter. Phosphorylation of CREB or CREM by PKA leads to recruitment of CBP/p300 to the CRE site to drive renin gene transcription. In the presence of 1,25(OH)2D3, ligand-activated VDR physically interacts with CREB, blocking CREB binding to the CRE and thus disrupting the formation of CREB-CBP/p300 complex on the CRE site. As a consequence, renin transcription is stopped. This is at least part of the molecular mechanism by which 1,25(OH)2D3 inhibits renin production. This mechanism is a basis to use vitamin D and its analogs as inhibitors to inhibit renin production.
Therapeutic Potentials of Vitamin D Analogs in Cardiovascular Disease
Because of its profound effect on the cardiovascular system, the RAS has been a major therapeutic target for prevention and intervention of cardiovascular disease. Small molecules that target the RAS, including ACE inhibitors (ACEI), Ang II type 1 receptor blockers (ARB) and renin inhibitors, are widely used anti-hypertensive drugs 36-38. The notion that 1,25(OH)2D3 suppresses renin biosynthesis provides a molecular basis to explore vitamin D and vitamin D analogs as novel RAS inhibitors for therapeutic purposes. Bodyak et al reported that treatment with paricalcitol (19-nor-1,25-dihydroxyvitamin D2), an activated vitamin D analog, blocked high salt-induced cardiac hypertrophy in the Dahl salt-sensitive rat model 39. Surrogate biomarkers of hypertrophy such as ANP and BNP were significantly suppressed by the treatment, together with a marked reduction of cardiac renin expression. In the rat model of chronic renal failure (5/6 nephrectomy) paricalcitol treatment significantly lowered blood pressure and suppressed the RAS in the remnant kidney 40. In an early study, 15-weeks intravenous infusion of calcitriol (1,25(OH)2D3) was shown to regress left ventricular hypertrophy in hemodialysis patients 41. Interestingly, in this study the reduction in the left ventricular mass was accompanied by a significant reduction in plasma renin activity, plasma Ang II and ANP levels in these patients, consistent with the suppressive effect of 1,25(OH)2D3 on the RAS.
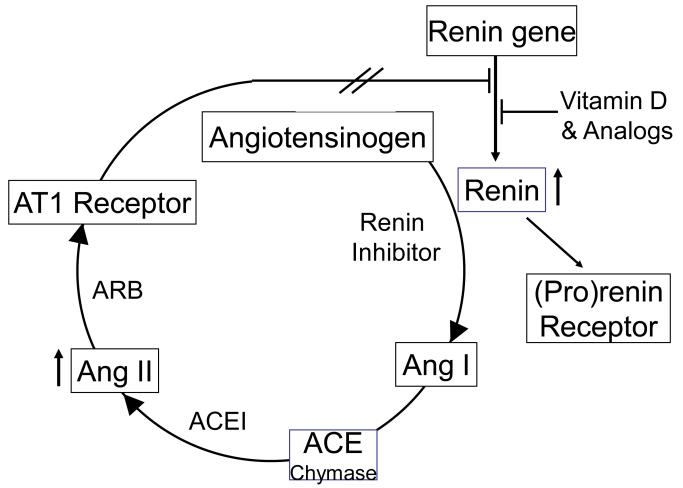
A major problem associated with the current RAS inhibitors is the compensatory increase of renin concentration 42. As the rate-limiting step of the RAS, renin homeostasis is maintained by the negative feedback loop mediated by the AT1 receptor. Blockade of the RAS simultaneously disrupts the negative feedback loop, leading to up-regulation of renin expression. The huge increase in renin concentration and activity in the plasma and tissue interstitial space stimulates the conversion of Ang I, which can ultimately lead to the build-up of Ang I, Ang II and other angiotensin metabolites in the body, through ACE-dependent and -independent pathways (Figure 1). Ang II accumulation compromises the efficacy of RAS inhibition and may explain why the current RAS inhibitors are only clinically suboptimal.
Figure 1.
Vitamin D analogs block the compensatory induction of renin in combination therapy. The homeostasis of renin production is maintained by the negative feedback loop mediated by AT1 receptor. Inhibition of the RAS by the three classes of inhibitors (renin inhibitors, ACE inhibitors and ARB) disrupts the feedback loop leading to increased renin production. Ultimately Ang II levels can be raised, which reduces reduced the efficacy of the drugs. In combination therapy, vitamin D analogs are able to block the compensatory increase of renin by suppressing renin gene expression, and that improves the therapeutic efficacy.
One important application of vitamin D analogs is to block the compensatory renin increase at the transcriptional level in combination therapy with the classic RAS inhibitors 43 (Figure 1). The combination is expected to enhance the efficacy of RAS inhibition and achieve better therapeutic outcomes 44. Recently we demonstrated that a combination of paricalcitol or doxercalciferol (1α-hydroxyvitamin D2) with losartan blocks cardiac hypertrophy in spontaneously hypertensive rats much more effectively than each mono-treatment 33. Spontaneously hypertensive rats, a model of human essential hypertension, develop age-dependent left ventricular hypertrophy. The rats were treated for 2 months with losartan, paricalcitol, doxercalciferol, losartan and paricalcitol combination or losartan and doxercalciferol combination. Left ventricular mass was quantified by echocardiography, and hypertrophic markers such as ANP and BNP were also measured. While paricalcitol or doxercalciferol reduced left ventricular hypertrophy as effectively as losartan in mono-treatment, the combinations had much better therapeutic efficacy than the three mono-therapies. Indeed, the compensatory increase of renin in the kidney and heart was blocked in the combination therapy 33. Similar findings were observed with the combination therapy in models of diabetic nephropathy 45-47. In humans, a recently reported large randomized clinical trial (the VITAL Study) confirmed that paricalcitol was able to reduce albuminuria and blood pressure in patients with diabetic nephropathy who were already on RAS inhibitor therapy 48. Direct renin inhibitors such as aliskiren also have the problem of inducing a huge increase in plasma renin concentration 49, and it is expected that combination of aliskiren with a vitamin D analog should also lead to blockade of the compensatory renin induction and improvement of the therapeutic efficacy of aliskiren 44 (Figure 1). Therefore, the combination of vitamin D analogs and RAS inhibitors has broad therapeutic potentials. Given the wide use of RAS inhibitors in cardiovascular disease, the combination strategy warrants further investigations in clinical settings. In fact, the ongoing PRIMO study (ClinicalTrials.gov Identifier: NCT00497146; Enrollment: 220 stage 3 and 4 CKD patients), a randomized double-blind placebo control clinical trial that investigates the effect of paricalcitol on the progression of left ventricular hypertrophy in stage 3 and 4 CKD patients who are already on RAS inhibitors, will assess the cardiac outcome of the combination of vitamin D analog and RAS inhibitors in humans.
Conclusion
The finding of the vitamin D hormone as a negative endocrine regulator of the RAS has important physiological and clinical significance. It suggests that the vitamin D endocrine system may maintain the homeostasis of the cardiovascular system through suppressing the RAS, and vitamin D-deficiency or insufficiency may lead to activation of the RAS, thus increasing the risk of cardiovascular disease. Vitamin D analogs as novel renin inhibitors have great therapeutic potentials for cardiovascular disease. Combination therapy with vitamin D analogs and RAS inhibitors can enhance the therapeutic efficacy in the treatment of cardiovascular disease.
Abbreviations
- VDR
vitamin D receptor
- RAS
renin-angiotensin system
- AGT
angiotensinogen
- Ang
angiotensin
- ACE
angiotensin-converting enzyme
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 5.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 6.He J, Klag MJ, Wu Z, et al. Stroke in the People’s Republic of China. I. Geographic variations in incidence and risk factors. Stroke. 1995;26:2222–2227. [PubMed] [Google Scholar]
- 7.Spencer FA, Goldberg RJ, Becker RC, et al. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31:1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 8.Harburg E, Gleibermann L, Roeper P, et al. Skin color, ethnicity, and blood pressure I: Detroit blacks. Am J Public Health. 1978;68:1177–1183. doi: 10.2105/ajph.68.12.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klag MJ, Whelton PK, Coresh J, et al. The association of skin color with blood pressure in US blacks with low socioeconomic status. Jama. 1991;265:599–602. [PubMed] [Google Scholar]
- 10.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 12.Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 13.Burgaz A, Orsini N, Larsson SC, et al. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2010 doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 14.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Liu Y, Hollis BW, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 19.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 20.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- 21.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 22.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 23.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 24.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kong J, Li YC. Effect of Angiotensin II Type I Receptor Antagonist and Angiotensin-Converting Enzyme Inhibitor on Vitamin D Receptor Null Mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R255–R261. doi: 10.1152/ajpregu.00517.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kong J, Zhang Z, Li D, et al. Loss of vitamin D receptor produces polyuria by increasing thirst. J Am Soc Nephrol. 2008;19:2396–2405. doi: 10.1681/ASN.2008010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C, Lu F, Cao K, et al. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 30.Kong J, Qiao G, Zhang Z, et al. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008;74:1577–1581. doi: 10.1038/ki.2008.452. [DOI] [PubMed] [Google Scholar]
- 31.Qiao G, Kong J, Uskokovic M, et al. Analogs of 1alpha,25-dihydroxyvitamin D3 as novel inhibitors of renin biosynthesis. J.Steroid Biochem. Mol. Biol. 2005;96:59–66. doi: 10.1016/j.jsbmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411:1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 33.Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177:622–631. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan W, Pan W, Kong J, et al. 1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 35.Castrop H, Hocherl K, Kurtz A, et al. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 36.August P. Initial treatment of hypertension. N Engl J Med. 2003;348:610–617. doi: 10.1056/NEJMcp010357. [DOI] [PubMed] [Google Scholar]
- 37.Cheung BM. Blockade of the renin-angiotensin system. Hong Kong Med J. 2002;8:185–191. [PubMed] [Google Scholar]
- 38.Nussberger J, Wuerzner G, Jensen C, et al. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 39.Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freundlich M, Quiroz Y, Zhang Z, et al. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 41.Park CW, Oh YS, Shin YS, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 42.Muller DN, Luft FC. Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol. 2006;1:221–228. doi: 10.2215/CJN.01201005. [DOI] [PubMed] [Google Scholar]
- 43.Li YC. Renoprotective effects of vitamin D analogs. Kidney Int. 2010;78:134–139. doi: 10.1038/ki.2009.175. [DOI] [PubMed] [Google Scholar]
- 44.Li YC. Inhibition of renin: an updated review of the development of renin inhibitors. Curr Opin Investig Drugs. 2007;8:750–757. [PubMed] [Google Scholar]
- 45.Zhang Y, D.K. D, Kong J, et al. Long-Term Therapeutic Effect of Vitamin D Analog Doxercalciferol on Diabetic Nephropathy: Strong Synergism with AT1 Receptor Antagonist. American Journal of Physiology Renal Physiology. 2009;297:F791–F801. doi: 10.1152/ajprenal.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Zhang Y, Ning G, et al. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deb DK, Sun T, Wong KE, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 2010;77:1000–1009. doi: 10.1038/ki.2010.22. [DOI] [PubMed] [Google Scholar]
- 48.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 49.Azizi M, Menard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–3133. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]