Abstract
Purpose
To describe the efficacy and safety of hydromorphone extended-release tablets (OROS hydromorphone ER) during dose conversion and titration.
Patients and methods
A total of 459 opioid-tolerant adults with chronic moderate to severe low back pain participated in an open-label, 2- to 4-week conversion/titration phase of a double-blind, placebo-controlled, randomized withdrawal trial, conducted at 70 centers in the United States. Patients were converted to once-daily OROS hydromorphone ER at 75% of the equianalgesic dose of their prior total daily opioid dose (5:1 conversion ratio), and titrated as frequently as every 3 days to a maximum dose of 64 mg/day. The primary outcome measure was change in pain intensity numeric rating scale; additional assessments included the Patient Global Assessment and the Roland–Morris Disability Questionnaire scores. Safety assessments were performed at each visit and consisted of recording and monitoring all adverse events (AEs) and serious AEs.
Results
Mean (standard deviation) final daily dose of OROS hydromorphone ER was 37.5 (17.8) mg. Mean (standard error of the mean [SEM]) numeric rating scale scores decreased from 6.6 (0.1) at screening to 4.3 (0.1) at the final titration visit (mean [SEM] change, −2.3 [0.1], representing a 34.8% reduction). Mean (SEM) change in Patient Global Assessment was −0.6 (0.1), and mean change (SEM) in the Roland–Morris Disability Questionnaire was −2.8 (0.3). Patients achieving a stable dose showed greater improvement than patients who discontinued during titration for each of these measures (P < 0.001). Almost 80% of patients achieving a stable dose (213/268) had a ≥30% reduction in pain. Commonly reported AEs were constipation (15.4%), nausea (11.9%), somnolence (8.7%), headache (7.8%), and vomiting (6.5%); 13.0% discontinued from the study due to AEs.
Conclusion
The majority of opioid-tolerant patients with chronic low back pain were successfully converted to effective doses of OROS hydromorphone ER within 2 to 4 weeks.
Keywords: chronic low back pain, noncancer pain, extended-release opioids, OROS hydromorphone ER, opioid rotation, conversion and titration
Introduction
Approximately 14% of the United States population suffers from chronic pain resulting from a widespread collection of etiologies.1,2 Pain is often an undertreated condition despite various treatment options and increased recognition of its impact on patients’ quality of life.3–5 Opioid analgesics are frequently prescribed for patients with moderate to severe pain.6,7 Around-the-clock opioid therapy with an extended-release (ER) formulation may benefit individuals requiring prolonged analgesia.
Drug plasma levels induced by ER opioids are more stable than immediate-release (IR) formulations, and plasma levels can remain within the therapeutic range for extended periods of time.8,9 These pharmacokinetic characteristics of ER opioids translate into clinical advantages for the patient, such as sustained analgesia and decreased incidence of certain adverse reactions.10–12 Patients may also experience more restorative sleep, which may decrease subsequent pain.13–15 Less frequent dosing regimens typical of ER opioids may offer greater convenience to patients, potentially increasing compliance to the treatment regimen.16,17 Decreasing the overall pill burden is attractive to patients with chronic pain, who are often taking a number of concomitant medications to manage other conditions.18
Opioids commonly used in the management of chronic pain include fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, and oxymorphone.19–28 Adverse reactions commonly associated with opioid analgesics include constipation, nausea, vomiting, dizziness, and somnolence.7,29,30 Gastrointestinal (GI)-related adverse reactions are of particular concern, especially constipation, which is reported by over 40% of patients treated with strong opioids and is a common reason for discontinuing opioid therapy.7,29,31 Recently published treatment guidelines suggest stool softeners, laxatives, and increased intake of fluids and fiber as prophylactic treatment prior to initiating opioid therapy.7
Hydromorphone, a semisynthetic mu-opioid agonist, has been widely used in the management of pain for over 80 years and is currently available in both IR and ER formulations.32 Compared with hydromorphone IR, once-daily hydromorphone ER tablets provide consistent drug plasma concentrations over 24 hours once a steady state has been achieved, with less peak-to-trough fluctuation.10,33 Hydromorphone is released at a controlled rate through an oral osmotic drug delivery system (OROS® Push-Pull™; Alza Corporation, Mountain View, CA, USA), which permits the once-daily dosing regimen.34,35 OROS hydromorphone ER is highly potent, with a 5:1 equianalgesic ratio to morphine,36–43 and exhibits a tolerability profile consistent with other strong opioid analgesics.44 The efficacy and safety of OROS hydromorphone ER has been established in both short- and long-term controlled trials in patients with chronic cancer and noncancer pain (including chronic low back pain [LBP], musculoskeletal pain, neuropathic pain, and other chronic pain conditions).42,43,45–47
Because patients exhibit variability in sensitivity to different opioids,7,48,49 clinicians often need to try several options before finding an agent that provides effective analgesia at a tolerable dose. In a retrospective chart review of patients with chronic noncancer pain, 36% of patients achieved an effective and tolerable dose of the first opioid prescription given; however, 45% required between two and five opioid trials to achieve a stable, effective opioid regimen.50 Whether early in the treatment process or after a period of chronic treatment when efficacy declines or adverse events (AEs) increase in response to dose escalation, many patients receiving long-term opioid therapy are likely to require rotation from one agent to another at some point.51 It is therefore essential for clinicians to understand the dosing parameters for converting patients from prior opioid therapy to any new opioid agent or formulation, as well as the expected safety and efficacy profile during dose titration.
A multicenter, double-blind, randomized, placebo-controlled trial in patients with chronic moderate to severe LBP showed that OROS hydromorphone ER was effective and well tolerated.41 Results from the double-blind phase of the study showed that the reduction in pain intensity was maintained over 12 weeks with continued use of OROS hydromorphone ER, and that this was significantly superior to placebo.41 Reported here are the efficacy and safety data obtained during the open-label conversion and titration phase of this trial, which reflects usual clinical practice and may provide useful insights for clinicians incorporating OROS hydromorphone ER into opioid rotation programs for patients on chronic opioid therapy.
Methods
Study design
This was the open-label conversion and titration phase of a double-blind, placebo-controlled, randomized withdrawal study of OROS hydromorphone ER in patients with chronic LBP (Figure 1). The conversion and titration phase lasted 2 to 4 weeks and consisted of up to five visits. The study protocol was approved by the institutional review boards at all centers and performed in accordance with Good Clinical Practice guidelines. All patients gave written informed consent prior to undergoing any study procedure.
Figure 1.
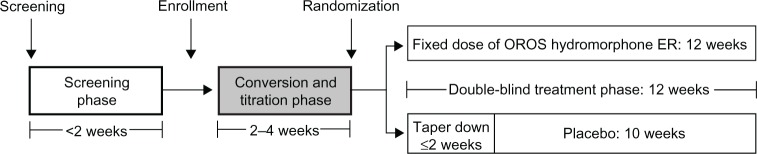
Study design, highlighting the conversion and titration phase.
Abbreviation: ER, extended release.
Patients
Study patients were 18 to 75 years of age and had moderate to severe chronic LBP for at least 20 days per month, for a minimum of 3 hours per day, for at least 6 months. Patients were required to have: (1) non-neuropathic (Class 1 and 2) or neuropathic (Class 3, 4, 5, or 6) LBP based on the Quebec Task Force Classification of Spinal Disorders; and (2) a daily opioid requirement of ≥60 mg oral morphine equivalent (≥12 mg of hydromorphone), but ≤320 mg oral morphine equivalent (≤64 mg hydromorphone) per day within the 2 months prior to the screening visit.
Exclusion criteria included: (1) an allergic reaction or hypersensitivity to opioids; (2) an active diagnosis of fibromyalgia, complex regional pain syndrome, acute spinal cord compression, back pain because of a secondary tumor, or pain caused by a confirmed or suspected neoplasm; (3) having undergone a surgical procedure for back pain within 6 months prior to the screening visit; (4) nerve or plexus block, including epidural steroid injections or facet blocks within 1 month prior to screening; or (5) preexisting severe narrowing of the GI tract secondary to prior GI surgery or GI disease resulting in impaired GI function.
Eligible patients were entered into the screening phase and trained on how to record their average pain in the past 24 hours in pain diaries every evening between 7:00 pm and 11:59 pm. Patients were required to document their daily pain intensity for two consecutive practice days using an eleven-point numeric rating scale (NRS), where 0 indicated no pain and 10 indicated the worst possible pain. Patients who met entrance requirements and enrolled in the study returned to the clinic within 14 days to begin the conversion and titration phase.
Dosing schedule and stabilization
During conversion, patients were converted to a dosage of OROS hydromorphone ER (Exalgo®; Mallinckrodt Brand Pharmaceuticals, Inc, Hazelwood, MO, USA) that was approximately 75% of the equianalgesic dose of their prior total daily opioid dose. Morphine conversion tables were used and assumed a morphine equivalent:hydromorphone potency ratio of 5:1. The lowest starting dose of OROS hydromorphone ER was 12 mg/day and the highest was 48 mg/day. OROS hydromorphone ER tablets, titrated to response and tolerability for each individual, were administered orally once daily in total daily doses of 12 mg, 16 mg, 24 mg, 32 mg, 40 mg, 48 mg, or 64 mg.
Titration was determined by daily pain intensity NRS scores and occurrence of AEs. OROS hydromorphone ER dosage could be titrated upward as frequently as every 3 days to the next available dosage (16 mg/day, 24 mg/day, 32 mg/day, 40 mg/day, 48 mg/day, or 64 mg/day of OROS hydromorphone ER) and twice per week. Only one dosage adjustment by telephone was allowed between each weekly visit. Decreases in OROS hydromorphone ER dosage were permitted only once, and not to below 12 mg/day. Dosages were not to exceed 64 mg/day during the course of the study. If the mean pain intensity NRS score during the last seven consecutive days (between visits) was ≤4 and the patient met stable dosing criteria within the 4-week timeframe, the patient was entered into the double-blind phase of the study.
Entry criteria into the double-blind phase included the following: final titrated doses of OROS hydromorphone ER that were ≥12 mg/day and ≤64 mg/day; patients were on the same dose without change for ≥7 consecutive days (stable dose period) and required a mean of ≤2 tablets of rescue medication per day; patients answered “yes” to the question, “Has this medication helped your pain enough so that you would continue to take the medication?”; and patients were free of adverse effects that were intolerable or that could impact their ability to complete the study. The final visit in the conversion and titration phase was considered the baseline visit of the double-blind phase for patients reaching stable doses and the termination visit for those who did not.
Efficacy analyses
The primary efficacy variable was the change in pain intensity NRS, recorded daily in patients’ diaries, from baseline to the final visit of the conversion and titration phase. The proportion of patients with 30% and 50% reductions in pain from screening to the final visit were calculated.52 Reduction in pain was calculated as: (reduction in pain intensity from screening to final visit/pain intensity at screening) × 100.
Additional efficacy assessments included mean changes from baseline in Patient Global Assessment (PGA) scores, providing patients’ overall impression of the study drug based on a five-point scale (1 = excellent, 2 = very good, 3 = good, 4 = fair, 5 = poor), as well as the Roland–Morris Disability Questionnaire (RDQ). The RDQ is a 24-item questionnaire used to evaluate patients’ ability to perform routine tasks, with scores ranging from 0 (highest ability) to 24 (lowest ability).
Safety analyses
The Clinical Opiate Withdrawal Scale (COWS), consisting of eleven questions to assess patients’ withdrawal symptoms, was completed at all visits by the investigators. Total scores range from 0 to 48, with higher scores indicating more severe withdrawal symptoms, and are grouped as mild (5–12), moderate (13–24), moderately severe (25–36), and severe (>36).53 Investigators determined if symptoms were attributed to an etiology other than opioid withdrawal. The Subjective Opiate Withdrawal Scale (SOWS), consisting of 16 questions to assess patients’ withdrawal symptoms, was also completed at all visits. Patients rated the degree to which they experienced each of the 16 symptoms (0 = not at all, 1 = a little, 2 = moderately, 3 = quite a bit, and 4 = extremely); total scores range from 0 to 64, with higher scores indicating more severe withdrawal symptoms.54
Safety assessments were performed at each visit and consisted of recording and monitoring all AEs and serious AEs (SAEs). An SAE was considered any medical occurrence at any dose of study medication that resulted in death, was life threatening, required inpatient hospitalization or prolonged existing hospitalization, resulted in persistent or significant disability or incapacity, or was a congenital anomaly/birth defect. Treatment-emergent AEs were summarized by event intensity (mild, moderate, severe, or not reported) and relationship to study drug.
To prevent constipation, patients were permitted to use prophylaxis, such as osmotic laxatives (ie, lactulose, sorbitol) and peristalsis-increasing agents (ie, senna, bisacodyl).
Rescue medication use
Throughout the study, IR hydromorphone hydrochloride tablets (2 mg, 4 mg, and 8 mg) were used as rescue medication for breakthrough pain. IR hydromorphone tablet strength was determined as between 5% to 15% of an individual patient’s daily dose of OROS hydromorphone ER. Rescue medication use was unrestricted for the first 3 days, but was restricted to two tablets per day after day 3. Overuse of rescue medication did not subject patients to discontinuation prior to randomization in the double-blind phase of the study.
Statistical analyses
Prior opioids were coded using the World Health Organization encoding dictionary, and the numbers and percentages of patients receiving each opioid were summarized. The primary population for the efficacy analyses was the intent-to-treat population. Weekly NRS scores were calculated from daily patient diary entries, and a weekly mean change from baseline was calculated for each patient. For a patient’s mean pain score in a given week to be included in the analysis, there had to be at least one daily pain intensity NRS score in the patient’s diary for the week. If a patient discontinued due to opioid withdrawal symptoms, the baseline pain intensity NRS score was carried forward to the final visit. For those who discontinued due to an AE, the pain intensity score at screening was carried forward to the final visit. If a patient discontinued due to lack of efficacy or other reasons (eg, administrative, withdrawal of consent), the last observation (mean pain score over the last week in the study), was carried forward to the final visit (last observation carried forward). Descriptive statistics for PGA and RDQ scores are presented by treatment group at each visit, and for changes from baseline at each visit.
Total scores were calculated for COWS and SOWS by visit. The scales were imputed with the mean of nonmissing items if up to 25% of the items in the scale were missing. Missing items on a scale were imputed using last observation carried forward methodology. The total scores at each visit and change from screening at each visit were summarized using descriptive statistics and stabilized OROS hydromorphone ER dose.
The mean number of rescue medication tablets used per day was compared for patients reaching stable doses of OROS hydromorphone during conversion and titration and those who discontinued using a t-test with unequal variances.
Results
Patients
Of the 806 patients screened for study entry, 459 met the inclusion criteria and were enrolled into the conversion and titration phase. The safety population included 447 patients who received ≥1 dose of OROS hydromorphone ER. Overall, 179 patients discontinued during the conversion and titration phase after receiving OROS hydromorphone (at 75% equianalgesic dose of their prior opioid), most commonly due to AEs (13.0%, 58 patients) and a lack of analgesic efficacy (12.5%, 56 patients).
The mean (standard deviation [SD]) age of patients in the safety population was 49.0 (10.43) years, and 6.0% of patients were ≥65 years of age. About half of the patients were male, and the majority were Caucasian (Table 1). Patients entered the trial with moderate to severe pain as indicated by a mean (SD) NRS score of 6.6 (1.8). Prior opioid medications included hydrocodone, oxycodone, morphine, fentanyl, methadone, tramadol, propoxyphene, hydromorphone, and oxymorphone.
Table 1.
Demographic and baseline characteristics
| Characteristic | OROS hydromorphone ER (N = 447) |
|---|---|
| Age, years | |
| Mean (SD) | 49.0 (10.43) |
| Age group, n (%) | |
| 18–64 years | 420 (94.0) |
| 65–75 years | 27 (6.0) |
| Sex, n (%) | |
| Male | 227 (50.8) |
| Race, n (%) | |
| Caucasian | 383 (85.7) |
| Black | 37 (8.3) |
| Other | 27 (6.0) |
| Weight, kg | |
| Mean (SD) | 90.33 (23.35) |
| Height, cm | |
| Mean (SD) | 170.95 (10.75) |
| Body mass index, kg/m2 | |
| Mean (SD) | 30.85 (7.36) |
| Etiology, n (%) | |
| Non-neuropathic low back paina | 276 (61.7) |
| Neuropathic low back painb | 167 (37.4) |
| Missing | 4 (0.9) |
| Prior opioid, n (%) | |
| Hydrocodone | 151 (33.8) |
| Oxycodone | 123 (27.5) |
| Morphine | 73 (16.3) |
| Fentanyl | 26 (5.8) |
| Methadone | 25 (5.6) |
| Tramadol | 22 (4.9) |
| Propoxyphene | 8 (1.8) |
| Hydromorphone | 8 (1.8) |
| Oxymorphone | 7 (1.6) |
Notes:
Class 1 or 2 based on the Quebec Task Force Classification of Spinal Disorders;
class 3, 4, 5, or 6 based on the Quebec Task Force Classification of Spinal Disorders.
Abbreviations: ER, extended release; N, total number; SD, standard deviation.
Conversion and titration
Sixty percent (n = 268) of patients successfully reached a stabilized dose of OROS hydromorphone ER within 4 weeks of screening. With the exception of patients previously receiving oxymorphone, there was a ≥50% success rate in conversion to OROS hydromorphone ER from all prior opioids (Table 2). The mean (SD) duration of exposure to OROS hydromorphone ER was 20.1 (9.58) days in the overall safety population (Table 3). Patients achieving a stable dose during conversion and titration had a mean (SD) duration of exposure of 23.4 (7.84) days (range, 8 to 47 days), versus 15.2 (9.84) days (range, 1–49 days) in patients discontinuing during titration. The total number of titration visits did not appear to influence patients’ ability to achieve a stabilized dose of OROS hydromorphone ER.
Table 2.
Patients with response by prior opioid compound
| Prior opioid compound | OROS hydromorphone ER n/N (%) |
|---|---|
| Hydromorphone | 6/8 (75.0) |
| Fentanyl | 19/26 (73.1) |
| Tramadol | 15/22 (68.2) |
| Hydrocodone | 95/151 (62.9) |
| Morphine | 42/73 (57.5) |
| Methadone | 14/25 (56.0) |
| Oxycodone | 68/123 (55.3) |
| Propoxyphene | 4/8 (50.0) |
| Oxymorphone | 3/7 (42.9) |
| Missing data | 2/4 (50.0) |
Abbreviations: ER, extended release; n, number; N, total number.
Table 3.
Duration of exposure
| Duration of exposure | OROS hydromorphone ER (N = 447) |
|---|---|
| Duration, daysa | |
| Mean (SD) | 20.1 (9.58) |
| Median (range) | 22.0 (1–49) |
| Duration rangeb, n (%) | |
| <1 week | 34 (7.6) |
| 1 to <2 weeks | 75 (16.8) |
| 2 to <3 weeks | 97 (21.7) |
| 3 to <4 weeks | 107 (23.9) |
| ≥4 weeksc | 134 (30.0) |
Notes:
Duration in days calculated as the difference between the first date medication was dispensed and the date of the last dose in the conversion and titration phase;
duration in weeks calculated as the difference between the first date medication was dispensed and the date of the last dose in the conversion and titration phase divided by seven;
last office visit, if necessary, occurred at week 4/day 29.
Abbreviations: ER, extended release; N, total number; SD, standard deviation.
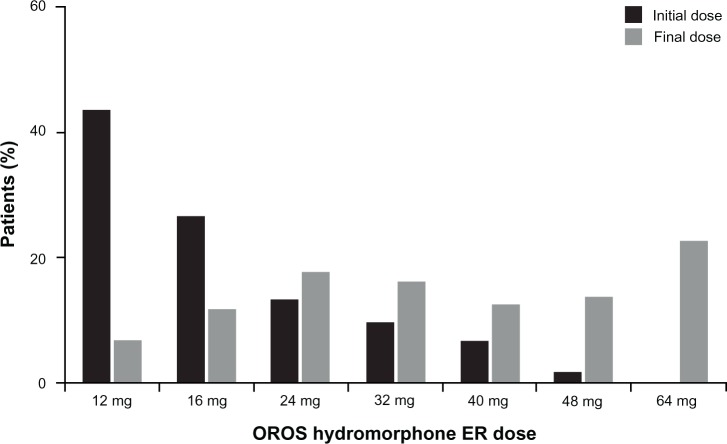
Approximately 43% of patients began the conversion and titration phase at a 12 mg dose of OROS hydromorphone ER, and the most common final daily dose was 64 mg (22.1%). The mean (SD) final dose of OROS hydromorphone ER was 37.5 (17.8) mg in the overall patient population, and was similar in patients reaching a stabilized dose or discontinuing during titration (37.8 [17.5] mg and 37.1 [18.2] mg, respectively). The distribution of initial and final doses of OROS hydromorphone ER in patients reaching a stabilized dose is shown in Figure 2; the final titration dose was 16 mg or higher for 94% of patients achieving a stabilized dose, and 32 mg or higher for 65% of patients.
Figure 2.
Distribution of initial and final doses of OROS hydromorphone ER in patients who achieved an effective dose.
Abbreviation: ER, extended release.
Efficacy
In the overall population, mean (standard error of the mean [SEM]) NRS score decreased from 6.6 (0.1) at screening to 4.3 (0.1) at the final visit of the conversion and titration phase (mean [SEM] change from screening to final visit, −2.3 [0.1]), representing a 34.8% reduction. Likewise, mean (SEM) PGA score improved from 3.6 (0.04) at visit 1 to 3.0 (0.05) at the final visit (overall mean [SEM] change, −0.6 [0.1]), and mean (SEM) RDQ scores showed an improvement from 14.0 (0.2) at screening to 11.2 (0.3) at the final visit (mean change [SEM], −2.8 [0.3]).
When patients achieving a stable dose and patients discontinuing during titration were analyzed separately, those achieving a stable dose showed greater improvement. Mean (SEM) change in NRS score during the conversion and titration phase was −3.2 (0.1) for patients achieving a stable dose (representing a 50% reduction from screening) versus −0.7 (0.2) for dropouts during titration (P < 0.001). Mean (SEM) NRS score at baseline of the double-blind phase or termination visit was 3.3 (0.1) and 6.1 (0.2) in patients achieving a stable dose versus dropouts, respectively. The number of titration visits did not appear to influence the overall change in NRS score. The mean (SEM) PGA score decreased from 3.6 (0.1) to 2.5 (0.1) during the conversion and titration phase for patients achieving a stable dose, while increasing from 3.6 (0.1) at visit 1 to 3.8 (0.1) at the termination visit in patients discontinuing during titration (P < 0.001 for change from baseline in patients achieving a stable dose versus dropouts). Patients achieving a stable dose also had a mean (SEM) change in RDQ of −4.3 (0.3) [from 13.5 (0.3) at screening to 9.3 (0.4) at baseline of the double-blind phase], compared with a mean (SEM) change of −0.4 (0.3) for dropouts during titration (P < 0.001).
Almost 80% of patients (213/268) achieving a stable dose had a ≥30% reduction in pain, compared with 21.9% (37/179) of dropouts during titration. Among patients achieving a stable dose who had a ≥30% reduction in pain, 64.3% (137/213) were taking ≥32 mg of OROS hydromorphone ER. Approximately 52% of patients achieving a stable dose (140/268) had a ≥50% reduction in pain (among whom 64% [90/140] were taking a dose ≥ 32 mg), compared with 7.7% of dropouts during titration (13/179).
The mean number of rescue medication tablets per day was 2.7 during the first 3 days of conversion and titration, and ,1 tablet per day by the time a stable dose of OROS hydromorphone ER was achieved. The daily mean (SD) number of rescue medication tablets in patients achieving a stable dose and in those discontinuing during titration was 1.5 (0.89) and 2.4 (1.3), respectively (P < 0.001).
Opioid withdrawal symptoms
Mean (SD) COWS decreased from 1.0 (1.9) at visit 1 to 0.7 (1.4) at the final visit. The ability to achieve a stabilized dose or starting doses of OROS hydromorphone ER did not influence the mean change in COWS. Similar to COWS, SOWS decreased in the overall patient population from 7.2 (8.1) to 4.3 (6.1) from visit 1 to the final visit, respectively. In patients achieving a stable dose, mean (SD) change in SOWS from visit 1 to baseline of the double-blind phase was −3.5 (6.4). In contrast, the mean (SD) change in patients discontinuing during titration was −1.4 (8.3) from visit 1 to the termination visit.
Safety and tolerability
Approximately 55% of patients (n = 247) experienced ≥1 AE. The most commonly reported AEs were constipation, nausea, somnolence, headache, and vomiting, and were consistent among those who did or did not achieve a stable dose during titration (Table 4). About 5% of patients reported drug withdrawal syndrome. Of patients who reported AEs, the majority (90.6%) experienced AEs assessed with a maximum severity of mild or moderate (57.3% and 33.3%, respectively). Compared with dropouts during titration, those achieving a stable dose were less likely to report AEs (51.9% versus 60.3%, respectively).
Table 4.
Most common adverse events in the safety population (>5%) overall and according to achievement of a stable dose
| Adverse event, n (%) | Safety population (N = 447) | Achieved a stable dose (n = 268) | Did not achieve stable dose (n = 179) |
|---|---|---|---|
| Any adverse event | 247 (55.3) | 139 (51.9) | 108 (60.3) |
| Constipation | 69 (15.4) | 43 (16.0) | 26 (14.5) |
| Nausea | 53 (11.9) | 27 (10.1) | 26 (14.5) |
| Somnolence | 39 (8.7) | 21 (7.8) | 18 (10.1) |
| Headache | 35 (7.8) | 19 (7.1) | 16 (8.9) |
| Vomiting | 29 (6.5) | 13 (4.9) | 16 (8.9) |
Abbreviations: n, number; N, total number.
Aside from patient sex, baseline demographics such as age and race did not impact the incidence of AEs. Female patients, however, experienced more AEs than male patients (60.5% versus 50.2%), and reported constipation (18.2% versus 12.8%), nausea (15.5% versus 8.4%), and vomiting (9.5% versus 3.5%) more frequently.
In total, 13.0% of patients (n = 58) discontinued from the study due to an AE. A small percentage of patients (4.3%) withdrew due to GI disorders. Approximately 4% of patients withdrew due to nervous system disorders, such as somnolence (1.8%) and headache (1.6%). Most AEs that led to discontinuation were considered to be possibly or probably related to the study drug.
OROS hydromorphone ER dosage was reduced in 6.9% of patients. Approximately 2% of patients required dose reductions due to AEs. The incidence of AEs in relation to OROS hydromorphone ER dosage strength is presented in Table 5. The 24 mg dose of OROS hydromorphone ER was associated with the greatest incidence of AEs (30.2%).
Table 5.
Summary of all AEs by OROS hydromorphone ER dose (safety population)
| Evaluationa | OROS hydromorphone ER doseb
|
||||||
|---|---|---|---|---|---|---|---|
| 12 mg (n = 192) | 16 mg (n = 268) | 24 mg (n = 265) | 32 mg (n = 235) | 40 mg (n = 200) | 48 mg (n = 167) | 64 mg (n = 103) | |
| Patients with any AE, n (%) | 47 (24.5) | 67 (25.0) | 80 (30.2) | 59 (25.1) | 44 (22.0) | 43 (25.7) | 30 (29.1) |
| Patients with any SAEc, n (%) | 1 (0.5) | 0 (0.0) | 5 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuation due to AEs, n (%) | 8 (4.2) | 9 (3.4) | 13 (4.9) | 8 (3.4) | 9 (4.5) | 8 (4.8) | 1 (1.0) |
| Total number of AEsd, n | 85 | 121 | 203 | 132 | 88 | 99 | 52 |
Notes:
An AE may be counted multiple times for an individual patient under these circumstances: dose at onset of AE and dose at the time the AE increased in intensity;
n is the number of patients who were exposed to each dose;
an AE of headache for Patient 018019 was marked as a serious AE;
each occurrence of an AE is counted (eg, multiple occurrences of the same AE in one patient are counted as multiple AEs).
Abbreviations: AE, adverse event; ER, extended release; n, number; SAE, serious adverse event.
GI-related AEs
GI-related AEs, specifically constipation, nausea, and vomiting, were most commonly reported (30.4%). Incidence of GI-related AEs was similar between patients who achieved a stable dose and those who discontinued during titration (29.9% versus 31.3%, respectively), and was similar across all doses (12–48 mg) of OROS hydromorphone ER. Over 90% of GI-related AEs were considered mild or moderate in severity.
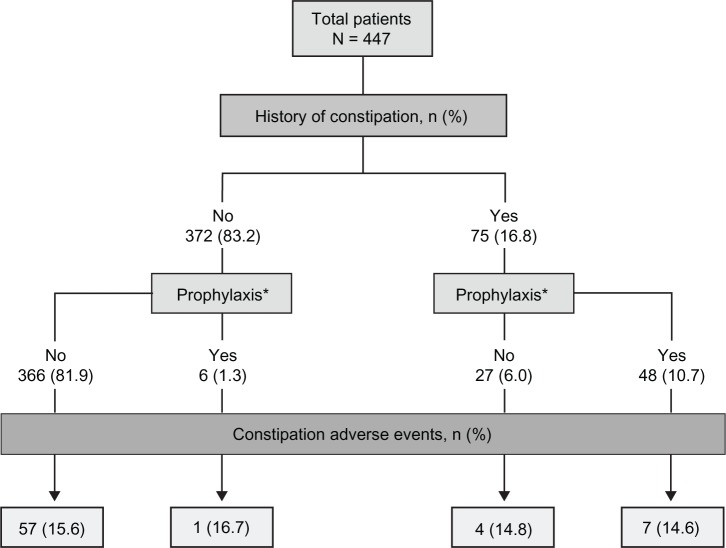
Constipation was reported by 69 patients (15.4%), 38 (8.5%) of whom received treatment for constipation during the conversion and titration phase. The incidence of constipation was similar in patients with or without a history of constipation (Figure 3). In patients with a history of constipation, eleven (14.7%) reported an AE of worsening constipation. In patients without a history of constipation, 58 (15.6%) reported an AE of constipation. The incidence of constipation was similar in patients who were or were not treated prophylactically for constipation (14.8% versus 15.5%, respectively) prior to the conversion and titration phase.
Figure 3.
Adverse events of constipation, by history of constipation and prophylaxis.
Note: *Prophylactic treatment for constipation included osmotic laxatives and peristalsis-increasing agents.
Abbreviations: N, total number; n, number.
Nineteen patients (4.3%) discontinued study participation because of GI-related AEs, most commonly due to nausea (1.8%), constipation (1.1%), and vomiting (0.9%). Additionally, gastritis and rectal hemorrhage each occurred in one patient (0.2%).
Treatment-related AEs and SAEs
Treatment-related AEs were reported by 43.0% of patients; 11.4% discontinued due to a treatment-related AE. The most commonly reported treatment-related AEs were constipation (14.3%), nausea (9.6%), somnolence (8.1%), headache (6.0%), and vomiting (4.5%). Five patients reported SAEs, none of which were determined by investigators to be related to OROS hydromorphone ER (Table 5). SAEs included meningitis, herpes, pneumonia, deep vein thrombosis, and diabetic ketoacidosis (one patient each). One patient experienced all of the following SAEs: hypokalemia, dizziness, nausea, and vomiting. No deaths occurred during the study.
Discussion
The objective of this study was to examine dosing parameters, efficacy, and safety in patients being converted to OROS hydromorphone ER from other ER opioid therapies. Results indicate that the majority of patients can be safely converted to OROS hydromorphone ER and titrated to effective doses within 2 to 4 weeks of treatment. The 5:1 morphine equivalent:hydromorphone conversion ratio used in this study is consistent with other published trials of OROS hydromorphone ER in patients with chronic cancer or non-cancer pain,36–43 supporting growing evidence for the use of this ratio in clinical practice. Although the 5:1 ratio indicates that hydromorphone ER is a potent opioid, it is important to note that the ratio is lower than the conversion ratio for oral hydromorphone IR, which has been reported to be as high as 8:1.55
Patients included in the current study had previously received other strong opioids, such as other formulations of hydromorphone, fentanyl, hydrocodone, morphine, methadone, oxycodone, propoxyphene (no longer available), and oxymorphone. An analysis of conversion success according to prior opioid therapy revealed that the proportion of patients able to achieve a stable dose of hydromorphone ER did not depend to any appreciable extent on the specific opioid from which they were converting. This has clinical implications in the context of opioid rotation, which is often necessary for patients with chronic pain who experience a decline in therapeutic efficacy with their current opioid or inadequate efficacy or tolerability during conversion and titration of a new opioid therapy.52 Results presented here lend additional support for the consideration of OROS hydromorphone ER as an appropriate option in well-selected patients rotating from any of the opioid therapies commonly used in clinical practice.
As reported previously, overall mean pain intensity was reduced by 50% with OROS hydromorphone ER treatment during the conversion and titration phase of this study, a reduction that was maintained in the active-treatment group during the 12-week double-blind phase.41 When the current analysis of the conversion and titration phase reported results separately for patients who did or did not achieve a stable dose of hydromorphone ER, those who successfully converted to OROS hydromorphone ER from prior opioid therapy experienced significantly greater reductions in pain intensity, as well as improved functional abilities and treatment satisfaction.
Approximately 80% of patients achieving a stable dose of OROS hydromorphone ER experienced at least a 30% pain reduction, which meets an accepted threshold of clinically meaningful pain relief.56,57 Furthermore, 52% of patients in this group experienced a ≥50% pain reduction. These reductions were maintained over time, as 60.6% and 42.4% of patients showed ≥30% and ≥50% reductions in pain, respectively, at the end of the double-blind phase of the trial.40
The safety profile of OROS hydromorphone ER is consistent with other strong (World Health Organization Step 3) opioids.31 As expected, the most common AEs during conversion and titration were GI-related, as well as somnolence and headache. GI-related AEs, specifically constipation, warrant further attention. Overall incidence of constipation was approximately 15%, which is substantially lower than what was reported in a systematic review of strong opioids (41%).31 The incidence of constipation did not vary according to a patient’s history of constipation or use of prophylactic medications, suggesting that OROS hydromorphone ER has a good GI tolerability profile under a variety of conditions. However, this study was not specifically designed to evaluate the true incidence of constipation or the effects of prophylactic or reactive management of constipation.
GI-related AEs were more common in female patients, as previously noted in the literature. Gender differences in AEs may be partially influenced by social factors that affect the way men and women communicate distress and perceive bodily experiences.58 Other baseline characteristics, including age, did not appear to influence the incidence of AEs. There was no strong evidence for an increased incidence of AEs at higher doses, although the data should be interpreted with caution because the duration of exposure to each dose was not accounted for in the analysis. In the ten patients who required a dose reduction due to AEs, there was no apparent relationship between the AE and OROS hydromorphone ER dose. The lack of a dose-response relationship for AEs was likely due to the individualized dosing strategy employed in the conversion and titration phase, as dose-dependent AEs are more likely to be seen with fixed dosing.59
There were no reports of abuse, overdose, or misuse during this trial. COWS and SOWS scores showed that most patients in the conversion and titration phase did not experience drug withdrawal, suggesting that the conversion and titration method used in this study was appropriate. It should be noted, however, that a small percentage of patients did report drug withdrawal syndrome as an AE.
This study had a number of limitations. Although the open-label, flexible-dose design of this phase of the study reflects usual clinical practice, there may have been factors in the design of this study that limited the ability of some patients to reach a stable dose of OROS hydromorphone ER. The time window for dose conversion and titration was limited to 2 to 4 weeks – a shorter time span than may be required in clinical practice to achieve an effective dose – and the maximum daily allowable dose of OROS hydromorphone ER was limited to 64 mg. The observation that 64 mg was the most common dose (taken by 22.1% of patients) at the final visit of the conversion and titration phase, and that 12.5% of patients discontinued due to lack of analgesic efficacy, suggests that the upper dose limit may not have been sufficient for a subset of patients. The mean final dose of OROS hydromorphone ER was approximately 38 mg/day in the conversion and titration phase of this study, which is lower than what has been reported in other studies of chronic noncancer and cancer pain (56.6 mg/day and 61.6 mg/day, respectively).39,40 In addition, the current study was performed in patients who were opioid-tolerant, and results may not be generalizable to opioid-naïve patients (it should be noted that OROS hydromorphone ER is indicated for use only in opioid-tolerant patients26). Conversion and titration of opioids should be implemented with particular caution in people without prior opioid exposure; medication should be initiated at a low dosage and titrated slowly to decrease the risk for adverse effects.7
In conclusion, the detailed analysis of results from this conversion and titration phase confirm the findings of previous studies evaluating the efficacy and safety of OROS hydromorphone ER.42,43,45–47 Data collected during this phase emphasize the need for individual titration of each patient to a stabilized dose of OROS hydromorphone ER. OROS hydromorphone ER was associated with clinically meaningful reductions in pain in the majority of patients and was generally well tolerated. GI tolerability was good; the rate of GI-related AEs was low across doses, and most were considered mild or moderate in severity. These findings suggest that OROS hydromorphone ER is an appropriate option in an opioid rotation program for the management of chronic pain.
Acknowledgments
This study was supported by Neuromed Pharmaceuticals, Inc. Technical editorial and medical writing support for the preparation of this manuscript was provided by Amanda McGeary, Synchrony Medical Communications, LLC, West Chester, PA, USA. Funding for this support was provided by Mallinckrodt Inc, a Covidien company, Hazelwood, MO, USA.
Footnotes
Disclosure
Portions of these data were included in a poster presentation at PAINWeek 2011, September 7–10, 2011, Las Vegas, NV, USA.
Dr Hale discloses that he has served as a consultant or on an advisory board for Cephalon, Covidien, Neuromed, and Purdue Pharma, in addition to serving on speakers’ bureaus for Covidien and Purdue Pharma. Dr Nalamachu discloses that he has served as a consultant, on an advisory board, or on a speakers’ bureau for, and received honoraria from, Cephalon, Covidien, Endo Pharmaceuticals, Lilly, and ProStrakan. In addition, Dr Nalamachu has received research grants from Covidien, Endo Pharmaceuticals, and ProStrakan. Dr Khan and Mr Kutch do not have conflicts of interest to disclose.
References
- 1.United States Census Bureau Preliminary annual estimates of the resident population for the United States, regions, states, and Puerto Rico: April 1, 2000 to July 1, 2010 (NST-PEST2010-01) [webpage on the Internet] 2002[updated Feb 2011]Washington, DC; United States Census Bureau; Available from: http://www.census.gov/popest/research/eval-estimates/eval-est2010.htmlAccessed October 22, 2012 [Google Scholar]
- 2.National Center for Health Statistics Health, United States, 2006. With Chartbook on Trends in the Health of Americans Hyattsville, MD: National Center for Health Statistics; 2006DHS Publication No 2006-1232. Available from: http://www.cdc.gov/nchs/data/hus/hus06.pdfAccessed March 8, 2012 [Google Scholar]
- 3.Haanpää ML, Backonja MM, Bennett MI, et al. Assessment of neuropathic pain in primary care. Am J Med. 2009;122(Suppl 10):S13–S21. doi: 10.1016/j.amjmed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Khouzam RH. Chronic pain and its management in primary care. South Med J. 2000;93(10):946–952. [PubMed] [Google Scholar]
- 5.McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123(2):119–130. doi: 10.3810/pgm.2011.03.2270. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Huffman LH, American Pain Society, American College of Physicians Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Fanciullo GJ, Fine PG, et al. American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell JR. Avinza – 24-h sustained-release oral morphine therapy. Expert Opin Pharmacother. 2004;5(2):469–472. doi: 10.1517/14656566.5.2.469. [DOI] [PubMed] [Google Scholar]
- 9.Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9(6):468–479. doi: 10.1111/j.1533-2500.2009.00320.x. [DOI] [PubMed] [Google Scholar]
- 10.Moore KT, St-Fleur D, Marricco NC, et al. Steady-state pharmacokinetics of extended-release hydromorphone (OROS hydromorphone): a randomized study in healthy volunteers. J Opioid Manag. 2010;6(5):351–358. doi: 10.5055/jom.2010.0032. [DOI] [PubMed] [Google Scholar]
- 11.Hays H, Hagen N, Thirlwell M, et al. Comparative clinical efficacy and safety of immediate release and controlled release hydromorphone for chronic severe cancer pain. Cancer. 1994;74(6):1808–1816. doi: 10.1002/1097-0142(19940915)74:6<1808::aid-cncr2820740625>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Bruera E, Sloan P, Mount B, Scott J, Suarez-Almazor M. A randomized, double-blind, double-dummy, crossover trial comparing the safety and efficacy of oral sustained-release hydromorphone with immediate-release hydromorphone in patients with cancer pain. J Clin Oncol. 1996;14(5):1713–1717. doi: 10.1200/JCO.1996.14.5.1713. [DOI] [PubMed] [Google Scholar]
- 13.McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther. 2008;15(4):312–320. doi: 10.1097/MJT.0b013e31818164f2. [DOI] [PubMed] [Google Scholar]
- 14.Mystakidou K, Clark AJ, Fischer J, Lam A, Pappert K, Richarz U. Treatment of chronic pain by long-acting opioids and the effects on sleep. Pain Pract. 2011;11(3):282–289. doi: 10.1111/j.1533-2500.2010.00417.x. [DOI] [PubMed] [Google Scholar]
- 15.Brennan MJ, Lieberman JA., 3rd Sleep disturbances in patients with chronic pain: effectively managing opioid analgesia to improve outcomes. Curr Med Res Opin. 2009;25(5):1045–1055. doi: 10.1185/03007990902797790. [DOI] [PubMed] [Google Scholar]
- 16.Fine PG, Mahajan G, McPherson ML. Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 2009;10(Suppl 2):S79–S88. doi: 10.1111/j.1526-4637.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 17.Pergolizzi JV, Taylor R, Jr, Raffa RB. Extended-release formulations of tramadol in the treatment of chronic pain. Expert Opin Pharmacother. 2011;12(11):1757–1768. doi: 10.1517/14656566.2011.576250. [DOI] [PubMed] [Google Scholar]
- 18.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Oramorph SR (morphine sulfate) Sustained-Release Tablets, CII [package insert] Newport, KY: Xanodyne Pharmaceticals, Inc; 2006. [Google Scholar]
- 20.MS Contin (morphine sulfate controlled-release) Tablets CII [package insert] Stamford, CT: Purdue Pharma LP; 2009. [Google Scholar]
- 21.Kadian (morphine sulfate) Extended-Release Capsules, for oral use, CII [package insert] Morristown, NJ: Actavis Elizabeth LLC; 2010. [Google Scholar]
- 22.Opana ER (oxymorphone hydrochloride) Extended-Release tablets, for oral use, CII [package insert] Chadds Ford, PA: Endo Pharmaceuticals, Inc; 2008. [Google Scholar]
- 23.Avinza (morphine sulfate extended-release capsules) CII [package insert] Bristol, TN: King Pharmaceuticals, Inc; 2008. [Google Scholar]
- 24.OxyContin (oxycodone hydrochloride controlled-release) Tablets CII [package insert] Stamford, CT: Purdue Pharma LP; 2010. [Google Scholar]
- 25.Embeda (morphine sulfate and naltrexone hydrochloride) Extended-Release Capsules, for oral use, CII [package insert] Bristol, TN: King Pharmaceuticals; 2009. [Google Scholar]
- 26.Exalgo (hydromorphone HCl) Extended-Release Tablets (CII) [package insert] Hazelwood, MO: Mallinckrodt Brand Pharmaceuticals, Inc; 2012. [Google Scholar]
- 27.Duragesic (fentanyl transdermal system) CII [package insert] Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009. [Google Scholar]
- 28.Vicodin (hydrocodone bitartrate and acetaminophen tablets, USP) [package insert] North Chicago, IL: Abbott Laboratories; 2011. [Google Scholar]
- 29.Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–1369. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Murray A, Hagen NA. Hydromorphone. J Pain Symptom Manage. 2005;29(5 Suppl):S57–S66. doi: 10.1016/j.jpainsymman.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Drover DR, Angst MS, Valle M, et al. Input characteristics and bioavailability after administration of immediate and a new extended-release formulation of hydromorphone in healthy volunteers. Anesthesiology. 2002;97(4):827–836. doi: 10.1097/00000542-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Turgeon J, Gröning R, Sathyan G, Thipphawong J, Richarz U. The pharmacokinetics of a long-acting OROS hydromorphone formulation. Expert Opin Drug Deliv. 2010;7(1):137–144. doi: 10.1517/17425240903386658. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Sathyan G. Providing constant analgesia with OROS® hydromorphone. J Pain Symptom Manage. 2007;33(2 Suppl):S19–S24. [Google Scholar]
- 36.Bruera E, Pereira J, Watanabe S, Belzile M, Kuehn N, Hanson J. Opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. Cancer. 1996;78(4):852–857. doi: 10.1002/(SICI)1097-0142(19960815)78:4<852::AID-CNCR23>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38(3):426–439. doi: 10.1016/j.jpainsymman.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Palangio M, Northfelt DW, Portenoy RK, et al. Dose conversion and titration with a novel, once-daily, OROS osmotic technology, extended-release hydromorphone formulation in the treatment of chronic malignant or nonmalignant pain. J Pain Symptom Manage. 2002;23(5):355–368. doi: 10.1016/s0885-3924(02)00390-1. [DOI] [PubMed] [Google Scholar]
- 39.Wallace M, Rauck RL, Moulin D, Thipphawong J, Khanna S, Tudor IC. Once-daily OROS hydromorphone for the management of chronic nonmalignant pain: a dose-conversion and titration study. Int J Clin Pract. 2007;61(10):1671–1676. doi: 10.1111/j.1742-1241.2007.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace M, Rauck RL, Moulin D, Thipphawong J, Khanna S, Tudor IC. Conversion from standard opioid therapy to once-daily oral extended-release hydromorphone in patients with chronic cancer pain. J Int Med Res. 2008;36(2):343–352. doi: 10.1177/147323000803600218. [DOI] [PubMed] [Google Scholar]
- 41.Hale M, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26(6):1505–1518. doi: 10.1185/03007995.2010.484723. [DOI] [PubMed] [Google Scholar]
- 42.Hanna M, Thipphawong J, 118 Study Group A randomized, doubleblind comparison of OROS(R) hydromorphone and controlled-release morphine for the control of chronic cancer pain. BMC Palliat Care. 2008;7:17. doi: 10.1186/1472-684X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binsfeld H, Szczepanski L, Waechter S, Richarz U, Sabatowski R. A randomized study to demonstrate noninferiority of once-daily OROS® hydromorphone with twice-daily sustained-release oxycodone for moderate to severe chronic noncancer pain. Pain Pract. 2010;10(5):404–415. doi: 10.1111/j.1533-2500.2009.00342.x. [DOI] [PubMed] [Google Scholar]
- 44.Quigley C, Wiffen P. A systematic review of hydromorphone in acute and chronic pain. J Pain Symptom Manage. 2003;25(2):169–178. doi: 10.1016/s0885-3924(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 45.Hanna M, Tuca A, Thipphawong J. An open-label, 1-year extension study of the long-term safety and efficacy of once-daily OROS(R) hydromorphone in patients with chronic cancer pain. BMC Palliat Care. 2009;8:14. doi: 10.1186/1472-684X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace M, Skowronski R, Khanna S, Tudor IC, Thipphawong J. Efficacy and safety evaluation of once-daily OROS hydromorphone in patients with chronic low back pain: a pilot open-label study (DO-127) Curr Med Res Opin. 2007;23(5):981–989. doi: 10.1185/030079907x182040. [DOI] [PubMed] [Google Scholar]
- 47.Wallace M, Thipphawong J. Open-label study on the long-term efficacy, safety, and impact on quality of life of OROS hydromorphone ER in patients with chronic low back pain. Pain Med. 2010;11(10):1477–1488. doi: 10.1111/j.1526-4637.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 48.Galer BS, Coyle N, Pasternak GW, Portenoy RK. Individual variability in the response to different opioids: report of five cases. Pain. 1992;49(1):87–91. doi: 10.1016/0304-3959(92)90192-E. [DOI] [PubMed] [Google Scholar]
- 49.Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86(9):1856–1866. doi: 10.1002/(sici)1097-0142(19991101)86:9<1856::aid-cncr30>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 50.Quang-Cantagrel ND, Wallace MS, Magnuson SK. Opioid substitution to improve the effectiveness of chronic noncancer pain control: a chart review. Anesth Analg. 2000;90(4):933–937. doi: 10.1097/00000539-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25(9):2133–2150. doi: 10.1185/03007990903120158. [DOI] [PubMed] [Google Scholar]
- 52.Fine PG, Portenoy RK, Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38(3):418–425. doi: 10.1016/j.jpainsymman.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 54.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 55.Mahler DL, Forrest WH., Jr Relative analgesic potencies of morphine and hydromorphone in postoperative pain. Anesthesiology. 1975;42(5):602–607. doi: 10.1097/00000542-197505000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 57.Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25(5):406–411. doi: 10.1016/s0885-3924(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 58.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74(2):102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 59.Ting N. Dose Finding in Drug Development. New York, NY: Springer; 2006. [Google Scholar]