Abstract
The 118A>G single nucleotide polymorphism (SNP) in the μ-opioid receptor (OPRM1) gene has been the most described variant in pharmacogenetic studies regarding opioid drugs. Despite evidence for an altered biological function encoded by this variant, this knowledge is not yet utilized clinically. The aim of the present review was to collect and discuss the available information on the 118A>G SNP in the OPRM1 gene, at the molecular level and in its clinical manifestations. In vitro biochemical and molecular assays have shown that the variant receptor has higher binding affinity for β-endorphins, that it has altered signal transduction cascade, and that it has a lower expression compared with wild-type OPRM1. Studies using animal models for 118A>G have revealed a double effect of the variant receptor, with an apparent gain of function with respect to the response to endogenous opioids but a loss of function with exogenous administered opioid drugs. Although patients with this variant have shown a lower pain threshold and a higher drug consumption in order to achieve the analgesic effect, clinical experiences have demonstrated that patients carrying the variant allele are not affected by the increased opioid consumption in terms of side effects.
Keywords: μ-opioid receptor, opioids, pharmacogenetics, pain, analgesia
Introduction
Opioid analgesics are widely used for the treatment of moderate–severe acute and chronic pain in the clinic. However, the occurrence of opioid-related side effects, such as respiratory depression, nausea, vomiting, constipation, and sedation may limit the dosing and affect effectiveness of opioid treatment. This may lead to poor patient compliance, discontinuation of therapy, drug underdosing, and inadequate analgesia. In contrast, prolonged use of opioids, as in the case of chronic pain treatment, may also lead to tolerance and adverse effects, such as hyperalgesia and addiction, which may limit their effectiveness.1,2
The analgesic efficacy of opioids varies greatly among individuals, leading to a scenario where some patients either receive inadequate therapy or experience severe side effects at standard doses.3 Similarly, interindividual variability concerning the development of tolerance and addiction following a chronic treatment may be hypothesized, as was suggested in human healthy volunteers in controlled experimental settings in the mid-1990s.4 For these reasons, it is important to understand the factors underlying this variability in response to opioids – in order to predict the clinical outcome and, thus, to personalize therapy for the individual patient.
Individual differences in opioid consumption may be caused by nongenetic (ie, gender, age, ethnic origin, hepatic and/or renal function, and emotional status) and genetic factors.5–9 Genetic variations, such as single nucleotide polymorphisms (SNPs), in genes involved in the pharmacodynamics and pharmacokinetics of opioids may lead to interindividual differences in response to opioid treatment.10 These genes can encode metabolic enzymes, drug transporters, receptors, or intracellular targets, such as transcription factors.
Among the genes involved in the pharmacodynamics of opioids, the μ-opioid receptor gene (OPRM1) has been investigated in different pharmacogenetic studies. OPRM1 codes for the μ-opioid receptor, which is the main target of both endogenous and clinically relevant opioids, such as morphine and fentanyl. The general aim of this review was to collect and organize the available information on the OPRM1 118A>G SNP, trying to correlate the predicted changes in the receptor protein with its activity at the molecular level and to better understand the relationship between the latter and clinical manifestations.
Molecular consequences of the 118A>G polymorphism (in vitro studies)
Effect of 118A>G on mechanisms related to an acute exposure to an opioid
The μ-opioid receptor belongs to the rhodopsin family of the G protein-coupled receptors (GPCRs) and consists of an extracellular N-terminus, seven transmembrane helices, three extra- and intracellular loops, and an intracellular C-terminus. The human OPRM1 is located on chromosome 6q24-q25 and spans over 200 Kb, with at least 9 exons and 19 different splice variants under the control of multiple promoters, and comprises more than a hundred SNPs.11,12 In particular, 118A>G (SNP database [dbSNP] Accession No rs1799971) has been the most studied variant in the pharmacogenetic research on opioid drugs. This SNP is located in the exon 1 of the gene and consists of the substitution of an adenine (A) with a guanine (G) that in turn, causes the amino acid exchange at position 40 of the μ-opioid receptor protein from asparagine to aspartic acid (N40D), leading to the loss of a N-glycosylation site in the extracellular region of the receptor.13 The variant allele (118G) has a frequency of 27%–48% in Asians, 11%–17% among Caucasians, 2.2% in African Americans, and 0.8% in sub-Saharan Africans (dbSNP Short Genetic Variations database of the American National Center for Biotechnology Information; NCBI, Bethesda, MD, USA, Accessed Dec 1, 2012), thus, it is carried sufficiently often to be clinically interesting for opioid therapy. Despite the fact that many authors have provided evidence for a biological function of this variant (summarized below), a recent meta-analysis showed that OPRM1 118A>G has little clinical relevance.14 The reasons for the discrepancy between functional experimental and clinical observational studies are not yet understood.
The receptor can be activated by both endogenous ligands, such as β-endorphins (the peptide derived from the precursor pro-opiomelanocortin), and opioid drugs (ie, morphine, fentanyl, and methadone). The acute agonist binding results in a conformational change of the receptor that triggers the G protein (particularly the pertussis toxin-sensitive Gi/Go proteins) activation/inactivation cycle. Hence, the signal transduction pathway includes the inhibition of adenylyl cyclase activity, a reduction in the voltage-gated calcium channel opening, and the stimulation of G protein-activated inwardly rectifying potassium channels, and finally results in a reduction of membrane potential, neuronal excitability, and neurotransmitter release.15 This inhibitory action on neurons, when located in the pain-processing circuits of the central nervous system, is responsible for the analgesic effects of opioids.
Few studies have evaluated the molecular consequences of the 118A>G polymorphism on the binding affinity of μ-opioid receptors and on μ-opioid receptor-evoked signal transduction pathways using in vitro methods. Using Syrian hamster adenovirus-12-induced tumor (AV-12) cells stably expressing the human μ-opioid receptor variants, Bond et al16 first showed that the 118A>G SNP affects the binding property of the μ-opioid receptor. Particularly, the variant receptor showed a threefold higher binding affinity for β-endorphins than the wild-type receptor (coded by the 118A allele), whereas it showed an unaltered binding affinity for methionine (Met)- and leucine (Leu) enkephalin (small endogenous opioid agonists), endomorphin-1 and -2 (selective endogenous μ-opioid receptor agonists), the μ-selective synthetic agonist [D-ala2, MePhe4, Gly-ol5]-enkephalin (DAMGO), dynorphin A (the endogenous ligand for k-opioid receptors, which also has some affinity for μ-opioid receptors), morphine, fentanyl, methadone, and naloxone (an opioid antagonist). Moreover, using Xenopus oocytes injected with in vitro–transcribed messenger (m)RNAs for the 118A or the 118G alleles, the authors showed that β-endorphins were three times more potent in agonist-induced activation of G protein-coupled potassium channels at the variant receptor compared with the wild-type.16 These observations are particularly intriguing, since they suggest that the 118G allele is associated to an increased sensitivity of μ-opioid receptors to the endogenous opioids. Hence, this gain of function for the 118G allele may be related to an interindividual variability in sensitivity to pain rather than to the interindividual variability in analgesic response to opioid drugs. Beyond analgesic therapy, this gain of function of the 118G allele may also affect the rewarding properties of nicotine and alcohol, which are mediated by the activation of the endogenous opioid system.17 Other in vitro studies did not confirm the results by Bond et al. In fact, in other studies of both COS (monkey kidney–derived) and HEK293 (human 293 embryonic kidney) cells, no differences in β-endorphin-binding activity between the variant and the wild-type receptors were detected.18,19
Studies evaluating the effects of the 118A>G SNP on the intracellular signaling cascades triggered by exogenous opioids binding to μ-opioid receptors have shown conflicting results. Both DAMGO and morphine were twofold more potent in inhibiting calcium channel currents in sympathetic neurons transfected with the 118G allele than in neurons expressing the wild-type receptors.20 However, in two different cell lines (HEK293 and AV-12 cells), stable expression of the 118G variant was associated to decreased agonist-mediated cyclic adenosine monophosphate (cAMP) signaling (and the half-maximal effective concentration [EC50]) for morphine, methadone, and DAMGO, but not for β-endorphin.21 These results suggest that the cellular environment may influence the phenotype associated with the variant receptor. More recently, in a study on human postmortem brain tissue, it was shown that the variant receptor is coupled to less efficient DAMGO-induced receptor signaling in the secondary somatosensory area, a pain-relevant brain region.22 Therefore, concerning exogenous opioids, these latter results show a loss of function of the variant receptor, probably resulting in reduced opioid drug effects.
Altogether, the results discussed above suggest that the variant receptor is associated with a decreased effect of exogenous opioids, while increasing the effect of endogenous opioids. Hence, carriers of the 118G allele should have an higher threshold to pain (due to increased sensitivity to endogenous opioids) but they may require increased μ-opioid drug doses in order to get analgesic effect and, subsequently, they may also be at risk for opioid-related side effects. On the other hand, the altered receptor sensitivity to endogenous/exogenous agonists should also be evaluated, considering that the expression of the receptor can be conditioned by the genotype (see below).
In conclusion, the 118A>G SNP has biological consequences at the molecular level that are strictly dependent on the experimental settings and opioid agonist used.
Effect of 118A>G on μ-opioid receptor desensitization
In the case of acute (minutes to hours) exposure to an opioid, desensitization of the μ-opioid receptors occurs, probably involving phosphorylation of the receptor and subsequent uncoupling of the receptor from its G protein, followed by internalization of the receptor.15 It has been suggested that the desensitization and internalization of μ-opioid receptors may play a role in the initiation of chronic tolerance.23
The OPRM1 118G variant seems to affect neither desensitization nor internalization of the μ-opioid receptor. In fact, it was shown that after prolonged treatment with either morphine, morphine-6-glucuronide (M6G) (an active metabolite of morphine with greater analgesic potency but reduced potency in inducing respiratory depression than morphine), or β-endorphin, both the variant and the wild-type receptors showed similar desensitization and resensitization time courses.19 Moreover, both the variant and wild-type receptors showed a robust internalization following DAMGO and β-endorphin administration, which was not observed when using morphine or M6G.19
Therefore, it can be concluded that interindividual differences in the occurrence of opioid-related tolerance are not explained (from a mechanistic point of view) by an effect of 118A>G on agonist-induced μ-opioid receptor endocytosis, desensitization, and resensitization.
Effect of 118A>G on mechanisms related to chronic exposure to opioids
The sustained administration of an opioid (days to weeks) leads to a progressive loss of the drug effect. This tolerance refers to a decrease in the apparent effectiveness of a drug with continuous or repeated agonist administration. Tolerance is surmountable with higher doses of the opioid and is reversible over time but, contrary to desensitization, it disappears over several weeks following the removal of the agonist, thus suggesting the existence of long-term adaptive mechanisms. During the state of tolerance, dependence is usually observed. Dependence represents a state of adaptation showed by receptor/drug class-specific withdrawal syndrome due to drug abstinence or the administration of an antagonist (ie, naloxone). As far as the cellular mechanisms underlying the state of tolerance and dependence, study results have been controversial. The main hypothesis concerns the adaptive counter-regulatory cellular change that occurs following chronic opioid exposure, namely the rebound increase in cellular cAMP levels produced by both upregulation and the increased activation of adenylyl cyclase. This takes place in the neurons of different brain areas, including those processing physical symptoms of withdrawal and reward (ie, the ventral tegmental area, locus coeruleus, and nucleus accumbens).23 This upregulation of the cAMP pathway observed after chronic morphine treatment triggers other intracellular adaptations, including the activation of cAMP-dependent protein kinase (PKA), increased levels of phosphorylated extracellular signal regulated kinase (ERK), and phosphorylation of the transcription factor, cAMP response element-binding protein (CREB), at serine (Ser)133.24–26 Altogether, such adaptive counter-regulatory changes impinge upon synaptic activity, altering its response to signaling and inducing a cell excitatory state (due to increased cation current through activation of PKA) and increased neurotransmitter release when the opioid treatment is discontinued. The final result is the occurrence of physical dependence to opioids, due to the sustained activation of bulbospinal pathways that increases the excitability of spinal dorsal horn pain transmission. The unpleasant feeling related to withdrawal may lead to a behavioral pattern characterized by compulsive drug seeking and drug taking (addiction).27 Indeed, increased CREB activity, together with changes in other transcription factors, has been hypothesized to induce changes in neuronal and synaptic morphology in the rewarding circuits of the brain, and these changes may be important for addiction.28,29
Given the role of cAMP, PKA, ERK, and CREB in the development of chronic opioid-related tolerance, dependence, and addiction, a recent study evaluated the effect of 118A>G SNP on these signaling molecules.30 In this paper, murine neuroblastoma Neuro 2 A cells stably transfected with cDNA containing the 118G variant did not show the upregulation of PKA activity and showed a differential response of ERK phosphorylation compared with cells transfected with the 118A variant, following chronic treatment (6 days) with 1 μM morphine. Hence, the 118A>G SNP may genetically determine patient sensitivity to tolerance and dependence.
Effect of 118A>G on the levels of expressed receptor
Different studies have evaluated the effect of polymorphism on the expression of OPRM1 and on the levels of μ-opioid receptor, using in vitro, ex vivo, and in silico methods. One analysis of 87 human brain tissue samples derived from autopsies, associated to in vitro experiments on Chinese hamster ovary (CHO) cells, showed that the amount of messenger (m)RNA transcribed from the 118G allele was twofold lower compared with the mRNA derived from the 118A allele. In addition, the levels of variant protein were tenfold lower compared with those of the wild-type receptor.31 Moreover, a lower cell-surface receptor binding site availability (Bmax) (measured with [3H]-DAMGO) was observed in both HEK293 and AV-12 cell lines stably expressing the 118G variant compared with cells expressing the 118A receptor.19,21 These effects on the expression of OPRM1 are particularly interesting. If the distribution and extension of the changes on OPRM1 expression differed at various anatomical sites, a variable loss of function of the 118G allele would occur. In turn, this might differently affect individual sensitivity to pain, opioid efficacy, and opioid-related side effects and reward, depending upon the brain area and the peripheral tissue involved. In this regard, a recent in vivo study using 11C-carfentanil positron emission tomography (PET) in smokers suggests that the decreased levels of μ-opioid receptor protein associated to the 118G allele may not be extended to the whole brain.32 The authors showed that smokers who were heterozygous for the 118G allele had lower levels of μ-opioid receptor availability compared with those who were homozygous for the 118A allele, in the amygdala, thalamus, and anterior cingulated cortex, but not in the striatum. Interestingly, these findings may partly explain the reduced nicotine reward, withdrawal, and relapse risk associated with the 118A>G polymorphism.33
As for the mechanisms underlying the effects of the 118A>G SNP on gene expression and levels of the receptor protein, this may be explained in different ways. Since the 118A>G SNP is not located in the gene promoter but in the coding region, the effects on gene expression may be due to another functional SNP that is in strong linkage disequilibrium with 118A>G. However, genotype and haplotype studies have failed to recognize any known SNP of sufficient frequency in linkage disequilibrium with 118A>G that may regulate gene expression.34,35 One in silico study showed that the substitution of the A with a G in position 118 of the OPRM1 gene was predicted to abolish three transcription factor binding sites while creating a novel exon splice enhancer as well as p53 and a zinc finger protein binding sites, thus suggesting a possible direct effect of 118A>G on gene expression and on the processing of heterogeneous nuclear RNA into mature mRNA.36 The effects of the 118A>G SNP on the level of OPRM1 mRNA may also be explained by a genetic–epigenetic interaction, as shown by a recent interesting paper.37 In fact, the substitution of an A with a G at gene position +118 introduces a new –C–phosphate–G– (CpG)-methylation site at position +117, which leads to an enhanced methylation of OPRM1 (at this site and downstream) and, in turn, this leads to a decreased gene expression.37 Altered levels of mRNA and receptor protein may be explained by the effects of the 118G on mRNA turnover, but this seems not be the case. After transcription into CHO cells of a complementary (c)DNA representing only the coding region of the OPRM1 and inhibition of transcription with actinomycin D, the mRNA turnover was the same for 118A and 118G variants.31 Using mfold software, which predicts mRNA secondary structure for different sequences, it was shown that the 118G variant demonstrated altered folding compared with other permutations that could affect mRNA stability.38 Finally, it has been hypothesized that the 118G variant may affect OPRM1 gene expression in addition to mRNA translation or posttranslational processing or turnover of the μ-opioid receptor protein.31 A recent paper described a role of the 118A>G SNP in posttranslational mechanisms.13 It suggested that N-glycosylation may affect receptor expression, since it plays an important role in correct folding of receptors in the endoplasmic reticulum and, hence, their sorting from the endoplasmic reticulum to the plasma membrane. It was shown that in CHO cells stably expressing the human μ-opioid receptor, the variant receptor had lower relative molecular mass than the wild-type one, which may be explained by a differential glycosylation status between the two receptors. Pulse-chain experiments on these cells revealed that the two expressed receptors have different protein stability, since the half-life of the mature form of the variant receptor (almost 12 hours) was shorter than that of the wild-type receptor (almost 28 hours).13
Summary of in vitro evidence
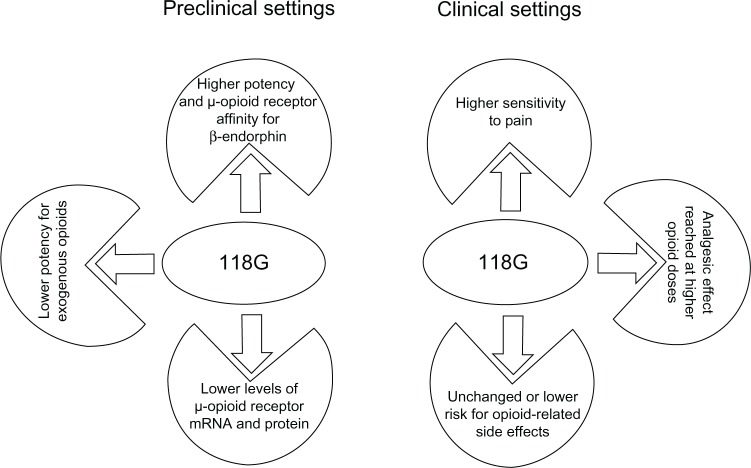
The 118A>G SNP has biological consequences at the molecular level, this being alteration of μ-opioid receptor binding affinity for β-endorphins, alteration in the signal transduction pathway downstream to μ-opioid receptors, and alteration of the levels of μ-opioid receptor mRNA and protein (Figure 1, Table 1). These effects have been strictly dependent on the experimental settings and opioid agonist used, which may explain the conflicting results.
Figure 1.
Consequences of OPRM1 118A>G SNP.
Abbreviations: mRNA, messenger RNA; SNP, single nucleotide polymorphism.
Table 1.
Molecular consequences of the 118A>G polymorphism (in vitro studies)
| Author | Experimental setting | Opioid analyzed | Results |
|---|---|---|---|
| Bond et al16 | • Radioligand-binding assay in AV-12 cells stably expressing the human μ-opioid receptor variants • Electrophysiological analysis in Xenopus oocytes injected with in vitro transcribed mRNAs for the 118A or the 118G alleles |
β-endorphins, Met-enkephalin, Leu-enkephalin, endomorphin-1, endomorphin-2, DAMGO, dynorphin A morphine, fentanyl, methadone, naloxone | • Compared to the wild-type the variant receptor had 3-fold higher binding affinity for β-endorphins but it showed unaltered binding affinity for all the other opioid analyzed • β-endorphins are 3 times more potent in agonist-induced activation of G protein–coupled potassium channels at the variant receptor compared to the wild-type |
| Befort et al18 | • Studies of ligand-binding and [35S]GTPγS binding assay using COS cells • Study of receptor desensitization using HEK293 cells |
β-endorphin, morphine, DAMGO, CTOP, heroin, Met-enkephalin, dynorphin A | • No differences in the agonist-binding property between the variant and the wild-type receptors • No differences in the agonist-induced stimulation between the variant and the wild-type receptors at the G protein level • No differences in receptor downregulation between the variant and the wild-type receptors |
| Beyer et al19 | HEK293 cells stably expressing the human μ-opioid receptor variants | β-endorphin, morphine, morphine-6-glucuronide | • Lower cell-surface receptor binding site availability in cells expressing the 118G variant compared to cells expressing the 118A one • No differences in the opioids-binding property between the variant and the wild-type receptors • No differences in opioid-induced receptor response between the variant and the wild-type receptors • Wild-type and variant receptors do not differ in their time courses of agonist-induced desensitization and resensitization |
| Margas et al20 | Evaluation of 118A>G SNP on intracellular signaling cascades downstream μ-opioid receptors using sympathetic neurons transfected with either 118G or 118A allele | Endomorphin-l, DAMGO, morphine, morphine-6-glucuronide | DAMGO and morphine were more potent in inhibiting calcium channel currents downstream variant μ-opioid receptor activation compared to the wild-type |
| Kroslak et al21 | • AV-12 cells stably expressing the human μ-opioid receptor variants • HEK293 cells stably expressing the human μ-opioid receptor variants |
β-endorphin, morphine, methadone, DAMGO | • Lower cell-surface receptor binding site availability in cells expressing the 118G variant compared to cells expressing the 118A one • Forskolin-induced cAMP-levels in 118G transfected cells were lower than in cells expressing the wild-type receptors • 118G variant was associated to a less potent agonist-mediated cAMP signaling for morphine, methadone and DAMGO, but not for β-endorphin |
| Oertel et al22 | Analysis of μ-opioid receptor expression, binding affinity and signaling in human post-mortem brain tissue from both the secondary somatosensory area and the ventral posterior part of the lateral thalamus | DAMGO | In secondary somatosensory area DAMGO was less efficient in agonist-induced receptor signaling in 118G carriers than 118A homozygous |
| Deb et al30 | Murine neuroblastoma Neuro 2A cells stably transfected with cDNA containing the 118G | Morphine | • No differences in pCREB levels upon both chronic and acute treatment are shown in both wild-type and mutants • 118G expressing cells exhibited lower basal level of pERK 1/2 and a higher level of PKA compared to the wild-type • Acute treatment causes inhibition of PKA and induction of pERK 1 activity for both the mutant and the wild-type (hence, both the variant and the wild-type triggered a similar response during acute treatment) • Differential regulation of PKA and pERK 1/2 occurs between the mutant and the wild-type cells during chronic treatment: PKA activity and pERK 1/2 level remained unaltered in cells expressing the mutant |
| Zhang et al31 | • Human autopsy brain tissue • Transfected CHO cells |
• 118A mRNA was 1.5–2.5 fold more abundant than the 118G mRNA in both human tissues and CHO cells. mRNA turnover was the same for 118A and 118G variants • The levels of the variant receptors were 10-fold lower than the wild-type in CHO cells |
|
| Huang et al13 | • Ex vivo studies on tissue derived from the knock-in mouse model 112A>G • CHO cells stably expressing the human μ-opioid receptor |
• The variant receptor had lower relative molecular mass than the wild-type one which was due to differences in N- glycosylation in both experimental settings • Pulse-chase studies in CHO cells revealed that the half life of the mature form of variant receptor was shorter than that of the wild-type |
Abbreviations: AV-12 cells, Syrian hamster adenovirus-12-induced tumor cell line; DAMGO, [D-ala2,MePhe4,Gly(ol)5]enkephalin; HEK293 cells, human 293 embryonic kidney cells; COS cells, cells being CV-1, simian, in Origin, and carrying the SV40 genetic material; CTOP, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; pCREB, phosphorylated cAMP response element-binding protein; pERK, phosphorylated extracellular signal regulated kinase; PKA, protein kinase A; CHO, Chinese hamster ovary.
Altogether, the OPRM1 118A>G SNP affects mechanisms related to individual sensitivity to pain, opioid efficacy, and opioid-related side effects, tolerance, dependence and reward. Particularly, carriers of the 118G allele should require increased μ-opioid drug doses in order to get analgesic effects, and once the analgesic effect is reached, they should show opioid-related side effects. Patients carrying the 118G allele may show either an unaltered or a higher sensitivity to pain compared with patients homozygous for the 118A allele, depending upon the individual endogenous opioid tone. In fact, the 118G allele has been associated both to low levels of μ-opioid receptors and to increased sensitivity to endogenous opioids. It has thus been related to two effects that may compensate each other.
Animal models for OPRM1 118A>G
Given the discrepant in vitro findings concerning the molecular consequences of 118A>G, a nonhuman primate orthologue model has been described and two different transgenic mouse models have been created for this SNP. Animal models may be particularly useful, to describe the SNP-related phenotypes following the administration of opioid drugs, and subsequently, to investigate the SNP-related biochemical and molecular changes by means of ex vivo experiments. In this regard in vivo studies may describe phenotypes, which are the final result of compensatory mechanisms at molecular level.
Nonhuman primate orthologue model
Rhesus monkeys are currently studied because of their physiological similarity to humans and are used in order to model a wide variety of human behaviors and neurobiological disorders. Moreover, they are also used as a model system of choice as preclinical platforms for both drug discovery and validation studies. A conserved polymorphism in the rhesus macaque consisting of a substitution of a cytosine (C) with a G at position 77 and resulting in a substitution of an arginine with a proline in the orthologue μ-opioid receptor has been suggested to be comparable with the human 118A>G SNP.39 In fact, both the 77C>G and 118A>G SNPs cause an amino acid change in the same region (N-terminal arm) of the orthologue μ-opioid receptors. However, unlike the human 118 A>G SNP, the 77C>G SNP does not affect the N-linked glycosylation sites of the rhesus monkey μ-opioid receptor.39 Monkeys carrying the 77G allele show physiological measures (stress response) as well as behavioral measures (predilection towards alcohol consumption) similar to humans carrying the 118G allele.39,40 Interestingly, the expression of 77G-containing rhesus monkey μ-opioid receptor clones in HEK293 cells was found to be related to a ~3.5-fold increase of μ-opioid receptor affinity for ß-endorphin but not for exogenous opioid ligands, similar to the data found in vitro by Bond et al concerning 118A>G SNP.16,39
Transgenic mouse models
Because of the high homology of the nucleotide (86.9%) and the amino acid sequences (92.3%) between mouse and human OPRM1 gene and μ-opioid receptor protein, respectively, Mague et al41 created a knockin mouse model in which a point mutation (112A>G) (equivalent to the human 118A>G variant) has been inserted in the mouse μ-opioid receptor gene. The 112A>G variant leads to the substitution of an asparagine with aspartic acid at position 38 of the amino acid sequence of the mouse receptor protein and causes the elimination of a N-glycosylation site, similarly to that in human 118A>G SNP. In these mice, behavioral assays and ex vivo molecular and biochemical experiments showed that mice homozygous for the 112G allele had a normal sensitivity to pain but showed a lower analgesic effect of subcutaneous morphine (1.0–2.0 mg/Kg) than mice homozygous for the 112 A allele.13,41,42 Both genotypes showed the same tolerance after a twice daily injection of morphine for 7 days. A sex × genotype interaction was evident in the behavioral responses associated to hedonia, with female mice homozygous for the 112G allele showing a reduction in both the rewarding properties of morphine and in the aversive components of naloxone-precipitated morphine withdrawal. These results are particularly intriguing since they suggest sex-dependent effects of 118A>G SNP on morphine-related behavior that are so far unexplored in clinical studies.41 Concerning the biochemical and molecular experiments on tissues derived from 112A>G mice, the presence of the 112G allele was shown to lead to a reduction in μ-opioid receptor gene expression and μ-opioid receptor protein levels in a brain region-specific manner (ie, the periaqueductal gray, hypothalamus, ventral tegmental area, and cortex were involved but not the hippocampus), though there were no alterations in μ-opioid receptor affinity for either β-endorphin or exogenous ligands such as morphine and naloxone.41,42
Recently, two transgenic mouse lines with humanized mouse genes for the μ-opioid receptor were created.43 In these mice, the first exon of the mouse μ-opioid receptor gene has been replaced by the corresponding human sequence carrying either the 118A or 118G allele. These mouse models, first characterized in the context of studies on alcoholism, share a specific neurochemical pattern with humans: both mice and humans carrying the 118G allele show an increase in dopamine release in the striatum (a brain area important for reward) in response to alcohol. In the case of alcohol administration, the μ-opioid receptor activation in the ventral tegmental area suppresses the activity of inhibitory gamma-aminobutyric acid (GABA)ergic interneurons, resulting in the disinhibition of dopamine neurons and increased dopamine release from their terminals in the ventral striatum.44 Increased striatal dopamine release is important for both alcohol and opioid drug reward, in rodents and in humans.45–47 Hence, it is possible that both humanized transgenic mice and humans carrying the 118G allele may be more prone to alcohol and opioid abuse; this hypothesis should be explored in appropriate experimental and clinical settings. Interestingly, the effects of the 118G allele on striatal dopamine release were not explained by altered affinity, signaling, or density of the μ-opioid receptors.43 Consequently, the authors hypothesized an alternative mechanism, ie, the loss of a glycosylation site, induced by the 118G allele, may alter the proper μ-opioid receptor oligomerization, which is critical for receptor trafficking.43 Hence, the 118G allele may have molecular consequences yet unexplored, and it is possible that other factors may concur in the 118G-related increase in striatal dopamine release in vivo.
Another study analyzing the same mouse model showed that morphine-mediated analgesia (on a hotplate assay) was significantly reduced in 118G homozygous humanized mice compared with 118A homozygous ones.48 Interestingly, sensory neurons isolated from 118G homozygous humanized mice showed a fivefold reduced potency of morphine, but not of fentanyl, in inhibiting voltage-gated calcium channels downstream to μ-opioid receptors compared with neurons isolated from 118A homozygous mice, despite the fact that the biophysical parameters (cell size, current density, and peak current amplitude potential) were the same in both group of neurons.48
Summary of in vivo evidence
The experiments on animal models show a gain of function of the 118G allele concerning responses mediated by the endogenous opioid system and confirm the loss of function of the 118G allele in the case of morphine administration, as suggested by in vitro studies (Table 2). However, there is no consensus on the underlying mechanisms of these effects. The theory of an exclusive 118G-related decrease in both OPRM1 expression and μ-opioid receptor protein levels may not explain such dual effect of the 118G allele.
Table 2.
Animal models for the 118A>G SNP
| Author | Animal model | Phenotypes | Results of either ex vivo or in vitro-related analyses |
|---|---|---|---|
| Miller et al35 | 770G Rhesus macaque. These non-human primates show the substitution of a C with a G at position 77 resulting in a substitution of an arginine with a proline in the orthologue μ-opioid receptor | • 77G macaques showed lower basal and ACTH-stimulated plasma Cortisol levels • 77G macaques had higher aggressive threat scores than 77C macaques |
The expression of 77G-containing rhesus monkey μ-opioid receptor clones in HEK293 cells was related to a ~3.5 fold increase of μ-opioid receptor affinity for β-endorphin but not for exogenous opioid ligands |
| Barr et al40 | 77C>G Rhesus macaque. These non-human primates show the substitution of a C with a G at position 77 resulting in a substitution of an arginine with a proline in the orthologue μ-opioid receptor | • 77G macaques had higher alcohol preference than 77C homozygous subjects • After naltrexone administration 77G carriers decreased their preference compared to vehicle and no longer differed from 77C homozygous subjects |
|
| Mague et al41 | 112A>G mice: knock-in mouse model in which a point mutation (substitution of a A with a G in position 112) has been inserted in the mouse μ-opioid receptor gene. The 112A>G leads to the substitution of an asparagine with aspartic acid at position 38 of the amino acid sequence of the mouse receptor protein and causes the elimination of a N- glycosylation site, similarly to human 118A>G SNP | • In contrast to 112AA mice, homozygous 112G mice did not exhibit hyperactivity following acute morphine administration. In contrast to 112AA mice, 112GG mice did not develop locomotor sensitization following repeated morphine administration • Hot-plate assay: 112GG mice showed lower analgesic effect of morphine than 112AA mice. At high temperatures 112GG mice showed both higher sensitivity to pain and lower analgesic effect of morphine than 112AA mice. Following 7 days of repeated morphine injections all animals showed the occurrence of tolerance • A sex x genotype interaction is evident in behavioral responses associated to hedonia, with female mice homozygous for the 112G allele showing a reduction in both the rewarding properties of morphine and in the aversive components of naloxone-precipitated morphine withdrawal |
• Ex vivo analyses of μ-opioid receptor expression and levels: mRNA was reduced in 112GG mice in several brain regions related to pain, stress and reward (PAG, hypothalamus, VTA NAc and cortex). Receptor protein levels were reduced in 112GG animals compared to 112AA mice, particularly in the thalamus • Variant receptors showed binding affinity for β-endorphin, morphine and naloxone comparable to the wild-type |
| Ramchandani et al43 | Humanized mouse genes for the μ-opioid receptor: in these mice the first exon of the mouse μ-opioid receptor gene has been replaced by the corresponding human sequence carrying either 118A or 118G allele | • Microdialysis on humanized mice: 118GG mice showed a 4-fold increase in DA release in striatum in response to alcohol compared to 118AA mice • PET study on humans using [11C]-raclopride (an antagonist of D2 receptors): 118AG subjects showed greater DA release than 118AA individuals in striatum after an intravenous alcohol challenge • This mouse model shares a specific neurochemical pattern with humans |
• Expression of humanized μ-opioid receptor mouse gene in CHO cells: variant receptors showed binding affinity for β-endorphin comparable to the wild-type • Electrophysiological analyses in isolated trigeminal ganglion neurons: there were no genotype differences in Ca++ currents in response to the μ-opioid receptor agonists DAMGO and β-endorphin • [3H]-DAMGO binding on mouse brain sections did not show genotype differences in receptor densities across a number of brain regions examined (ventral and dorsal striatum and VTA) |
| Mahmoud et al48 | Humanized mouse genes for the μ-opioid receptor: in these mice the first exon of the mouse μ-opioid receptor gene has been replaced by the corresponding human sequence carrying either 118A or 118G allele | 118AA mice had a greater analgesic response to morphine compared to 118GG mice | • Sensory neurons isolated from 118G homozygous humanized mice show a fivefold reduced potency of morphine, (but not of fentanyl) in inhibiting voltage-gated calcium channels downstream μ-opioid receptors compared with neurons isolated from 118A homozygous mice • Biophysical parameters (cell size, current density and peak current amplitude potential) were the same in both groups of neurons |
Abbreviations: ACTH, adrenocorticotropic hormone; HEK293, human 293 embryonic kidney cells; PAG, periaqueductal gray; VTA, ventral tegmental area; NAc, nucleus accumbens; DA, dopamine; PET, positron emission tomography; CHO, Chinese hamster ovary; DAMGO, [D-ala2,MePhe4,Gly(ol)5]enkephalin.
While there are many in vitro and clinical studies (see the following section, “Observed association/clinical consequences of the 118A>G SNP”) concerning the 118A>G SNP, only few data are available from animal models. The need to use transgenic mice has probably limited the number of available studies. Moreover, a further full characterization of the model is needed to determine whether the mice expressing the variants of the human OPRM1 118A>G SNP do compensate by modifying the expression of other murine receptors. On the other hand, such animal models could help to clarify whether the expression of the variants of a single opiate receptor subtype has relevant consequences on analgesia, rewarding systems, smooth muscle contractions, etc.
Observed association/clinical consequences of the 118A>G SNP
118A>G SNP and interindividual sensitivity to pain
The putative effects of the 118A>G SNP on interindividual variability in physical pain threshold are interesting, as they may affect analgesic request, thus contributing to variability in opioid consumption. Individual differences in sensitivity to pain have been examined in healthy subjects in experimental pain settings. The 118G allele has been associated to higher heat pain ratings and to lower pain tolerance threshold following electrical stimulation among healthy women but also to a decreased responsiveness to nociceptive stimuli in a cohort of healthy male and female patients.49–51 Moreover, the 118G allele has also been related to a higher pressure pain threshold although this last association was not confirmed in another study.49,52 Interesting, for the purpose of this review, are studies that have coupled data obtained in experimental pain protocols with clinical observations on the same patients upon surgery. Fukuda et al53 first analyzed the effect of the 118A>G SNP on both pain sensitivity and the analgesic effect of fentanyl in experimental pain settings (cold pressor-induced pain test), and following orofacial cosmetic surgery (mandibular sagittal split ramus osteotomy), they also evaluated the effect of the SNP on the efficacy of fentanyl delivered by patient controlled analgesia (PCA) in the same cohort of Japanese subjects. The authors showed that carriers of the 118G allele had a lower basal pain threshold and were more resistant to fentanyl effect during the experimental pain test. However, the authors failed to show an association of the SNP with postoperative fentanyl consumption. Interestingly, in another study with similar design, the same authors confirmed the associations previously observed during the cold pressor-induced pain test, but this time, they also showed an association between the presence of the 118G allele and higher fentanyl consumption in the first postoperative 24 hours.54 The discrepancy between the two subsequent studies may be due to the nonhomogeneity of the two cohorts (the second cohort showed a higher frequency of 118G carriers than the first one) and to differences in the type of nociceptive inputs (if the postoperative pain is not high, it is more difficult to find differences in opioids consumption). However, other genetic and nongenetic factors probably contributed to such discrepancy.
Altogether, these results obtained in experimental pain settings indicate that the 118G allele is related to a lower threshold of pain perception.
118A>G SNP and analgesic effect of opioid drugs
Most of the pharmacogenetic studies have evaluated the effect of 118A>G SNP on opioid consumption (doses) and/or pain control (assessed using different scales) during opioid treatment, in patients suffering from acute postoperative pain. However, these studies have given rise to conflicting results. Analyzing 120 Taiwanese patients following total knee arthroplasty, Chou et al55 showed that homozygous 118G patients consumed significantly more morphine (40.4 ± 22 mg) by PCA during the first 48 hours postoperatively compared with heterozygous (25.6 ± 11.7 mg) and homozygous patients for the 118A allele (25.3 ± 15.5 mg). The PCA device records the number of opioid doses demanded (number of times patients press the button in order to achieve better analgesia), and the pump is set with a lockout period within two different administered boluses of drug to avoid overdose. Interestingly, 118GG patients demanded more doses than 118AA and 118AG patients. There were no significant differences in perceived pain (measured on the visual analogue scale [VAS]) during opioid treatment among the three genotype groups.55 In order to exclude sex-related differences in morphine analgesia, in a similar study design, the same authors analyzed a cohort of 80 Taiwanese women and showed that homozygous 118G patients consumed significantly more morphine (33 ± 10 mg) by PCA in the first 24 hours following total abdominal hysterectomy compared with homozygous 118A patients (27 ± 10 mg).56 Similarly, an analysis of 588 female obstetric Chinese patients showed that 118GG subjects consumed more morphine by PCA (mean 9.4 mg; 95% confidence interval, 7.3–11.5 mg) than 118AG (mean 8.0 mg; 95% confidence interval, 6.9–9.1 mg) and 118AA ones (mean 5.9 mg; 95% confidence interval, 5.1–6.8 mg) and had a worse control of pain than the other two groups (as measured on VAS), following 24 hours from cesarean delivery.57 Hence, this study revealed a dose-dependent effect of the 118G allele, with each additional copy increasing the total need for morphine, despite physiological changes due to advanced stage pregnancy. However, the extension of the study to obstetric Malays and Indian patients did not confirm the effects of the 118G allele observed in the Chinese patients (despite that the 118G allele frequency in the study was higher in the Malay and Indian than in Chinese patients), further strengthening the concept that ethnicity is an important factor in pharmacogenetic studies.58 Analyzing a cohort of 74 patients with mixed ethnicity (White and Black subjects), no statistical significant association was observed between 118A>G SNP and morphine doses by PCA during the 24-hour postoperative (colorectal surgery) period, probably because of a lack of statistical power of the study.59 Moreover, another study evaluating the analgesic requirement with oral morphine following cesarean delivery failed to show any effect of the 118A>G SNP.60
As for fentanyl administration by PCA for acute postoperative pain, Fukuda et al53,54 showed both the absence and the presence of an association between the 118G allele and higher analgesic consumption in two subsequent studies on male and female Japanese patients, as described above (see the section “118A>G SNP and interindividual sensitivity to pain”). Moreover, a correlation between 118A>G genotypes and fentanyl consumption by PCA in the first 24 hours following surgery (homozygotes for 118G patients consumed more than did either heterozygous or homozygous for 118A) was observed in two different cohorts of Chinese gynecologic patients.50,61 In the case of fentanyl administration as a bolus injection following laparoscopic abdominal surgery, Chinese patients carrying the 118GG and 118AG genotypes had significantly less control of pain (higher VAS pain scores) than carriers of the 118AA genotype.62 The SNP was also associated to intrathecal fentanyl effective dose in half of patients (ED50) for labor analgesia, but in this case, women carrying the G allele were more sensitive to the opioid, requiring less analgesic drug.63 Moreover, another study failed to show a correlation of the SNP with analgesic effect (duration) of intrathecal fentanyl for labor analgesia.60
The 118G allele seems related to reduced analgesic effect of oxycodone, as measured during single electrical nerve stimulation (experimental pain).64 However one study evaluated the effect of 118A>G on analgesic efficacy of oxycodone in Caucasian patients affected by acute postoperative pain, and in this case, the authors showed lack of association.65
Also, in the case of opioid administration in order to treat chronic pain, studies have given conflicting results. Klepstad et al66 showed that oncologic Caucasian patients (mainly males) who were homozygous for the 118G allele required significantly higher oral morphine doses (225 ± 143 mg/24 h) compared with either heterozygous (66 ± 50 mg/24 h) or homozygous patients for the 118A allele (97 ± 89 mg/24 h). In the homozygous patients, the serum concentrations of morphine and its metabolites, M6G and morphine-3-glucuronide (M3G), were significantly higher than in other patients. Hence, the authors concluded that the loss of morphine efficacy may have been partly explained by the loss of analgesic contribution from M6G. In this regard, the 118G allele reduced the potency of M6G, assessed by pupil constriction in humans.67 However, the genetic variability in M6G efficacy is expected to contribute to the analgesic effect of morphine only during chronic morphine administration (as in the case of chronic pain treatment), since this metabolite is slowly transported through the blood–brain barrier and subsequently, has little effect after short-term exposure.68 Another study of oncologic Caucasian patients failed to report an association between the 118A>G SNP and variation in response to chronic morphine treatment.69 In this study, all patients had been treated with morphine as the first-line choice to control cancer-related pain, and those patients who had not tolerated the opioid (because of high pain scores at the Brief Pain Inventory [BPI] or side effects) were switched to oxycodone. There was no difference in the genotype or allelic frequencies for the 118A>G SNP between patients who had tolerated morphine and those who had switched. Janicki et al70 showed that the 118G allele may also alter the analgesic response to opioids (oxycodone, morphine, methadone, and fentanyl) in chronic noncancer pain patients. Finally, in a multicenter study, Lötsch et al71 evaluated the influence of the OPRM1 118A>G SNP on the analgesic efficacy of various opioids in a cohort of 352 patients on therapy for chronic pain of different origins. The authors observed a small dose-dependent effect for the 118G allele in reducing the daily control of pain (assessed on an 11-point numerical rating scale) during chronic opioid therapy, although also in this case, there could have been a bias in the selection of patients (different types of pain pathophysiology that could justify different response to opioids).
The comparison of all the described clinical studies is difficult, since they differ for the choice of the opioid used, the outcomes selected and the rating scale used to measure them, the characteristics of the cohorts analyzed (number of patients, ethnicity, gender, age, pathology, type of pain, and type of surgery), and the design of the study. Moreover, some studies have internal bias due to nonadherence of the genotype distribution to Hardy–Weinberg equilibrium or inadequate statistical power. All together, the majority of these clinical trials suggest a loss of function of the 118G allele concerning the analgesic effects of opioid drugs, in line with the data obtained by preclinical studies. A recent meta-analysis showed a weak association between 118GG genotype and increased opioid dosage requirements.14
Haplotypes containing the 118A>G SNP and analgesic effect of opioid drugs
Beyond the evaluation of the 118A>G SNP alone, the association among SNPs within the OPRM1 may be particularly interesting for the pharmacogenetic analysis of opioid treatment. Four substantial linkage disequilibrium blocks represented by 118A>G and four other tag SNPs (IVS2+G691C − rs2075572; IVS3+G5953A − rs599548; IVS3+A8449G − rs9384179; TAA+A2109G in 3′UTR) have been identified in human OPRM1. After having analyzed the influence of 118A>G alone, Hayashida et al72 evaluated whether the haplotypes created by the combination of these five tag SNPs could influence the epidural opioid (morphine or fentanyl) requirement, following major abdominal surgery in a cohort of 138 adult Japanese patients. The authors found that patients who were homozygous for the 118G allele required more analgesics during the first 24 hours postoperatively compared with heterozygous and homozygous patients for the 118A allele. Moreover, they found the existence of one 118G allele-containing haplotype, which was the most common haplotype (frequency of 44.6% ± 2.9%) in the population analyzed. Interestingly, the patients carrying this haplotype required more opioids in order to get the same analgesic effect compared with patients carrying the other existing haplotypes. The paper by Hayashida et al is particularly important, since it showed that haplotypes of OPRM1 gene polymorphisms were more significantly associated with analgesic requirements than the 118A>G SNP alone.
Combined effects of 118A>G in OPRM1 and 1947G>A in COMT with respect to analgesic effect of opioid drugs
Genetic variants in catechol-O-methyltransferase (COMT) may contribute to the interindividual variability in pain sensitivity since COMT enzymes metabolize neurotransmitters, such as dopamine and noradrenalin, that are involved in the control of pain signaling.73 In particular, the COMT 1947G>A SNP (rs4680), coding for a substitution of a valine (Val) with a Met in position 158 of the amino acid sequence (Val158Met), results in three- to fourfold reduced enzyme activity and has been associated with several pain phenotypes.74,75 PET studies showed that homozygous carriers of the COMT 1947A allele (low enzymatic activity) had increased μ-opioid receptor density in different brain regions.75 Some authors evaluated the effects of the association between the OPRM1 118A>G SNP and the COMT 1947G>A SNP on opioid request.76 A study on oncologic Caucasian patients showed that carriers of 1947GG and 1947AG genotypes (COMT) required 63% and 23%, respectively, higher morphine doses compared with carriers of the 1947AA genotype. Homozygous patients for the OPRM1 118G allele required a 93% higher morphine dose compared with those who were homozygous for the 118A allele.76 Interestingly, in the same study, the combination of 118A and 118G alleles in OPRM1 with 1947G and 1947A alleles in COMT showed that carriers of both 118AA and 1947AA genotypes required the lowest morphine dose (mean = 87 mg/24 h; 95% confidence interval, 57–116 mg) to achieve adequate analgesic effect, whereas those carrying neither 118AA nor 1947AA genotypes needed the highest opioid dose (mean = 147 mg/24 h; 95% confidence interval, 100–180 mg).
Association between 118A>G SNP and opioid-related side effects
The studies described above simultaneously evaluated the effect of 118A>G SNP on the analgesic efficacy of opioids and on the occurrence of opioid-related side effects. This paragraph will particularly focus on nausea/vomiting and respiratory depression, which are the most important and studied clinical side effects in pharmacogenetic trials and the primary cause of opioid poisoning.14,77 At the end of the paragraph, gastrointestinal side effects will be discussed (for other opioid-related side effects, see Table 3).
Table 3.
Clinical trials evaluating the 118A>G SNP
| Author | Type of pain | Drug | Routes of administration | No of patients | Ethnicity | Effect of 118A>G SNP on individual sensitivity to pain | Effect of 118A>G SNP on the analgesic effect of the drug | Effect of 118A>G SNP on the occurrence/ severity of drug-related side effects |
|---|---|---|---|---|---|---|---|---|
| Fillingim et al45 | EP: response to thermal, mechanical and ischemic pain | – | – | 167 healthy subjects | Mixed | • 118G carriers showed higher pressure pain threshold than 118AA individuals• The 118G allele was associated with lower pain ratings among man but higher pain ratings among women for heat pain | ||
| Lötsch et al51 | EP: evaluation of pain related cortical potential following a nociceptive stimulus (CO2) applied to the nasal mucosa | – | – | 45 healthy subjects | Caucasian | Amplitudes of nociceptive event-related potentials in carriers of 118G allele were half as high as dose of non-carriers | ||
| Huang et al52 | EP: pressor-induced pain test | – | – | 72 healthy women | Taiwanese | No differences due to genotype | ||
| Fukuda et al53 | • EP before surgery: cold pressor-induced pain test before and after opioid administration • AP due to orofacial surgery |
F | • EP: Bolus iv injection • AP: iv (PCA) |
280 | Japanese | 118G carriers showed a tendency (P = 0.064) for a lower EP threshold than 118AA individuals | • Carriers of 118G allele showed a lower analgesic effect of F during experimental pain • The 118A allele had no significant association with 24 h post-operative, perioperative, total perioperative F use and VAS at 3 and 24 h |
|
| Fukuda et al54 | • EP before surgery: cold pressor-induced pain test before and after opioid administration • AP due to orofacial surgery |
F | • EP: Bolus iv injection • AP: iv (PCA) |
60 | Japanese | 118G carriers are more sensitive to EP than 118AA | • Carriers of 118G allele showed a lower analgesic effect of F during EP• Homozygous 118G patients showed higher drug consumption in the first post-operative 24 hours | |
| Chou et al55 | AP due to total knee arthroplasty | Mo | iv (PCA) | 120 | Taiwanese | • No differences in perceived pain (VAS) during opioid treatment at any assessment throughout the first post-operative 24 h• Homozygous 118G consumed more drug than 118AG and 118AA in the first post-operative 48 hours • Homozygous 118G demanded more doses than 1118AG and 118AA in the first post-operative 48 hours |
• Patients were evaluated for: nausea (on a 4-point scale), pruritus (using the pain-track method), vomiting (assessed as events occurring in the first 24 h), sedation (Ramsey sedation score), respiratory rate and level of consciousness • There were no cases of respiratory depression • No influence of genotypes for adverse effects |
|
| Chou et al56 | AP due to total hysterectomy | Mo | iv (PCA) | 80 women | Taiwanese | • No differences in perceived pain (VAS) during opioid treatment at any assessment throughout the first postoperative 48 h • Homozygous 118G consumed more drug than 118AA in the first postoperative 24 hours • Homozygous 118G demanded more doses than 118AG and 118AA in the first post-operative 48 hours |
• Patients were evaluated for: nausea (on a 4-point scale), vomiting (assessed as events occurring in the first 24 h), sedation (Ramsey sedation score), respiratory rate and level of consciousness • There was a tendency for the 118A carriers for more vomiting episodes than 118G carriers • No influence of genotypes for other adverse effects |
|
| Sia et al57 | AP due to cesarean | Mo | iv (PCA) | 588 women | Chinese Singaporean | • Carriers of 118G allele self-administered more iv Mo (PCA) in the first postoperative 24 hours: each additional copy of the G allele increased total Mo intake of 1.87 mg • Carriers of 118G allele had a worse control of pain (VAS): each additional copy of the G allele increased pain scores by 0.51 units• Total Mo consumption was also influenced by patient’s age and paying status |
• Patients were evaluated for: nausea (on a 4-point scale), vomiting (assessed as events occurring in the first 24 h), CNS depression, respiratory depression and pruritus (on a 4-point scale) • No cases of respiratory and CNS depression • 118AA subjects were associated with the highest incidence of nausea than 118G carriers • No influence of genotypes for other adverse effects |
|
| Tan et al58 | AP due to cesarean | Mo | (PCA) | 994 women | Chinese, Malays, Indians | • 118GG subjects reported higher pain scores (VAS) and thus consumed more Mo than 118A carriers during the first post-operative 24 hrs • Pain score and Mo usage was also influenced by ethnicity, age and paying class • The 118G allelic frequency in the main ethnic groups considered were: 0.339 for Chinese, 0.49 for Malays and 0.441 for Indians. Separate analysis performed for each the 3 ethnic groups revealed that there was a statistically significant association between 118A>G SNP and Mo usage only for Chinese: every additional copy of the G allele results in an average increase in Mo usage by 0.025 mg |
• Patients were evaluated for: nausea (on a 3-point scale), vomiting (assessed as events occurring in the first 24 h), respiratory depression and pruritus (on a 3-point scale) • 118GG subjects reported both the lowest nausea scores and the lowest number of vomiting episodes |
|
| Coulbault et al55 | AP due to colorectal surgery | Mo | iv (PCA) | 74 | Mixed (black and white) | • The cumulative 24-hrs post-operative dose of Mo was influenced by age and regular use of psychotropic drugs before surgery • There was a trend for higher doses of Mo to be associated with 118G allele but it did not reach the statistic significance because of the low allele frequency of the 118G allelic variant |
• Patients were evaluated for: nausea (on a 4-point verbal scale), vomiting (assessed as events occurring in the first 24 h), respiratory depression and drowsiness • No cases of respiratory depression and drowsiness. • No association between 118A>G SNP and nausea and vomiting |
|
| Wong et al60 | Labor pain and AP due to cesarean | F (labor pain) Mo (postcesarean pain) | Intrathecal | 190 (labor pain) and 103 women (post-cesarean pain) | Mixed | • There was no difference in the median duration of intrathecal F labor analgesia between 118AA subjects and subjects carrying the 118G allele • 118A>G SNP did not influence either the supplemental analgesic requirements required to treat breakthrough pain within 72 h after intrathecal Mo analgesia or the duration of intrathecal Mo analgesia following cesarean delivery |
• In both studies patients were evaluated for: nausea (on a 4-point scale), vomiting and pruritus (on a 4-point scale) • Labor pain study: no association between the SNP and side effects • Post-cesarean study: the incidence of pruritus was lower for carriers of the 118G allele compare to 118AA subjects |
|
| Zhang et al50 | • EP before surgery: pain threshold and pain tolerance threshold following electric stimulation • AP due to hysterectomy or myomectomy |
F | (PCA) | 174 women | Chinese | • Carriers of the 118G allele had lower pain tolerance threshold following electrical stimulation than others • No differences in postoperative VAS pain scores |
118GG patients consumed significantly more F than other patients during the first 24-hrs post operatively. F consumption increased in accordance with the number of G alleles in the additive model | • Patients were evaluated for: nausea, vomiting (assessed as events occurring in the first 24 h) and sedation (Ramsay sedation score) • No association between 118A>G SNP and side effects |
| Zhang et al61 | AP due to hysterectomy or myomectomy | F | iv (PCA) | 165 women | Chinese | 118GG patients consumed significantly more F than other patients during the first 24-hrs post operatively | Patients were evaluated for nausea and vomiting (on a 4-point scale): no association between 118A>G SNP and side effects | |
| Wu et al62 | AP due to laparoscopic abdominal surgery | F | iv bolus injection | 189 | Chinese | Carriers of the 118G allele had significantly worse control of pain (VAS) 15 and 30 minutes after a bolus injection of F | • Patients were evaluated for respiratory depression (minute expiratory volume, end-tidal carbon dioxide concentration and respiratory rate) • No effect of the 118A>G SNP on the incidence of respiratory depression |
|
| Landau et al63 | Labor pain | F | Intrathecal | 223 women | Caucasian and Asians | 118AA women required significantly more F than carriers of the 118G allele | Patients were evaluated for pruritus (on a 4-point scale): no association | |
| Zwisler et al64 | EP: electrical nerve stimulation and cold pressure test before and after drug administration | O | Oral | 33 healthy subjects | • No volunteers had the genotype 118GG • 118AG subjects required a higher dose of O to show adequate pain control during the single electrical stimulation but not during the cold pressor test |
Patients were evaluated for dizziness, tiredness/drowsiness, nausea/vomiting, itching, reduced ability to keep focus: carriers of the 118G allele had a reduced ability to keep focus compared with the wild-type carriers | ||
| Zwisler et al65 | AP due to thyroidectomy or mastectomy or hysterectomy | O | iv (PCA) | 266 | Caucasian | No association between 118A>G SNP and the nonresponder rate, the need for rescue medication, O consumption, any of the pain measurement (NRS) | Patients were evaluated for sedation, tiredness/drowsiness, nausea/vomiting, skin itching: no association | |
| Klepstad et al66 | CCP | Mo | Oral | 207 | Caucasian | • 118AG patients had lower control of pain (BPI) than others • 118GG patients received significantly higher daily Mo doses compared to wild-type patients • The serum concentrations of Mo, M6G and M3G were significantly higher in 18GG patients |
Patients were evaluated for nausea/vomiting, constipation, fatigue, dyspnea, sleep disturbances, loss of appetite and cognitive function: no association | |
| Ross et al69 | CCP | Mo | 162 | Caucasian | • This study evaluated the contribution of 118A>G SNP to variability in responses to Mo treatment (good control of pain or need to switch to O): there were no significant differences in allelic and genotype frequencies for the SNP between responders and nonresponders • Also 6 haplotypes containing the 118A>G SNP were evaluated: no differences were observed in haplotype frequencies between switchers and responders |
One of the reasons for switch to O was the occurrence of drowsiness, hallucinations/confusion, nightmares, nausea/vomiting, myoclonus, pruritus: no significant differences in allelic and genotype frequencies for the 118A>G SNP between responders and nonresponders | ||
| Janicki et al70 | • AP due to abdominal surgery • CNCP |
AP: Mo CNCP: O, Mo, Me and F | CNCP: oral or transdermal or intrathecal | • AP: 101 • CNCP: 127 |
Mixed | • AP: no association between the118A>G SNP and total administered dose of Mo during the post-operative stay. No association between the SNP and average postoperative pain scores (11-point verbal NRS) • CNCP: no association between the SNP and opioid usage (expressed in Mo equivalents) • Comparison of the cohort of patients affected by chronic pain with the cohort comprising of AP patients: 118G allele is less common in chronic pain patients, particularly in those requiring higher doses of analgesics (thus, the 118G allele seems protective against chronic pain and may alter the response to opioid analgesics in chronic pain patients) |
||
| Lötsch et al71 | CCP and CNCP | F, Mo, O, Me and othersa | Transdermal, oral, iv, sc | 352 | Caucasian | The daily control of pain (on a 11-point NRS) was worse in carriers of the 118G allele than 118AA patients | Patients were evaluated for nausea/vomiting, constipation, tiredness and fatigue: no association | |
| Kolesnikov et al78 | AP due to prostatectomy or hysterectomy | Mo | (PCA) | 102 | Caucasian | • The 1l8A>G SNP was not associated with Mo consumption in the first postoperative 48-hrs. The 118A>G SNP was not associated with the average post-operative pain score • The combination of OPRM1 118A>G and COMT G I947A SNPs was associated with significant variability in drug consumption in the first postoperative 48 hrs |
• Patients were evaluated for nausea, sedation (Edmonton Symptom Assessment Scale), vomiting (number of events reported), respiratory depression and level of consciousness: the 118G allele was “protective” against morphine-induced nausea and sedation in the first postoperative 24 hrs • The combination of OPRM1 118A>G and COMT G1947A SNPs was associated with significant variability in scores for nausea and vomiting in the first post-operative 24 hrs |
Note:
Buprenorphine, dihydrocodeine, hydromorphone, piritramid, tilidine, tramadol.
Abbreviations: SNP, single nucleotide polymorphism; EP, experimental pain; AP, acute pain; CCP, chronic cancer pain; CNCP, chronic noncancer pain; F, fentanyl; Mo, morphine; O, oxycodone; Me, methadone; iv, intravenous; PCA, patient controlled analgesia; sc, subcutaneous; VAS, visual analogue scale; NRS, numerical rating scale; BPI, brief pain inventory; M6G, morphine-6-glucuronide; M3G, morphine-3-glucuronide; CNS, central nervous system.
As for nausea and vomiting, studies have given inconsistent results. In fact, some studies have reported a lack of association, in the case of acute postoperative pain treatment with morphine, oxycodone, and fentanyl, and in the case of chronic administration of morphine and other opioids.50,55,56,59,61,65,66,71 Others showed a “protective” effect of the 118GG genotype, the 118G allele, and the combination of 118AG (OPRM1) and 1947GA (COMT) genotypes on the occurrence of nausea and vomiting during postoperative morphine PCA.57,58,78 These contradictory results may be due to differences in the rating scales used to evaluate side effects among all the studies. Anyway, a recent meta-analysis confirmed the weak protective effect of the 118GG genotype against the occurrence of nausea.14
As for respiratory depression, following a bolus injection of fentanyl in the postoperative period, Chinese patients carrying the 118GG and 118AG genotypes had significantly less control of pain (higher VAS pain scores) but showed the same opioid effect on respiratory function compared to carriers of the 118AA genotype.62 A loss of analgesic effect due to the presence of the 118G allele but unmodified capability to induce respiratory depression was also observed following M6G administration in healthy volunteers during an experimental pain setting.79 However, a few pharmacogenetic studies showed that carriers of the 118G allele, even those receiving higher opioid doses, were not more prone to severe respiratory depression.55,57,66
Opioids have important effects upon all aspects of gastrointestinal function, and it has been estimated that 40%–95% of patients treated with opioids develop constipation.1 Interestingly, only two of the clinical studies described above considered the effects of 118A>G on constipation, showing lack of association.66,71
Association between 118A>G SNP and opioid-related dependence and rewarding property
Different studies have evaluated the 118A>G SNP as candidate for a genetic contribution to the risk of dependence on and the rewarding property of substances involving the activation of the endogenous opioid system, such as nicotine and alcohol.80–84 Altogether these trials have shown conflicting results. In fact, the 118G allele has been reported as either a risk or a protective factor for substance dependence, whereas some studies showed the lack of association.
As for opioid-related dependence, the majority of studies evaluated the effects of the 118A>G SNP on heroin dependence. Heroin is a semisynthetic compound that directly activates μ-opioid receptors when metabolized to morphine in the body. Here too, studies have shown conflicting results in the case of association between the 118G allele and heroin dependence, showing positive associations (in Swedish, in Chinese, and in Indian subjects), negative associations (in Hispanics and in Asians), or no associations (in Chinese subjects).16,30,85–90
Moreover, two meta-analyses showed lack of association between the 118A>G SNP and opioid dependence.89,91
Summary of clinical evidence
Determining the appropriate dose and achieving adequate analgesia without inducing adverse effects would be the breakthrough in the context of pain therapy. Pharmacogenetics may help in achieving this final purpose. As for pharmacogenetic studies evaluating the 118A>G SNP, the results obtained by some clinical trials (as summarized in Table 3) have suggested that patients carrying the 118G allele may be more sensitive to pain and that they may require higher opioid doses to get the analgesic response of the drug compared with carriers of the 118A allele. Despite the increased opioid consumption, nausea, vomiting, and constipation did not vary between carriers of the 118G allele and carriers of the 118A allele in many studies.55,56,59,61,65,66 Some studies of acute pain patients even showed that within the range of opioid doses leading to the adequate control of pain (VAS < 4 or numeric rating scale score < 4), the presence of the 118G allele was protective against gastrointestinal side effects.57,58,78 Although side effects were not systematically listed in all the analyzed studies, the available data show that within the range of opioid doses leading to the adequate control of pain (VAS < 4 in acute pain studies or average BPI pain scores < 4 in chronic pain studies), the 118G allele was not associated with the occurrence of severe respiratory depression.55,57,66 As for other opioid-related side effects (see Table 3), some studies evaluated the effects of 118A>G on the occurrence of pruritus, either showing a lack of association, or the protective effect of the 118G allele.55,57,58,60,63,69 Interestingly, Kolesnikov et al78 showed that carriers of the 118G allele reported significantly lower levels of sedation (evaluated by Edmonton Symptom Assessment Scale) compared with 118A homozygous patients. Moreover, the influence of the 118A>G SNP on the development of opioid dependence is still unclear. In order to draw final conclusions, future clinical studies should particularly investigate the influence of this SNP on opioid-related side effects. To date, the analysis of 118A>G SNP alone seems to have a poor clinical (predictive) utility.
Conclusion
The description of 118G-related phenotypes during clinical studies has revealed that the 118A>G SNP does not have the same influence on all opioid effects (Table 4). In fact, at standard opioid doses, carriers of the 118G allele do not show analgesic effects of the opioids, whereas they do show the same opioid-induced respiratory depression as carriers of the 118A allele. At increased doses, carriers of 118G allele show clinically adequate control of pain, but they are not more at risk of gastrointestinal side effects and severe respiratory depression than carriers of the 118A allele (Figure 1). In this regard, studies of the μ-opioid receptor gene in homozygous and heterozygous knockout mice suggest the existence of a functional μ-opioid receptor “reserve” that varies among the different neuronal populations controlling distinct opioid-related effects.92 Since the 118G allele results in decreased μ-opioid receptor levels, it may differentially affect opioid functions and drug response in the various target organs. In regard to the complexity of the scenario, the μ-opioid receptor-mediated functions depend upon the agonist used, and the same ligand can trigger different intracellular signaling pathways, depending upon the neuronal population considered.93 The 118G allele may affect signaling pathways that are specific for some μ-opioid receptor agonists and that are located in specific neuronal circuits. Moreover, due to the existence of different opioid receptor subtypes, the loss of function of the variant μ-opioid receptors might not be particularly relevant to a certain final phenotype, or it may unbalance the relation between various opioid receptor-mediated events. Finally, mechanisms beyond the opioid system may occur and counterbalance the loss of function of variant μ-opioid receptors in specific neuronal circuits in vivo.
Table 4.
Concluding summary
| • The 118A>G single nucleotide polymorphism (SNP) in OPRM1 results in amino acidic substitution at position 40 from asparagine to aspartic acid (N40D) that probably causes the loss of a N-glycosylation site in the extracellular region of the receptor. The 118G allele has a frequency of 27%–48% in Asians, of 11%–17% among Caucasians, of 2.2% in African-Americans and of 0.8% in Sub-Saharan Africans. |
| • In vitro experiments show that the variant receptors are associated to higher binding affinity and potency of the endogenous ligand β-endorphin, but, conversely, to lower potency of exogenous opioid ligands (i.e. morphine). The variant receptor was also less expressed than the wild-type. |
| • In vivo studies confirmed the higher binding affinity of the variant receptor for endogenous ligands and a lower potency of exogenous opioids observed in vitro. Transgenic mice carrying the variant allele show a lower analgesic effect of morphine compared to the wildtype. |
| • Studies on humans show that the effect of 118A>G SNP on interindividual sensitivity to pain and analgesic response to opioid is slight and not always confirmed. Despite patients carrying the 118G allele may require higher opioid doses to get the analgesic response of the drug compared to carriers of the 118 A allele they are not more at risk of opioid-related side effects. To date the analysis of 118A>G SNP alone seems to have a poor clinical (predictive) utility. |
| • Description of 118G-related phenotypes during clinical studies reveals that the 118A>G SNP has not the same influence on all opioid effects. The characteristics of variant μ-opioid receptors controlling gastrointestinal, respiratory and other opioid-related effects should be explored in future preclinical studies. |
| • Pain is a complex experience: the interaction of multiple genes, each with a small individual effect, in addition to emotional and environmental factors may influence opioid efficacy in clinical settings. Evaluation of the combined effects of OPRM1 118A>G and SNPs in other pain-related genes, as well as studies of 118A>G containing haplotypes emerge as intriguing tools in pharmacogenetics of opioids. |
As for genetic factors underlying the interindividual variability in analgesic responses, the clinical phenotypes may be the final result of the simultaneous interaction of genetic variants in genes related to receptors, transporters, and metabolizing enzymes of opioids, as shown by Bianchi et al94 in a particular case report. Consistent with a polygenic model for the complex phenotypes of pain-related traits, 118A>G may also interact with genetic variants in genes related to the physiological control of the pain signal, as in the case of the SNPs in COMT.76
Another interesting point to consider is whether the 118A>G SNP, in addition to altering the analgesic response to opioids, may also alter opioid-induced hyperalgesia. Different reviews underscored the importance of the problem of hyperalgesia, examining preclinical and clinical models, but no data were provided regarding the role of the 118A>G SNP in this.95,96 As for the clinical data, it has been suggested that endogenous opioid-mediated hyperalgesia (ie, stress-induced hyperalgesia) and the 118A>G SNP may contribute to pain symptoms in a particular condition that is recovery after sexual assault.97
This complexity strongly limits the predictive value of the 118A>G polymorphisms in the individual patient and has prevented its recommendation as a clinical tool for prescribing opioid drugs in pain therapy.98
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(Suppl 2):S105–S120. [PubMed] [Google Scholar]
- 2.Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490–518. doi: 10.1016/j.ejpain.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25(9):2133–2150. doi: 10.1185/03007990903120158. [DOI] [PubMed] [Google Scholar]
- 4.Zacny JP, Lichtor JL, Flemming D, Coalson DW, Thompson WK. A dose-response analysis of the subjective, psychomotor and physiological effects of intravenous morphine in healthy volunteers. J Pharmacol Exp Ther. 1994;268(1):1–9. [PubMed] [Google Scholar]
- 5.Glare PA, Walsh TD. Clinical pharmacokinetics of morphine. Ther Drug Monit. 1991;13(1):1–23. doi: 10.1097/00007691-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Macintyre PE, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1996;64(2):357–364. doi: 10.1016/0304-3959(95)00128-X. [DOI] [PubMed] [Google Scholar]
- 7.Cepeda MS, Farrar JT, Roa JH, et al. Ethnicity influences morphine pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2001;70(4):351–361. [PubMed] [Google Scholar]
- 8.Ozalp G, Sarioglu R, Tuncel G, Aslan K, Kadiogullari N. Preoperative emotional states in patients with breast cancer and postoperative pain. Acta Anaesthesiol Scand. 2003;47(1):26–29. doi: 10.1034/j.1399-6576.2003.470105.x. [DOI] [PubMed] [Google Scholar]
- 9.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103(1):156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Allegri M, De Gregori M, Niebel T, et al. Pharmacogenetics and postoperative pain: a new approach to improve acute pain management. Minerva Anestesiol. 2010;76(11):937–944. [PubMed] [Google Scholar]
- 11.Shabalina SA, Zaykin DV, Gris P, et al. Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18(6):1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasternak GW. Molecular insights into mu opioid pharmacology: From the clinic to the bench. Clin J Pain. 2010;26(Suppl 10):S3–S9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang P, Chen C, Mague SD, Blendy JA, Liu-Chen LY. A common single nucleotide polymorphism A118G of the μ opioid receptor alters its N-glycosylation and protein stability. Biochem J. 2012;441(1):379–386. doi: 10.1042/BJ20111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter C, Lötsch J. Meta-analysis of the relevance of the OPRM1 118A > G genetic variant for pain treatment. Pain. 2009;146(3):270–275. doi: 10.1016/j.pain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Jordan B, Devi LA. Molecular mechanisms of opioid receptor signal transduction. Br J Anaesth. 1998;81(1):12–19. doi: 10.1093/bja/81.1.12. [DOI] [PubMed] [Google Scholar]
- 16.Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108(3):183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276(5):3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- 19.Beyer A, Koch T, Schröder H, Schulz S, Höllt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89(3):553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 20.Margas W, Zubkoff I, Schuler HG, Janicki PK, Ruiz-Velasco V. Modulation of Ca2+ channels by heterologously expressed wild-type and mutant human micro-opioid receptors (hMORs) containing the A118G single-nucleotide polymorphism. J Neurophysiol. 2007;97(2):1058–1067. doi: 10.1152/jn.01007.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103(1):77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 22.Oertel BG, Kettner M, Scholich K, et al. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009;284(10):6530–6535. doi: 10.1074/jbc.M807030200. [DOI] [PubMed] [Google Scholar]
- 23.Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5(1):60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Fan P, Jiang Z, Diamond I, Yao L. Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol. 2009;76(3):526–533. doi: 10.1124/mol.109.057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narita M, Ioka M, Suzuki M, Narita M, Suzuki T. Effect of repeated administration of morphine on the activity of extracellular signal regulated kinase in the mouse brain. Neurosci Lett. 2002;324(2):97–100. doi: 10.1016/s0304-3940(02)00141-6. [DOI] [PubMed] [Google Scholar]
- 26.Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem. 1992;58(3):1168–1171. doi: 10.1111/j.1471-4159.1992.tb09377.x. [DOI] [PubMed] [Google Scholar]
- 27.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 28.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10(3):201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 29.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78(3):637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 30.Deb I, Chakraborty J, Gangopadhyay PK, Choudhury SR, Das S. Single-nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu-opioid receptor and may contribute to the genetic risk for addiction. J Neurochem. 2010;112(2):486–496. doi: 10.1111/j.1471-4159.2009.06472.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 32.Ray R, Ruparel K, Newberg A, et al. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci USA. 2011;108(22):9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman C, Wileyto EP, Patterson F, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4(3):184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- 34.Luo X, Kranzler HR, Zhao H, Gelernter J. Haplotypes at the OPRM1 locus are associated with susceptibility to substance dependence in European-Americans. Am J Med Genet B Neuropsychiatr Genet. 2003;120B(1):97–108. doi: 10.1002/ajmg.b.20034. [DOI] [PubMed] [Google Scholar]
- 35.Hoehe MR, Köpke K, Wendel B, et al. Sequence variability and candidate gene analysis in complex disease: association of mu opioid receptor gene variation with substance dependence. Hum Mol Genet. 2000;9(19):2895–2908. doi: 10.1093/hmg/9.19.2895. [DOI] [PubMed] [Google Scholar]
- 36.Pang GS, Wang J, Wang Z, Goh C, Lee CG. The G allele of SNP E1/A118G at the mu-opioid receptor gene locus shows genomic evidence of recent positive selection. Pharmacogenomics. 2009;10(7):1101–1109. doi: 10.2217/pgs.09.63. [DOI] [PubMed] [Google Scholar]
- 37.Oertel BG, Doehring A, Roskam B, et al. Genetic-epigenetic interaction modulates μ-opioid receptor regulation. Hum Mol Genet. 2012;21(21):4751–4760. doi: 10.1093/hmg/dds314. [DOI] [PubMed] [Google Scholar]
- 38.Johnson AD, Zhang Y, Papp AC, et al. Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: detection through allelic expression imbalance in human target tissues. Pharmacogenet Genomics. 2008;18(9):781–791. doi: 10.1097/FPC.0b013e3283050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9(1):99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- 40.Barr CS, Chen SA, Schwandt ML, et al. Suppression of alcohol preference by naltrexone in the rhesus macaque: a critical role of genetic variation at the micro-opioid receptor gene locus. Biol Psychiatry. 2010;67(1):78–80. doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci USA. 2009;106(26):10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. Reduced expression of the μ opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–184. doi: 10.1016/j.neuroscience.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramchandani VA, Umhau J, Pavon FJ, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89(2):649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 45.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- 46.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boileau I, Assaad JM, Pihl RO, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoud S, Thorsell A, Sommer WH, et al. Pharmacological consequence of the A118G μ opioid receptor polymorphism on morphine- and fentanyl-mediated modulation of Ca2+ channels in humanized mouse sensory neurons. Anesthesiology. 2011;115(5):1054–1062. doi: 10.1097/ALN.0b013e318231fc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Chang YZ, Kan QC, et al. Association of human micro-opioid receptor gene polymorphism A118G with fentanyl analgesia consumption in Chinese gynaecological patients. Anaesthesia. 2010;65(2):130–135. doi: 10.1111/j.1365-2044.2009.06193.x. [DOI] [PubMed] [Google Scholar]
- 51.Lötsch J, Stuck B, Hummel T. The human mu-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. Behav Neurosci. 2006;120(6):1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- 52.Huang CJ, Liu HF, Su NY, et al. Association between human opioid receptor genes polymorphisms and pressure pain sensitivity in females*. Anaesthesia. 2008;63(12):1288–1295. doi: 10.1111/j.1365-2044.2008.05760.x. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda K, Hayashida M, Ide S, et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. 2009;147(1–3):194–201. doi: 10.1016/j.pain.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda K, Hayashida M, Ikeda K, Koukita Y, Ichinohe T, Kaneko Y. Diversity of opioid requirements for postoperative pain control following oral surgery – is it affected by polymorphism of the μ-opioid receptor? Anesth Prog. 2010;57(4):145–149. doi: 10.2344/0003-3006-57.4.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou WY, Yang LC, Lu HF, et al. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50(7):787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 56.Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105(2):334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109(3):520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 58.Tan EC, Lim EC, Teo YY, Lim Y, Law HY, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Mol Pain. 2009;5:32. doi: 10.1186/1744-8069-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79(4):316–324. doi: 10.1016/j.clpt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Wong CA, McCarthy RJ, Blouin J, Landau R. Observational study of the effect of mu-opioid receptor genetic polymorphism on intrathecal opioid labor analgesia and post-cesarean delivery analgesia. Int J Obstet Anesth. 2010;19(3):246–253. doi: 10.1016/j.ijoa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Yuan JJ, Kan QC, Zhang LR, Chang YZ, Wang ZY. Study of the OPRM1 A118G genetic polymorphism associated with postoperative nausea and vomiting induced by fentanyl intravenous analgesia. Minerva Anestesiol. 2011;77(1):33–39. [PubMed] [Google Scholar]
- 62.Wu WD, Wang Y, Fang YM, Zhou HY. Polymorphism of the micro-opioid receptor gene (OPRM1 118A > G) affects fentanyl-induced analgesia during anesthesia and recovery. Mol Diagn Ther. 2009;13(5):331–337. doi: 10.1007/BF03256337. [DOI] [PubMed] [Google Scholar]
- 63.Landau R, Kern C, Columb MO, Smiley RM, Blouin JL. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139(1):5–14. doi: 10.1016/j.pain.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zwisler ST, Enggaard TP, Noehr-Jensen L, et al. The antinociceptive effect and adverse drug reactions of oxycodone in human experimental pain in relation to genetic variations in the OPRM1 and ABCB1 genes. Fundam Clin Pharmacol. 2010;24(4):517–524. doi: 10.1111/j.1472-8206.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 65.Zwisler ST, Enggaard TP, Mikkelsen S, et al. Lack of association of OPRM1 and ABCB1 single-nucleotide polymorphisms to oxycodone response in postoperative pain. J Clin Pharmacol. 2012;52:234–242. doi: 10.1177/0091270010397729. [DOI] [PubMed] [Google Scholar]
- 66.Klepstad P, Rakvåg TT, Kaasa S, et al. The 118A > G polymorphism in the human mu-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48(10):1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 67.Lötsch J, Skarke C, Grösch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12(1):3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Lötsch J, Kobal G, Geisslinger G. No contribution of morphine-6-glucuronide to clinical morphine effects after short-term administration. Clin Neuropharmacol. 1998;21(6):351–354. [PubMed] [Google Scholar]
- 69.Ross JR, Rutter D, Welsh K, et al. Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J. 2005;5(5):324–336. doi: 10.1038/sj.tpj.6500327. [DOI] [PubMed] [Google Scholar]
- 70.Janicki PK, Schuler G, Francis D, et al. A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain. Anesth Analg. 2006;103(4):1011–1017. doi: 10.1213/01.ane.0000231634.20341.88. [DOI] [PubMed] [Google Scholar]
- 71.Lötsch J, von Hentig N, Freynhagen R, et al. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenet Genomics. 2009;19(6):429–436. doi: 10.1097/fpc.0b013e32832b89da. [DOI] [PubMed] [Google Scholar]
- 72.Hayashida M, Nagashima M, Satoh Y, et al. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008;9(11):1605–1616. doi: 10.2217/14622416.9.11.1605. [DOI] [PubMed] [Google Scholar]
- 73.Argoff C. Mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27(10):2019–2031. doi: 10.1185/03007995.2011.614934. [DOI] [PubMed] [Google Scholar]
- 74.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158 met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 76.Reyes-Gibby CC, Shete S, Rakvåg T, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130(1–2):25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 78.Kolesnikov Y, Gabovits B, Levin A, Voiko E, Veske A. Combined catechol-O-methyltransferase and mu-opioid receptor gene polymorphisms affect morphine postoperative analgesia and central side effects. Anesth Analg. 2011;112(2):448–453. doi: 10.1213/ANE.0b013e318202cc8d. [DOI] [PubMed] [Google Scholar]
- 79.Romberg RR, Olofsen E, Bijl H, et al. Polymorphism of mu-opioid receptor gene (OPRM1:c.118A > G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102(3):522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Kendler KS, Chen X. The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct. 2006;2:28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ray R, Jepson C, Patterson F, et al. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 2006;188(3):355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- 82.Perkins KA, Lerman C, Grottenthaler A, et al. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behav Pharmacol. 2008;19(5–6):641–649. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, Ray R, Faith M, et al. Nicotine abstinence-induced cerebral blood flow changes by genotype. Neurosci Lett. 2008;438(3):275–280. doi: 10.1016/j.neulet.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Zwaluw CS, van den Wildenberg E, Wiers RW, et al. Polymorphisms in the mu-opioid receptor gene (OPRM1) and the implications for alcohol dependence in humans. Pharmacogenomics. 2007;8(10):1427–1436. doi: 10.2217/14622416.8.10.1427. [DOI] [PubMed] [Google Scholar]
- 85.Bart G, Heilig M, LaForge KS, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9(6):547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drakenberg K, Nikoshkov A, Horváth MC, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA. 2006;103(20):7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szeto CY, Tang NL, Lee DT, Stadlin A. Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport. 2001;12(6):1103–1106. doi: 10.1097/00001756-200105080-00011. [DOI] [PubMed] [Google Scholar]
- 88.Tan EC, Tan CH, Karupathivan U, Yap EP. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport. 2003;14(4):569–572. doi: 10.1097/00001756-200303240-00008. [DOI] [PubMed] [Google Scholar]
- 89.Glatt SJ, Bousman C, Wang RS, et al. Evaluation of OPRM1 variants in heroin dependence by family-based association testing and meta-analysis. Drug Alcohol Depend. 2007;90(2–3):159–165. doi: 10.1016/j.drugalcdep.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi J, Hui L, Xu Y, Wang F, Huang W, Hu G. Sequence variations in the mu-opioid receptor gene (OPRM1) associated with human addiction to heroin. Hum Mutat. 2002;19(4):459–460. doi: 10.1002/humu.9026. [DOI] [PubMed] [Google Scholar]
- 91.Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 92.Sora I, Elmer G, Funada M, et al. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25(1):41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- 93.Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the μ-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63(4):1001–1019. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bianchi M, Fornasari D, Antonini RA, Beretta-Piccoli BT, Nava S, Neuenschwander H. The pharmacogenetics of morphine-induced analgesia: a case report. J Pain Symptom Manage. 2008;36(1):e10–e12. doi: 10.1016/j.jpainsymman.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 96.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–161. [PubMed] [Google Scholar]
- 97.Ballina LE, Ulirsch JC, Soward AC, et al. μ-Opioid receptor gene A118G polymorphism predicts pain recovery after sexual assault. J Pain. 2013;14(2):165–171. doi: 10.1016/j.jpain.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 98.Caraceni A, Hanks G, Kaasa S, et al. European Palliative Care Research Collaborative (EPCRC) European Association for Palliative Care (EAPC) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]