Abstract
Purpose
Pemetrexed-based chemotherapy represents the standard of care in firstline-treatment of advanced malignant pleural mesothelioma (MPM). However, there are no established predictors of clinical benefit. Pemetrexed inhibits multiple enzymes involved in pyrimidine and purine synthesis, but the main target is thymidylate synthase (TS). Following cellular uptake pemetrexed is converted into more effective polyglutamated forms by folylpoly-γ-glutamate synthetase (FPGS). We hypothesized that FPGS and TS protein expression is associated with clinical outcome following pemetrexed-based chemotherapy.
Patients and Methods
Pretreatment tumor samples from 84 patients with histologically confirmed MPM, who received pemetrexed combined with platinum (79/84) or single-agent pemetrexed (5/84) as firstline treatment, were retrospectively analyzed. FPGS and TS protein expression was semiquantitatively assessed by using the H-Scoring system (range: 0–300). H-scores were correlated with radiological response according to modified RECIST, progression-free survival (PFS) and overall survival (OS).
Results
Median H-score of the entire cohort was 230 for FPGS (range: 100–300), and 210 for TS (range: 100–300). High FPGS protein expression was significantly associated with longer PFS (PCOX=0.0337), better objective tumor response (PR vs. SD + PD; PKW=0.003), and improved disease control rate (PR + SD vs. PD; PKW=0.0208), but not with OS. In addition, high TS protein expression was associated with PD under pemetrexed-based therapy (P=0.0383), and shorter OS (PCOX=0.0071), but no association with PFS was observed.
Conclusion
FPGS and TS expression were associated with clinical response and outcome to pemetrexed-based firstline chemotherapy in MPM. Prospective evaluation of FGPS and TS expression and their prognostic/predictive power in MPM patients is warranted.
Keywords: Malignant Pleural Mesothelioma, Biomarker, FPGS, TS, Pemetrexed
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a rare tumor with a poor prognosis. Systemic therapy represents the primary treatment option for most cases1,2. In clinical practice, pemetrexed is used in combination with cisplatin3 or carboplatin4–6 or in patients who are medically unfit for platinum-containing chemotherapy as single agent7,8.
Pemetrexed is a multitarget antifolate that primarily inhibits thymidylate synthase (TS). At higher concentrations, pemetrexed also inhibits folate-dependent enzymes such as dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyl transferase (GARFT), all of which (Figure 1) are involved in the de novo biosynthesis of thymidine and purine nucleotides9–11. Once pemetrexed is taken up by cells, it undergoes ATP-dependent polyglutamylation catalyzed by folylpoly-γ-glutamate synthetase (FPGS). FPGS adds up to 10 glutamate residues, one at a time, to the γ-carboxyl residues of pemetrexed12–14. Polyglutamylation results in more negatively charged molecules which are less susceptible to normal influx/efflux pathways. This leads to higher intracellular concentration of pemetrexed11,13,14.
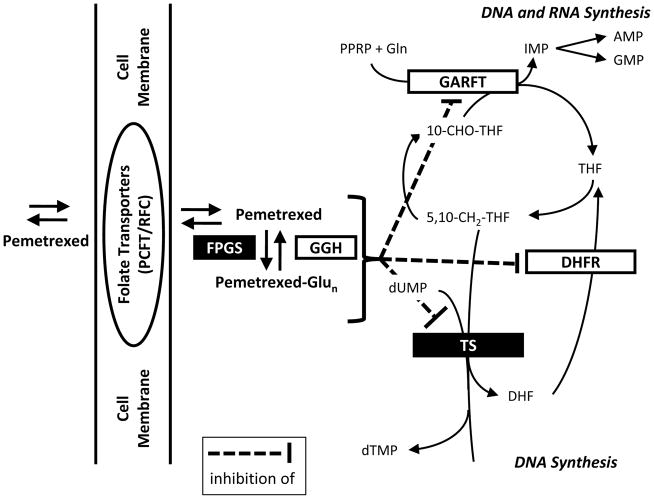
Figure 1.
Pemetrexed’s mechanism of action: After transfer into cells, pemetrexed undergoes polyglutamylation. Both the non-glutamated as well as the polyglutamated forms are able to inhibit DHFR, GARFT, and TS. AMP: adenosine monophosphate; DHF: dihydrofolate; DHFR: dihydrofolate reductase; DNA: desoxyribonucleid acid; dTMP: desoxythymidine monophosphate; FPGS: folylpoly-γ-glutamyl synthetase; GARFT: glycinamide ribonucleotide formyltransferase; GGH: γ-glutamyl hydrolase; Gln: glutamine; GMP: guanosine monophosphate; IMP: inosine monophosphate; PCFT: proton-coupled folate carrier; RFC: reduced folate carrier; RNA: ribonucleic acid; THF: tetrahydrofolate; TS: thymidylate synthase. Figure according to36.
Pemetrexed was found to be one of the best substrates of mammalian FPGS, with a Km of 0.8 μM. It is believed that polyglutamylation plays an important role in determining both selectivity and antitumor activity of this agent11,15. In its monoglutamyl form, pemetrexed is a weak inhibitor of TS (Ki=109 nM), DHFR (Ki=7 nM) and GARFT (Ki=9.3 μM). However, the Ki values of pemetrexed pentaglutamate decrease to 1.3 nM (TS) and 65 nM (GARFT), respectively16. Therefore, the pentaglutamate form is 100-fold more potent than monoglutamyl pemetrexed, making it one of the most potent folate-based TS inhibitors. Accordingly, loss of FPGS activity is an established mechanism of resistance to intermittent exposure to high-dose polyglutamable antifolates, including methotrexate, raltitrexed and pemetrexed, both in vitro and in vivo12,14,17–20.
Two studies retrospectively examined the association of TS mRNA and protein expression with clinical outcome in MPM patients treated with pemetrexed-based chemotherapy6,21. One study reported a significant correlation between low TS expression and disease control (DC), progression-free survival (PFS) and overall survival (OS) in MPM patients treated with carboplatin and pemetrexed6. In another study low TS protein levels were associated with improved PFS and OS following treatment with pemetrexed and carboplatin or cisplatin. However, no significant correlation of outcome with TS mRNA expression was found21.
Currently, there are no clinically validated biomarkers to predict objective response (OR) or outcome following pemetrexed-based chemotherapy. High FPGS expression levels were observed in several MPM cell lines such as MST0-211H, NCI-H28, and NCI-H226a22. Thus, in the current study we investigated the association of FPGS and TS expression, as detected by immunohistochemistry, to clinical outcome following pemetrexed-based chemotherapy in a cohort of advanced MPM patients.
MATERIALS AND METHODS
Study Population
The study included patients with advanced MPM, who received pemetrexed-based firstline chemotherapy at the West German Cancer Center between 2003 and 2010. Analysis of tumor samples from 104 patients (Suppl. table 1), which were available following routine diagnostic histopathological workup revealed qualitatively adequate pre-treatment biopsies from 84 patients for this study (Table 1). In addition, post-treatment samples were available from 27 of these 84 MPM patients (32%, suppl. table 2). All patients had undergone thoracoscopic biopsy and staging following published guidelines23,24. In all cases diagnosis of MPM was based on histological and immunohistochemical examination using antibodies detecting calretinin, cytokeratin 5/6, and mesothelin25. Clinicopathological data included gender, age, histology, complete history, and thoracoscopic findings. Tumor staging was based on the tumor, node, and metastases (TNM) staging system as proposed by the International Mesothelioma Interest Group (IMIG)26. As MPM is difficult to quantify radiographically due to non-radial and variable patterns of growth and response to therapy2,27–30, modified Response Evaluation Criteria in Solid Tumors (RECIST) were applied for evaluation of response. These criteria were used to classify the tumor response to treatment as: complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD)27–30. Patients were regarded as achieving disease stabilization if radiologic evaluations during treatment to the first evaluation after completion of chemotherapy treatment showed SD. PFS was calculated from the first day of chemotherapy treatment until progression, death from any cause, or the last visit where a patient was alive without progression. OS was defined as the time between the start of treatment until the date of death, or last follow-up. Patients were censored at last follow-up if still alive or lost to follow-up. Surveillance of PFS and OS was stopped on May 31, 2011. Surviving patients had provided written informed consent. The study was approved by the Ethics Committee of the Medical Faculty of the University Duisburg-Essen (no. 10-4404).
Table 1.
Clinicopathological data of MPM patients: CR: complete response; PR: partial reponse; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival.
| Epidemiologic and clinicopathological data | Number of patients (%) |
|---|---|
| 84 (100%) | |
| Age | |
| Median age at diagnosis in years (range) | 67 (44–85) |
| Sex | |
| Male | 70 (83%) |
| Female | 14 (17%) |
| Side of pleural involvement | |
| Left | 34 (40%) |
| Right | 50 (60%) |
| T stage | |
| T1 | 16 (19%) |
| T2 | 38 (45%) |
| T3 | 17 (20%) |
| T4 | 13 (16%) |
| N stage | |
| N0 | 55 (65%) |
| N1 | 3 (4%) |
| N2 | 25 (30%) |
| N3 | 1 (1%) |
| M stage | |
| M0 | 72 (86%) |
| M1 | 12 (14%) |
| IMIG stage | |
| I | 9 (11%) |
| II | 24 (29%) |
| III | 28 (33%) |
| IV | 23 (27%) |
| Histologic subtype | |
| Epithelioid | 72 (86%) |
| Non-epithelioid | 10 (12%) |
| Unspecified | 2 (2%) |
| Characteristics | Number of patients (%) |
| Chemotherapy | |
| Pemetrexed plus cisplatin | 66 (79%) |
| Pemetrexed plus carboplatin | 10 (12%) |
| Pemetrexed plus cisplatin and carboplatin | 3 (3%) |
| Pemetrexed single-agent | 5 (6%) |
| Median number of cycles (range) | 4 (1–8) |
| Median cumulative pemetrexed dose in mg (range) | 3745 (750–8400) |
| Median duration of pemetrexed-based therapy in months (range) | 2.8 (0.6–9.4) |
| Response to treatment | |
| CR | 0 (0%) |
| PR | 36 (43%) |
| SD | 36 (43%) |
| PD | 9 (11%) |
| NA | 3 (3%) |
| Survival | |
| Median PFS in months (95% CI) | 7.6 (6.6–9.1) |
| Median OS in months (95% CI) | 23.3 (18.2–29.8) |
FPGS detection in human MPM cell lines
Human MPM cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). NCI-H28, NCI-H2052, NCI-H2452, MeT-5A, and MSTO-211 were cultured in Roswell Park Memorial Institute (RPMI) medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum and 100 U/ml of penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) at 37°C in a 5% CO2-humidified atmosphere. Cells were grown until 80% to 95% confluency, then washed and trypsinized with 2 ml of 0.25% trypsin-0.03% ethylendiaminetetraacetic acid (EDTA, Sigma-Aldrich, St. Louis, MO, USA). Following centrifugation at 150 × g for 5 minutes cell pellets were washed twice with Phosphate-Buffered Saline (PBS, Mediatech Inc., Manassas, VA, USA) and centrifuged at 150 g for 5 minutes each. Pellets were resolved in fixating solution consisting of 50% ethanol and 50% of 10% buffered formalin and fixed for a minimum of 6 hours (at 20°C). After fixation, cells were collected by centrifugation, processed, and embedded in paraffin blocks. These formalin-fixed paraffin-embedded (FFPE) cell blocks were sectioned at 4 μm thickness and mounted on Plus GOLDR slides. Immunocytochemical staining and scoring with anti-FPGS and anti-TS antibodies was performed as described below.
Tissue micro array (TMA) preparation
Sections were prepared from each FFPE tumor block and stained with hematoxylin and eosin for histological examination to identify 3 tumor-rich foci, which were marked with a cytology pen. A Tissue Arrayer (Beecher Instruments, Inc., Sun Prarie, WI) was used to precisely generate core samples from specific regions of the donor FFPE blocks. The FFPE blocks were placed in the arrayer and the tumor-rich foci were sampled with a 1 mm needle and transferred in a recipient paraffin block.
Immunohistochemistry (IHC) in tumor specimens
4 μm sections of TMA blocks were mounted on PlusGOLD® slides for IHC staining. In cases of lost TMA cores or inadequate tumor samples for TMA preparation, whole tissue sections were used. For antigen retrieval, the sections were heated in a drying oven at 60°C for 60 minutes. Deparaffinization, rehydration, blocking of endogenous peroxidase activity with hydrogen peroxide and staining visualization was accomplished using an ultraView Universial DAB detection kit (Ventana, Tucson, AZ, USA) on an automated staining system (BENCHMARK XT, Ventana). The slides were incubated for 1 hour at 37°C or at room temperature with the respective primary antibodies: rabbit polyclonal anti-FPGS antibody (dilution 1:20, Spring Bioscience, Inc., Pleasanton, CA, USA) or mouse monoclonal anti-TS antibody (dilution 1:10, Invitrogen, Inc., Carlsbad, CA, USA). Negative controls were obtained by using non-immunized ready-to-use rabbit and mouse antibodies (Ventana, Tucson, AZ, USA) as the primary antibody (for FPGS and TS, respectively). Tissue sections of hepatocellular (FPGS) or colorectal carcinoma (TS) served as positive controls, and lymphocytes were used as an intra-specimen positive control.
IHC evaluations were performed independently by one pathologist (B.R.A) and one trained reader (D.C.C.). Ambiguous cases were reevaluated jointly until a consensus was reached. Immunoreactivity levels in each section were assessed under a light microscope and images were captured at 400-fold magnification. Tumor staining intensity was graded on a scale from 0+ to 3+, and in each intensity category the percentage of tumor cells was scored, ranging from 0 to 100% so that the sum of the percentages accross the different intensity categories adds up to 100. The percentaged score was then multiplied by its intensity category to obtain a final “Hybrid (H)-score”, ranging from 0 to 3006,21. Examples of tissue samples with different H-scores for FPGS and TS are shown in figure 2.
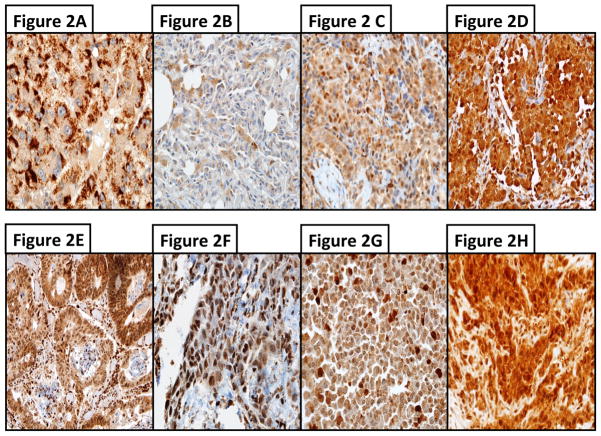
Figure 2.
Figure 2A–H. Examples of immunohistochemistry (IHC) for FPGS and TS in MPM samples with different protein expressions. A–D) IHC with an antibody against FPGS: A) hepatocellular carcinoma was used as positive control; B) weakly stained MPM sample (H-score of 90); C) moderately stained MPM sample (H-score of 170); and D) strongly stained MPM sample (H-score of 290). E–H), IHC with an antibody against TS (dilution 1:10); E) colorectal carcinoma was used as positive control; F) weakly stained MPM sample (H-score of 90); G) moderately stained MPM sample (H-score of 170); and H) strongly stained MPM sample (H-score of 290). Magnification 1:400.
Evaluation of FPGS protein expression was based on cytoplasmic staining. Conversely, TS protein expression is detectable mainly in the nucleus, but also in the cytoplasm. Therefore, TS staining was assessed in the cytoplasm and nucleus6.
Statistics
The objective of this study was to assess the impact of the two selected biomarkers (FPGS and TS) on treatment response and outcome of patients with MPM. Descriptive analyses were performed on FPGS and TS H-scores, as well as on patient’s clinical characteristics namely sex (female/male), age at diagnosis, pleural side of disease (left/right), histology (epithelioid/non-epithelioid), and IMIG stage (I–IV). Spearman’s test31 was used to test the correlations between continuous variables: FPGS H-score, TS H-score, and age at diagnosis. Distributional differences of H-scores between different levels of categorical variables (sex, pleural side of disease, histology, and stage) were analyzed using Kruskal-Wallis test32 (test is equivalent to Wilcoxon rank sum test when comparing two groups). Relationships between pre- and post-treatment protein expression levels on the same patient were also tested using Spearman’s correlation.
We defined “objective response (OR)” as a dichotomous variable: positive response=CR or PR, negative response=SD or PD. In addition, “disease control (DC)” was also defined as a dichotomous variable: positive response=CR or PR or SD, negative response=PD. Distributional differences of H-scores between response groups were tested using Kruskal-Wallis test. Receiver operating characteristics (ROC) curves33 were constructed by calculating the sensitivity and specificity at several cutoff values. The optimal cutoff value was then given by the maximum of the Youden index34.
Kaplan-Meier (KM) curves were generated for PFS and OS time within each level of stage, sex, and histology, respectively. Associations between PFS/OS and FPGS/TS were analyzed using Cox proportional hazards models. Diagnostics for the functional form of stage and FPGS and TS H scores suggested that all three variables could be analyzed as continuous35. Univariate and multivariable analyses of both PFS and OS were carried out using Cox proportional hazards model. Covariates of multivariate analyses include both proteins levels and IMIG stage. Hazard ratios, for an increment (or decrement) of 30 units in protein H-scores were computed (which refer to changes in the H-scores of units of 10%), as well as the HR’s corresponding 95% confidence intervals (CIs). A P-value less than 0.05 was considered statistically significant. Nevertheless, as this is a retrospective study all P-values or results of statistical tests should be regarded as exploratory. Statistical analyses were performed by the University of Colorado Cancer Center Biostatistics and Bioinformatics Core using SAS/BASE and SAS/STAT software, Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient cohort
Demographic and clinicopathologic data of the entire cohort and the study sample were equally balanced (Suppl. table 1, table 1). The main histologic subtype was epithelioid MPM in 72 patients (86%); a non-epithelioid subtype (sarcomatoid or biphasic) was found in 10 patients (12%). Two patients did not have available histological data (2%) The majority of patients received combination chemotherapy with pemetrexed plus cisplatin (n=66, 79%), pemetrexed plus carboplatin (n=10, 12%), or pemetrexed plus cisplatin switched to carboplatin (n=3, 3%). In the latter cases cisplatin was replaced by carboplatin during the course of treatment due to cisplatin-induced toxicities. Median PFS and OS of the entire cohort with pre-treatment samples were 7.6 months (95% CI, 6.6–9.1) and 23.3 months (95% CI, 18.2–29.8), respectively.
Expression of FPGS and TS in MPM cell lines
To validate the primary antibody and scoring system, FPGS protein expression was studied by immunocytochemistry in FFPE cell pellets from 5 different human MPM cell lines and compared with TS protein expression. FPGS and TS were moderately to highly expressed. H-scores varied significantly between cell lines (median H-Score for FPGS: 210; range: 190–225; median H-score for TS: 220; range: 180–280) (Table 2).
Table 2.
Different levels of FPGS/TS protein expression in 5 MPM cell lines. Formalin-fixed paraffin-embedded MPM cells were evaluated by immunohistochemistry using the H-score.
| MPM cell line | 0 | 1+ | 2+ | 3+ | FPGS H-score | 0 | 1+ | 2+ | 3+ | TS H-score |
|---|---|---|---|---|---|---|---|---|---|---|
| H28 | 0% | 10% | 70% | 20% | 210 | 0% | 20% | 40% | 40% | 220 |
| H2052 | 0% | 30% | 50% | 20% | 190 | 0% | 10% | 30% | 60% | 250 |
| H2452 | 0% | 15% | 45% | 40% | 225 | 0% | 0% | 20% | 80% | 280 |
| MeT-5A | 0% | 30% | 30% | 40% | 210 | 0% | 0% | 80% | 20% | 220 |
| MSTO-211 | 0% | 30% | 40% | 30% | 200 | 10% | 30% | 30% | 30% | 180 |
Association of histologic subtype, gender, side, IMIG stage with FPGS and TS expression
Median pre-treatment H-scores were 230 for FPGS (range: 100–300), and 210 for TS (range: 100–300) of the entire cohort. For further analyses of both markers, tumors with H-scores equal to or above the median H-score of the respective marker were assigned to “high expressors”, and tumors with H-score below the median were assigned to “low expressors”. There was no statistically significant correlation between age, sex, histology or side of pleural involvement with FPGS protein expression (Table 3A). Using Kruskal-Wallis tests, we found an association between IMIG stages and FPGS protein expression (PKW=0.0318). FPGS protein expression was higher in IMIG stage II (mean H-score: 247 ± 10, median 270) as compared to IMIG stage IV (mean H-score 217 ± 8, median 210). In contrast, TS expression failed to associate with IMIG stage (Table 3B, PKW=0.1913). Low TS protein expression was associated with female sex (P=0.0039; mean H-score 174 ± 14 vs. 221 ± 6, median 170 vs. 220). No further associations of TS expression with demographic or clinical parameters were found.
Table 3.
Associations between clinicopathological data and protein expression of FPGS (3A) or TS (3B).
| Table 3A | |||
|---|---|---|---|
| Characteristic | Number of patients (%) | Mean pretreatment FPGS H-score ± SEM, median | P-value |
| Histology | 0.1874 | ||
| Epithelioid | 72 (86%) | 235 ± 5, 235 | |
| Non-epithelioid | 10 (12%) | 222 ± 9, 220 | |
| Sex | 0.6379 | ||
| Female | 14 (17%) | 220 ± 17, 235 | |
| Male | 70 (83%) | 234 ± 4, 230 | |
| Side of pleural involvement | 0.7381 | ||
| Left | 34 (40%) | 228 ± 8, 235 | |
| Right | 50 (60%) | 235 ± 5, 230 | |
| Stage | 0.0318 | ||
| IMIG I | 9 (11%) | 221 ± 10, 230 | |
| IMIG II | 24 (29%) | 247 ± 10, 270 | |
| IMIG III | 28 (33%) | 235 ± 7, 240 | |
| IMIG IV | 23 (27%) | 217 ± 8, 210 | |
| Table 3B | |||
|---|---|---|---|
| Characteristic | Number of patients (%) | Mean pretreatment TS H-score ± SEM, median | P-value |
| Histology | 0.4793 | ||
| Epithelioid | 68 (86%) | 217 ± 6, 210 | |
| Non-epithelioid | 9 (11%) | 196 ± 21, 220 | |
| Sex | 0.0039 | ||
| Female | 14 (18%) | 174 ± 14, 170 | |
| Male | 65 (82%) | 221 ± 6, 220 | |
| Side of pleural involvement | 0.5381 | ||
| Left | 32 (40%) | 208 ± 9, 210 | |
| Right | 47 (60%) | 216 ± 7, 220 | |
| Stage | 0.1913 | ||
| IMIG I | 7 (9%) | 197 ± 14, 210 | |
| IMIG II | 24 (30%) | 206 ± 11, 210 | |
| IMIG III | 26 (33%) | 229 ± 10, 240 | |
| IMIG IV | 22 (28%) | 206 ± 10, 217 | |
Comparison of FPGS and TS expression
For technical reasons, IHC staining and H-scoring was successful for FPGS in 84 cases, but only in 79 cases for TS. There was no positive correlation between FGPS and TS protein expression in our cohort (Spearman’s correlation; r= 0.063; P=0.5808).
Longitudinal analyses of pre- and post-treatment samples
In 27 MPM patients tissue samples before and after pemetrexed-based chemotherapy were available for FGPS and TS expression analysis. The distribution of demographic and clinicopathologic parameters in this subcohort did not differ significantly from the entire study cohort, except as all of these patients had epithelioid tumors (Suppl. table 2). A moderate, but significant correlation (Spearman’s correlation; r=0.537; P=0.0039) of pre- and post-treatment FGPS expression was observed. In contrast, there was no correlation between pre- and post-treatment H-scores for TS expression (Spearman’s correlation; r=0.338; P=0.0843). Accordingly, the median differences in FPGS H-Scores (ΔFPGS) or TS H-Scores (ΔTS) protein levels before and after PMX-based treatment was lower for ΔFPGS (−10) compared to ΔTS (0) (Suppl. figure 1).
FPGS expression is associated with treatment response
High FPGS expression was associated with objective response (mean H-score 248 ± 6, median 255 for responders vs. 221 ± 6, median 220 for non-responders, P=0.0030) (Table 4A) and disease control (objective response or disease stabilization) (mean H-score 236 ± 5, median 240 for PR/SD vs. 209 ± 8, median 200 for PD, P=0.0208) (Table 4A). As we observed a significant association between high FPGS protein levels and objective response, we calculated ROC curves analyzing FPGS and OR and we received an area-under-the-curve (AUC) value of 0.692 (P=0.032). We found the optimal cutoff value of 235 (Youden’s index=0.333) before estimating the sensitivity, specificity, positive and negative predictive value for OR, which were 67%, 67%, 71%, and 62%, respectively. Similar analysis for disease control revealed an AUC value of 0.736 (P=0.022) and an optimal cutoff value of 225 (Youden’s index=0.375). Estimation of the sensitivity, specificity, positive and negative predictive value for DC resulted in values of 89%, 63%, 50%, and 98%, respectively. No statistically significant association between TS expression and OR was observed. However, high TS protein expression was associated with progressive disease (P=0.0383) (Table 4B).
Table 4.
Associations between protein expression of FPGS (4A) or TS (4B) and objective response (OR) or disease control (DC). Responding and non-responding patients were compared and significant differences in the mean or median FPGS H-Score for OR or DC as well as in the mean or median TS H-Score for DC were found.
| Table 4A | |||
|---|---|---|---|
| Number of patients | Mean pretreatment FPGS H-score ± SEM, median | P-value | |
| OR | 0.0030 | ||
| PR | 36 | 248 ± 6, 255 | |
| SD + PD | 45 | 221 ± 6, 220 | |
| DC | 0.0208 | ||
| PR + SD | 72 | 236 ± 5, 240 | |
| PD | 9 | 209 ± 8, 200 | |
| Table 4B | |||
|---|---|---|---|
| Number of patients | Mean pretreatment TS H-Score ± SEM, median | P-value | |
| OR | 0.6752 | ||
| PR | 34 | 211 ± 8, 210 | |
| SD + PD | 42 | 213 ± 8, 220 | |
| DC | 0.0383 | ||
| PR + SD | 67 | 208 ± 6, 210 | |
| PD | 9 | 246 ± 12, 260 | |
Association of FPGS and TS with survival
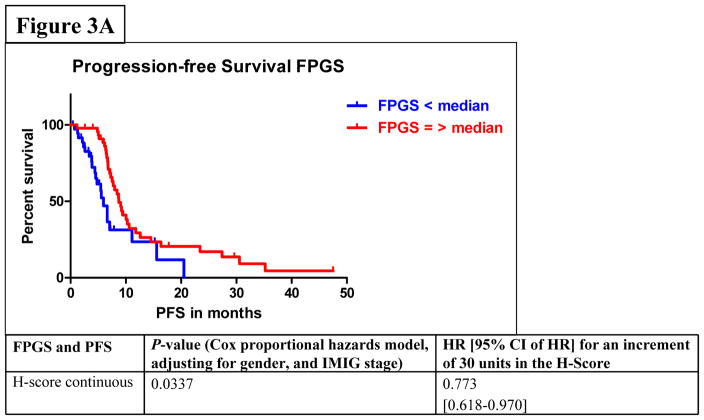
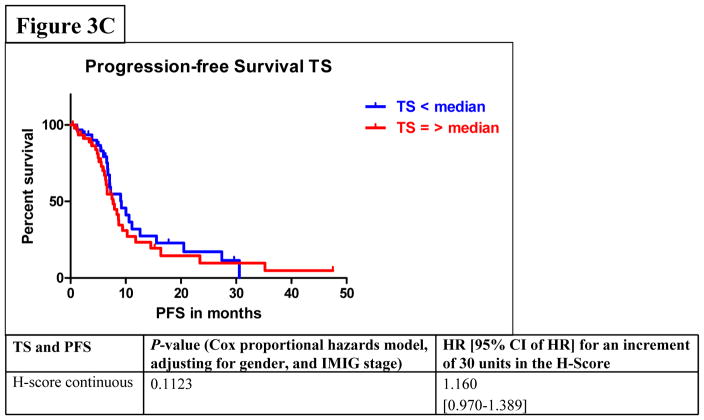
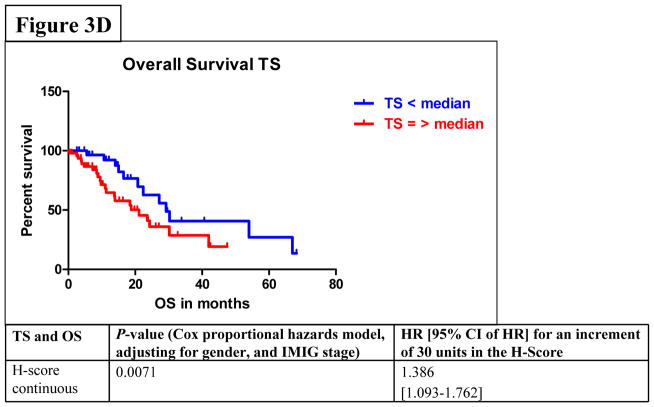
Among all stages of MPM patients, median PFS was 7.6 months (95% CI: 6.6–9.1) and median OS was 23.3 months (95% CI: 18.2–29.8). In the univariate analysis by Cox proportional hazards model considering FPGS, “high FPGS expressors” had a significantly longer PFS as compared to “low expressors” (9.1 vs. 6.1 months) (P=0.0233) (Figure 3A). Univariate analysis showed that the association between FPGS expression and OS was not significant (P=0.7043) (Figure 3B). The association between TS expression and PFS was not significant (P=0.1412) (Figure 3C). Patients whose tumors expressed TS levels below-median had significantly longer OS time (P=0.0036) (Figure 3D). As IMIG stages were associated with FPGS protein expression (PKW=0.0318) and significant associations between IMIG stages and PFS (PCOX=0.0103) or OS (PCOX=0.0178) were observed, we investigated the relationship between IMIG stages, PFS/OS and FPGS/TS expression by a Cox proportional hazards model. The significant association between PFS and FPGS levels was confirmed in multivariate analyses, when adjusting for IMIG stage and sex (HR for 30 units increment in FPGS: 0.773, 95% CI: 0.618–0.970], PCOX=0.0337). The association between OS and TS levels was also statistically significant when adjusting for IMIG stage and sex (HR for 30 units increment in TS: 1.386, 95% CI: 1.093–1.762, PCOX=0.0071).
Figure 3.
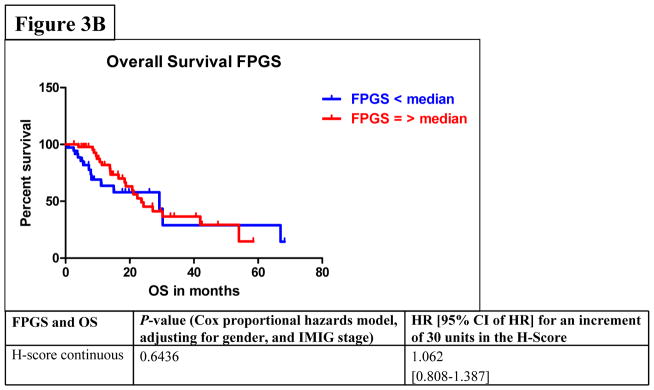
Figure 3A–D. Associations between protein expression of FPGS and progression-free survival (PFS) (3A) or overall survival (OS) (3B); associations between protein expression of TS and PFS (3C) or OS (3D).
DISCUSSION
For molecular targeted drugs, the identification of predictive markers of effective therapy is crucial for maximizing therapeutic efficacy and minimizing useless treatment in patients with cancer. Therefore, novel strategies for the prediction of clinical response are urgently required for individualized therapy.
We performed a retrospective study in a disease with unprecedented data about FPGS protein expression and we included all consecutive patients with MPM treated with pemetrexed-based firstline chemotherapy at our institution. In this clinical setting, almost no data are available on possible predictors of responsiveness.
By using modified RECIST27–30 to evaluate response and outcome to firstline pemetrexed-based chemotherapy, we found that high FPGS levels were associated with OR and DC. Furthermore, patients with high FPGS-expressing tumors had a median PFS time of 9.1 months compared to 6.1 months for patients with tumors with low expression (PCOX=0.0337). Analysis of TS protein expression revealed that high TS protein expression was associated with PD during chemotherapy and a shorter median OS time (20.8 vs. 28.8 months) (PCOX=0.0071).
Analysis of FPGS protein expression before pemetrexed-based chemotherapy showed that high FPGS levels were associated with OR and DC. Therefore we determined the predictive value of FPGS expression for OR and DC by analyses of ROC curves and received AUC values of 0.692 and 0.736, which indicate an association between OR or DC and FPGS expression. In our study, patients with high FPGS-expressing tumors had a prolonged median PFS. But PFS is a rather weak endpoint which might be affected by assessment schedules and other factors, although at our institution standard procedures for radiographic evaluations after completion of the chemotherapy are established. However, prospective evaluation of the predictive value for response and PFS is warranted.
Our results of the associations between TS protein expression and outcome are in parts in accordance with recently published studies6,21, as we only observed a statistically significant association between low levels of TS protein expression and improved DC (P=0.0383) or prolonged OS. Surprisingly, TS expression was not associated with PFS after pemetrexed treatment and this lacking association might reflect the above mentioned difficulties with PFS as an endpoint. Recently, a study reported an improved PFS of patients with tumors expressing low levels of TS (7 vs. 6 months)6. But in our study the difference in PFS of MPM-patients having tumors with low and high levels of FPGS was much larger.
In another study no significant correlation of outcome after pemetrexed-based treatment with TS mRNA expression was found21. This was attributed to methodological problems, as viable tumor cells growing in small nests or as single cells in biopsy specimens, they were not suitable for microdissection which would lead to the dilution of the tumor-derived RNA by RNA from stromal origin. Further, MPM are commonly infiltrated by TS-rich inflammatory cells. However, the predictive value of TS expression for pemetrexed treatment is still under discussion, and data about the prognostic value of TS expression in MPM are limited21.
Reduced antifolate polyglutamate accumulation is one mechanism by which tumor cells can display natural resistance or acquired resistance to pulse exposure to antifolates that require polyglutamylation for potent inhibition of their target enzymes (such as pemetrexed). Decreased polyglutamate accumulation is often a result of decreased expression of FPGS activity36–38, which doesn’t correlate with FPGS mRNA levels12,14. Post-transcriptional alterations such as aberrant splicing of FPGS mRNA, including intron retention and/or exon skipping, which results in premature translation termination, or alterations affecting ribosomal binding to FPGS mRNA, translation initiation or elongation reduce the translatability of the FPGS mRNA12,13,39. We expected that analysis of FPGS gene expression will not predict response or clinical outcome and focused on FPGS protein expression levels which can be easily assessed by IHC and is also reliable in small specimens due to its cytoplasmic localization22.
We analyzed FPGS and TS levels in 27 patients before and after pemetrexed-based treatment. There was no correlation between pre- and post-treatment TS levels (Spearman’s correlation; r=0.338; P=0.0843). One explanation might be that pemetrexed influences TS expression, as it was observed in vitro in lung cancer cell lines18,40 and pemetrexed administration might also have an impact on TS expression in vivo. In contrast, we found a moderate, but significant correlation in FPGS expression levels before and after treatment (Spearman’s correlation; r=0.537; P=0.0039).
Some limitations in our study should be mentioned. First, we used only IHC for the detection of FPGS and TS protein levels in MPM samples. FPGS and TS gene expression was not examined because the tumor samples are surrounded by TS-rich inflammatory cells and in a retrospective study no association between TS gene expression and outcome in MPM was noted21, but an association between TS protein expression and TTP/OS was observed21. Second, we did not evaluate the expression levels of glutamyl hydrolase, which is responsible for removing glutamate residues from polyglutamated molecules20. Pemetrexed polyglutamates are excellent substrates for glutamyl hydrolase. Therefore, an increase in the activity of this enzyme reduces the accumulation of these compounds and may also lead to pemetrexed resistance20. Further investigations of this enzyme may be warranted. Third, we were neither able to investigate FPGS and TS expression in a control cohort of MPM patients who did not receive pemetrexed-based chemotherapy nor could we investigate the protein expression in a homogenously treated cohort and the low numbers of patients in different subgroups limited further statistical analyses. Particularly, due to the low number of patients to whom pemetrexed as single-agent was administered, we were unable to perform this subgroup analysis for FPGS and TS expression. Finally, we did not analyze the expression of Excision Repair Cross-Complementation Group-1 (ERCC-1), as this enzyme is involved in resistance to alkylating agents like cisplatin or carboplatin6,21,41. The reported results were inconsistent, and the discussion about the specificity of the antibody (8F1) is ongoing42.
In conclusion, in patients with MPM treated with pemetrexed-based chemotherapy, high FPGS protein expression was associated with a better OR and DC as well as an improved PFS, but not with a prolonged OS. An association between DC or OS and TS expression was observed. This study is retrospective and hypothesis generating and the findings need to be further validated in a larger cohort of patients and in prospective studies. Furthermore, prospective trials might clarify the predictive versus the prognostic associations of FPGS and TS in MPM.
Supplementary Material
Comparison of pre- and post-treatment protein levels of FPGS (1A) and TS (1B). Median differences of the pre- and post-treatment samples for FPGS differed from the differences of TS. Axis of abscissa depicting patients’ ID.
Suppl. table 1: Clinicopathological data of all 104 MPM patients with available pre- and/or post-treatment samples. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival.
Suppl. table 2: Clinicopathological data of 27 MPM patients with available pre- and post-treatment samples for a paired analysis. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival.
Acknowledgments
Source of Funding:
This work was supported by an IASLC Fellowship Award (D.C.C., C.M.). C.M. is also supported by a Specialized Program of Research Excellence (SPORE) Career Development Award and a grant from the Louisiana Chapter of the National Lung Cancer Partnership. D.C.C. received a research grant and travel support from Lilly Germany, T.C.G. received consulting fee or honorarium and travel support from Lilly Germany. D.T. received consulting fee from Lilly Germany. W.E.E. received consulting fee and honoraria from Lilly Germany. F.R.H. received consulting fee from Eli Lilly.
This work was supported by IASLC Fellowship Awards (D.C.C., C.M.) and a research grant from Lilly Germany. C.M. is also supported by a Specialized Program of Research Excellence (SPORE) Career Development Award and a grant from the Louisiana Chapter of the National Lung Cancer Partnership. The authors would like to acknowledge A. Peglow, S. Fox, I. Perelmuter, and R. Daniels for collecting and preparing clinical data of the patients as well as J. Eckelberger for critically reading of the manuscript.
Footnotes
Conflicts of Interest
For the remaining authors no conflicts of interest were identified for this paper.
This work was presented in part at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, USA, June 4–8 (Poster Discussion Session, Lung Cancer - Local-regional and Adjuvant Therapy/Small Cell; abstract no. 7024) and at the 14th World Conference on Lung Cancer, Amsterdam, The Netherlands, July 3–7, 2011 (Poster Session 2; abstract no. 2.480).
References
- 1.Treasure T, Sedrakan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet. 2004;364:1183–1185. doi: 10.1016/S0140-6736(04)17108-0. [DOI] [PubMed] [Google Scholar]
- 2.Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol. 2006;24:1443–1448. doi: 10.1200/JCO.2005.04.3190. [DOI] [PubMed] [Google Scholar]
- 5.Ceresoli GL, Castagneto B, Zucali PA, et al. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer. 2008;99:5155–56. doi: 10.1038/sj.bjc.6604442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucali PA, Giovanetti E, Destro A, et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res. 2011;17:2581–2590. doi: 10.1158/1078-0432.CCR-10-2873. [DOI] [PubMed] [Google Scholar]
- 7.Jaenne PA, Wozniak AJ, Belani CP, et al. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol. 2006;1:506–512. [PubMed] [Google Scholar]
- 8.Taylor P, Castagneto B, Dark G, et al. Single-agent pemetrexed for chemonaive and pretreated patients with malignant pleural mesothelioma: results of an International Expanded Access Program. J Thorac Oncol. 2008;3:761–771. doi: 10.1097/JTO.0b013e31817c73ec. [DOI] [PubMed] [Google Scholar]
- 9.Adjei AA. Pharmacology and mechanism of action of pemetrexed. Clin Lung Cancer. 2004;5:S51–S55. doi: 10.3816/clc.2004.s.003. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Selvaggi G. Emerging drugs for mesothelioma. Expert Opinion Emerg Drugs. 2007;12:127–137. doi: 10.1517/14728214.12.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Shih C, Chen VJ, Gossett LS, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–1123. [PubMed] [Google Scholar]
- 12.Liani E, Rothem L, Bunni MA, et al. Loss of folylpoly-gamma-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer. 2003;103:589–599. doi: 10.1002/ijc.10829. [DOI] [PubMed] [Google Scholar]
- 13.McGuire JJ, Russell CA. Folylpolyglutamate synthetase expression in antifolate-sensitive and -resistant human cell lines. Oncol Res. 1998;10:193–200. [PubMed] [Google Scholar]
- 14.Stark M, Wichman C, Avivi I, et al. Aberrant splicing of folylpolyglutamate synthetase as a novel mechanism of antifolate resistance in leukemia. Blood. 2009;113:4362–4369. doi: 10.1182/blood-2008-08-173799. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 16.Zhao R, Titus S, Gao F, et al. Molecular analysis of murine leukemia cell lines resistant to 5, 10-dideazatetrahydrofolate identifies several amino acids critical to the function of folylpolyglutamate synthetase. J Biol Chem. 2000;275:26599–26606. doi: 10.1074/jbc.M002580200. [DOI] [PubMed] [Google Scholar]
- 17.Barnes MJ, Estlin EJ, Tayler GA, et al. Impact of polyglutamation on sensitivity to raltitrexed and methotrexate in relation to drug-induced inhibition of de novo thymidylate and purine biosynthesis in CCRF-CEM cell lines. Clin Cancer Res. 1999;5:2548–2558. [PubMed] [Google Scholar]
- 18.Ozasa H, Oguri T, Uemura T, et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010;101:161–166. doi: 10.1111/j.1349-7006.2009.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhao R, Goldman ID. Decreased expression of the reduced folate carrier and folypolyglutamate synthetase is the basis for acquired resistance to the pemetrexed antifolate (LY231514) in an L1210 murine leukemia cell line. Biochem Pharmacol. 2003;65:1163–1170. doi: 10.1016/s0006-2952(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 20.Rots MG, Pieters R, Perters GJ, et al. Role of folylpolyglutamate synthetase and folylpolyglutamate hydrolase in methotrexate accumulation and polyglutamylation in childhood leukemia. Blood. 1999;93:1677–1683. [PubMed] [Google Scholar]
- 21.Righi L, Papotti MG, Ceppi P, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010;28:1534–1539. doi: 10.1200/JCO.2009.25.9275. [DOI] [PubMed] [Google Scholar]
- 22.Quinn AE, Pinkney M, Piggott NH, et al. A monoclonal antibody for detection of folylpolyglutamate synthetase in paraffin embedded tissues. Hybridoma (Larchmt) 2009;28:415–421. doi: 10.1089/hyb.2009.0040. [DOI] [PubMed] [Google Scholar]
- 23.Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer. 1993;72:394–404. doi: 10.1002/1097-0142(19930715)72:2<394::aid-cncr2820720214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Borasio P, Berruti A, Bille A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33:307–313. doi: 10.1016/j.ejcts.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 25.Husain AN, Colby CV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 26.Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest. 1995;108:1122–1128. doi: 10.1378/chest.108.4.1122. [DOI] [PubMed] [Google Scholar]
- 27.Ceresoli GL, Chiti A, Zucali PA, et al. Assessment of tumor response in malignant pleural mesothelioma. Cancer Treat Rev. 2007;33:533–541. doi: 10.1016/j.ctrv.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Nowak AK. CT, RECIST, and malignant pleural mesothelioma. Lung Cancer. 2005;49:S37–S40. doi: 10.1016/j.lungcan.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol. 2008;18:1635–1643. doi: 10.1007/s00330-008-0918-9. [DOI] [PubMed] [Google Scholar]
- 30.Klaveren RJ van, Aerts JG, Bruin H de, et al. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;43:63–69. doi: 10.1016/s0169-5002(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 31.Spearman C. The proof and measurement of association between two things. Int J Epidemiol. 2010;39:1137–1150. doi: 10.1093/ije/dyq191. [DOI] [PubMed] [Google Scholar]
- 32.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. Am Stat. 1952;47:583–621. [Google Scholar]
- 33.Fawcett T. ROC graphs: notes and practical considerations for researchers. ReCALL. 2004;31:1–38. [Google Scholar]
- 34.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Lin DY, Ying Z. A simple nonparametric estimator of the bivariate survival function under univariate censoring. Biometrika. 1993;80:573–581. [Google Scholar]
- 36.Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26:153–181. doi: 10.1007/s10555-007-9049-z. [DOI] [PubMed] [Google Scholar]
- 37.McGuire JJ, Russell CA, Balinski M. Human cytosolic and mitochondrial folylpolyglutamate synthetase are electrophoretically distinct. Expression in antifolate-sensitive and -resistant human cell lines. J Biol Chem. 2000;275:13012–13016. doi: 10.1074/jbc.275.17.13012. [DOI] [PubMed] [Google Scholar]
- 38.Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 39.Roy K, Egan MG, Sirlin S, et al. Posttranscriptionally mediated decreases in folylpolyglutamate synthetase gene expression in some folate analogue-resistant variants of the L1210 cell. Evidence for an altered cognate mRNA in the variants affecting the rate of de novo synthesis of the enzyme. J Biol Chem. 1997;272:6903–6908. doi: 10.1074/jbc.272.11.6903. [DOI] [PubMed] [Google Scholar]
- 40.Wu MF, Hsiao YM, Huang CF, et al. Genetic determinants of pemetrexed responsiveness and nonresponsiveness in non-small cell lung cancer cells. J Thorac Oncol. 2010;5:1143–1151. doi: 10.1097/JTO.0b013e3181e0b954. [DOI] [PubMed] [Google Scholar]
- 41.Zimling ZG, Sorensen JB, Gerds TA, et al. Low ERCC1 expression in malignant pleural mesotheliomas treated with cisplatin and vinorelbine predicts prolonged progression-free survival. J Thorac Oncol. 2012;7:249–256. doi: 10.1097/JTO.0b013e318233d6a9. [DOI] [PubMed] [Google Scholar]
- 42.Niedernhofer LJ, Bhagwat N, Wood RD. ERCC1 and Non-Small Cell Lung Cancer. New Engl J Med. 2007;356:2540–2541. doi: 10.1056/NEJMc070742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of pre- and post-treatment protein levels of FPGS (1A) and TS (1B). Median differences of the pre- and post-treatment samples for FPGS differed from the differences of TS. Axis of abscissa depicting patients’ ID.
Suppl. table 1: Clinicopathological data of all 104 MPM patients with available pre- and/or post-treatment samples. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival.
Suppl. table 2: Clinicopathological data of 27 MPM patients with available pre- and post-treatment samples for a paired analysis. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival.