Abstract
The outer shell of the adenovirus capsid comprises three major types of protein (hexon, penton base and fiber) that perform the majority of functions facilitating the early stages of adenovirus infection. These stages include initial cell-surface binding followed by receptor-mediated endocytosis, endosomal penetration and cytosolic entry, and intracellular trafficking toward the nucleus. Numerous studies have shown that the penton base contributes to several of these steps and have supported the development of this protein into a delivery agent for therapeutic molecules. Studies revealing that the fiber and hexon bear unexpected properties of cell entry and/or nuclear homing have supported the development of these capsid proteins, as well into potential delivery vehicles. This review summarizes the findings to date of the protein–cell activities of these capsid proteins in the absence of the whole virus and their potential for therapeutic application with regard to the delivery of foreign molecules.
Adenovirus capsid roles in early infection
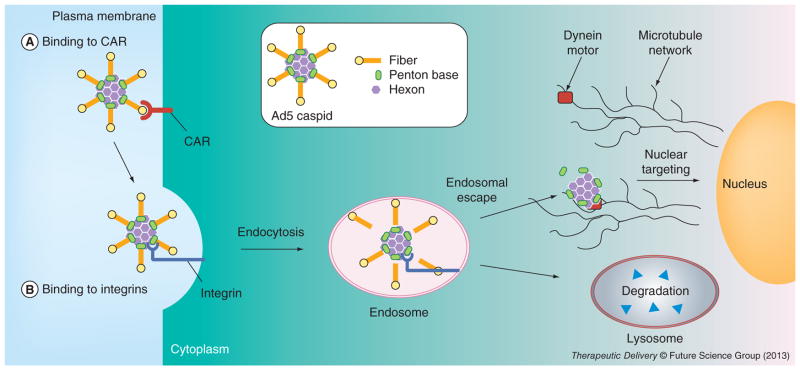
The adenovirus (Ad) is a non-enveloped, dsDNA virus whose outer shell of the icosahedral-shaped capsid is comprises three major types of proteins: hexon, penton base and fiber (Figure 1). The hexon comprises the majority of the outer shell of the Ad capsid, forming 240 homotrimers that encapsidate the majority of the virus, including the viral genome and associated proteins [1]. The fiber protrudes from each of the 12 vertices of the icosahedron, while the penton base lies at the base of each fiber. These three capsid proteins contribute to the majority of activities required for the early stages of Ad infection.
Figure 1. Representation of adenovirus capsid (inset) and the early stages of adenovirus infection.
(A) Binding of the virus to the primary receptor, CAR, which initiates infection. This is immediately followed by (B) secondary binding of the virus to integrins, which triggers receptor-mediated endocytosis.
CAR: Coxackievirus adenovirus receptor.
To date, seven Ad subgroups, now known as ‘species’, have been identified, designated A through to G [2]. Among the most studied are specific serotypes of species C (Ads2 and 5) and B (Ads3 and 7). Due to their common occurrence in human viral infection, these particular serotypes have been best characterized with regard to infection mechanism, genome sequencing, gene expression and regulation, protein structure and function, and vector–host interactions. Such studies have contributed to the development of these serotypes into gene delivery vectors. Subsequently, the capsid proteins of these serotypes have become the platform for novel nonviral approaches in nucleic acid and drug delivery for therapeutic applications, which is the focus of this review. Such approaches have used the minimal components necessary to mimic the high efficiency cell penetration of the virus while avoiding the concerns associated with using whole viruses for therapy.
Cell binding
The early stages of Ad infection entail initial cell binding mediated by interaction of the fiber with primary cell-surface receptors, followed by secondary binding of the penton base to cell-surface integrins, triggering integrin receptor-mediated endocytosis, subsequent endosomal escape (or endosomolysis) and intracellular trafficking to the nucleus (Figure 1) [3]. The fiber comprises of a homotrimer of proteins containing an amino (N terminal) tail domain, which interacts with the penton base, and a carboxy (C terminal) globular knob that binds to the primary receptor [4]. These two domains are separated by a shaft of differing length depending on serotype [5], and comprises a repeated sequence forming a triple β spiral in the fiber homotrimer [6].
The earliest identified primary receptor for Ad2 and 5, and most well-studied primary Ad receptor, is the coxackievirus adenovirus receptor (CAR) [7]. Since its initial isolation, it has been identified as the primary receptor for other Ad serotypes as well, including Ad4, 12 and 41 [8]. Crystallization of soluble Ad5 knob confirmed that it structurally trimerizes with threefold symmetry [9]. Interaction with CAR occurs between adjacent monomers, causing CAR trimerization at the cell surface [10]. CAR is a tight junction protein that under most circumstances does not internalize in response to ligation [11–14]. Other primary receptors have since been identified, including CD46 [15], heparan sulfate glycosaminoglycans [16–18] and desmoglein [19].
The penton base is composed of a homo-pentamer that noncovalently attaches to each Ad capsid vertex as well as to the tail domain of the fiber [20]. A solvent-exposed loop containing an arginine-glycine-aspartate motif confers binding to α-v integrins, whereas a less-exposed leucine-aspartate-valine motif enables binding to α-4 integrins [21]. CryoEM of Ads2 and 12 bound to integrins shows interaction of five integrin proteins per penton base pentamer [22]. Immunofluorescence studies on the cell entry of soluble Ad5 penton base demonstrate that integrins accumulate at sites of cell-surface interaction [23]. Taken together, these findings support the notion that penton–base interaction with integrins causes receptor clustering at the cell surface.
Studies in recent years have shown that the hexon may also participate in cell binding. Studies on liver cell entry of Ad have indicated that the hexon interacts with blood coagulation factor X and that this complex facilitates binding to HS-GAG on hepatocyte cell surfaces [24–26].
Cell entry & intracellular trafficking
Clustering of Ad particles at the cell surface upon integrin binding triggers formation of clathrin-coated pits and internalization into clathrin-coated vesicles [27,28]. Importantly, HS-GAG-bound virus is also capable of internalization. As the virus enters the cell, it begins to sequentially shed its capsid proteins, starting with the fibers [29,30]. Escape from the endosomal vesicle and entry into the cytoplasm avoids degradation in lysosomes. Studies on whole virus trafficking indicate that escape from endosomal vesicles is dependent on several viral and cellular factors, including endosomal pH [30–32], penton base-integrin interaction [33,34] and uncoating [35,36]. While endosomal escape occurs in response to acidification of the maturing endosome, the timing of escape appears to be determined by the fiber, which acts as a probe for determining the pH at which escape takes place [37,38]. The ability of the virus to uncoat is also important for endosomolysis, as temperature-sensitive mutants that are deficient at uncoating are unable to penetrate the endosomal membrane [35,36]. Penetration through the endosomal membrane has long been attributed to the penton base [39], though conflicting studies have suggested alternative proteins. Protein VI, which lies inside the Ad capsid, has been identified as a factor that becomes revealed by uncoating and whose processing by the Ad protease exposes putative helical membrane-lytic domains [40]. On the other hand, corroborating independent studies have shown that soluble recombinant penton base proteins can penetrate the endosomal membrane without the assistance of other viral proteins [23,41]. Additionally, it has been demonstrated that the interaction of the penton base with certain integrins determines endosomal escape, as mutation of a TVD motif on the cytosolic tail of α-v β-5 integrins prevents cytoplasmic entry while causing vesicle-accumulation of Ad2, suggesting that this motif is a cellular determinant for endosomolysis [33]. Studies comparing Ad with rhinovirus in facilitating the leakage of different-sized dextran molecules from endosomal vesicles have indicated that endosomal membrane penetration by Ad appears to engage a mechanism of general membrane disruption in contrast to pore formation, as used by rhinovirus [42].
Once the Ad has penetrated into the cytoplasm, it is capable of hijacking dynein proteins to traffic along microtubules toward the nucleus [43–45]. Once docked at nuclear pores, the virus extrudes its genomic DNA through the pores into the nucleus for expression of viral genes [30].
Capsid proteins as soluble delivery agents
Penton base
Due to its multiple functions in viral infection, including cell-surface binding, endocytosis and endosomal penetration, the penton base has been considered as a possible reagent for gene and drug delivery. Several studies have demonstrated that soluble recombinant penton base recapitulates the cell entry and intracellular trafficking of whole Ad. Immunofluorescence studies have shown that soluble Ad5 penton base proteins induce integrin clustering and accumulation into endosomal vesicles after cell surface binding [23]. Electron microscopy studies have shown that soluble recombinant Ad2 penton base (which shares high sequence identity with the Ad5 protein) penetrates through the endosomal membrane, as well as entering the nucleus through nuclear pores [41]. Immunofluorescence and subcellular fractionation of HeLa cells after exposure to soluble recombinant Ad5 penton base have supported its nuclear accumulation after cell entry [23]. Use of cytoskeletal inhibitors and trafficking mutants have shown that soluble Ad5 penton base traffics toward the nucleus after endosomal escape, requiring intact dynein and microtubules for this transit [23]. As with the whole virus, the exact mechanism of endosomal escape remains unclear. Karayan et al. identified several amino acid residues on the Ad2 penton base that are necessary for the release of co-internalized nucleic acid into the cytoplasm [46]. Our laboratory’s studies have suggested that endosomal escape by soluble recombinant Ad5 penton base is not necessarily pH-dependent, as gene delivery by a tumor-targeted version of this protein is not inhibited by bafilomycin [47].
These observations are not limited to the penton base of subgroup C viruses, as the penton base dodecahedrons (Dds) of Ad3 exhibit similar cell-penetration activities. The Ad3 penton base noncovalently self-assembles into structurally stable dodecahedral particles, which comprises 12 pentamers (or 60 penton base monomers) when expressed as a recombinant protein in insect cells [48,49]. These particles, called Dds, can mimic viral cell entry by binding to integrins as well as HS-GAG [50,51], undergoing receptor-mediated endocytosis, and trafficking toward the nuclear periphery where Dds accumulate inside the cell. The HS-GAG binding site comprises a KQKR motif located peripheral to the integrin-binding sequence on the arginine-glycine-aspartate loop of the Ad3 penton base linear sequence, and appears to cooperate with integrin binding for cell attachment [52]. Whereas the diameter of the Dd internal cavity is too small (8 nm) to accommodate genes [53,54], fusion of the fiber tail to a polylysine enabled DNA binding to the Dd surface and delivery of nucleic acids in vitro [48]. In comparison to gene delivery by similar numbers of recombinant Ad particles, low concentrations of Dd yielded higher levels of gene transfer whereas higher particle numbers equated similar gene transfer levels as whole Ad [48]. Other types of cargo could be delivered by taking advantage of two conserved PPxY motifs found at the N-terminus of the penton base sequence, which are recognized by proteins containing a WW motif, such as the ubiquitination enzyme, Nedd4 [55]. This interaction has been exploited for binding foreign proteins to Dd as a protein-delivery strategy [56]. More recently, Dd has been tested for in vitro delivery of the drug, bleomycin, which was conjugated to Dd by covalent linkage [57].
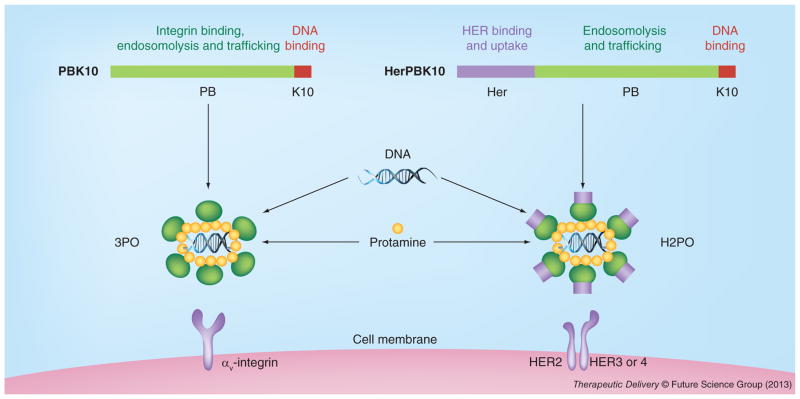
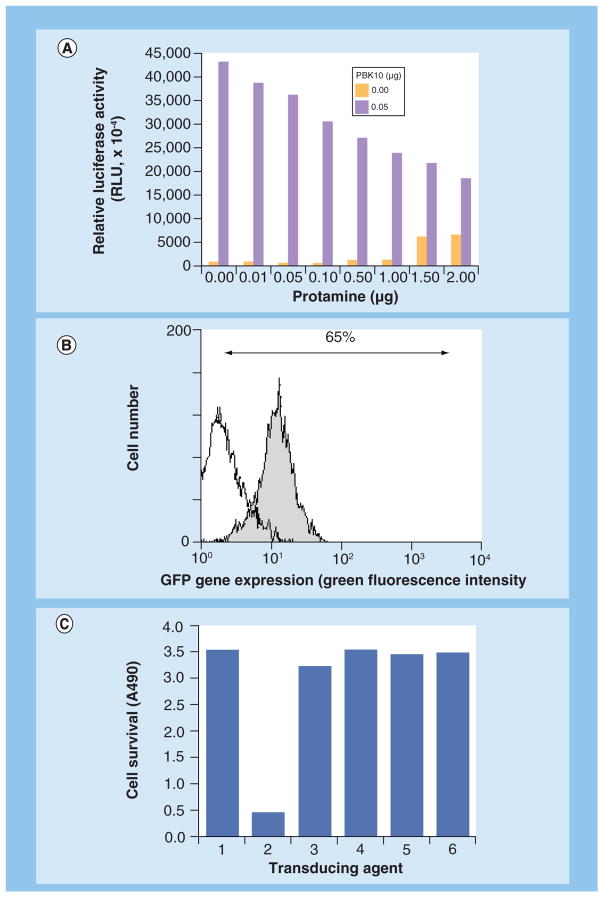
Our group has also shown that soluble recombinant Ad 5 penton base facilitates the cytosolic entry of several membrane-impermeable molecules, including genes [58], siRNA [23] and corroles [59,60]. Our gene-delivery approach has entailed modification of recombinant Ad5 penton base by appendage, through genetic fusion, of a polylysine tail at the carboxy (C)-terminus of the protein, forming the protein, PBK10 (Figure 2) [58]. This modification allowed assembly with nucleic acids through electrophilic interaction. Gene transfer complexes called 3PO, formed from the assembly of PBK10 with protamine-condensed plasmid DNA, targeted gene delivery to HeLa cells in culture through integrin binding and endocytosis [58] and yielded up to 65% GFP-plasmid transfection efficiency in 293 cells (Figure 3B). The addition of soluble recombinant fiber protein, which can noncovalently assemble with the penton base and 3PO, could augment gene transfer fivefold over 3PO and re-target gene transfer through CAR interaction [61]. In comparison to recombinant Ad5, with regard to cytotoxicity, 2 × 107 recombinant (nonreplicating) Ad reduced cell survival of HeLa by 80%, whereas 3PO −/+ fiber as well as individual penton base and fiber proteins produced undetectable cytotoxicity (Figure 3C). Importantly, these comparisons represented a 7.5-fold excess of penton base and 4.5-fold excess of fiber compared with that in 2 × 107 Ad, suggesting that human cells can better tolerate a high concentration of recombinant capsid proteins delivered through 3PO −/+ fiber rather than through Ad5, and that the cytotoxic effects of the virus are due to other additional factors.
Figure 2. Representation of gene delivery complexes, 3PO and H2PO.
Colored bars at the top of the figure represent the linear sequences of PBK10 and HerPBK10 from amino to carboxy termini (left to right). The structure of the assembled complex is speculated based on functional findings, including receptor-specific delivery and gene expression, DNA condensation (based on ethidium bromide exclusion), and DNA protection from serum nucleases.
Figure 3. 3PO gene transfer and comparison of cytotoxicity to Ad.
(A) 3PO-mediated delivery of luciferase-expressing plasmid to HeLa plated in a 96-well dish (0.1 μg of DNA/well used). (B) GFP gene transfer efficiency in 293 cells plated in a 24-well dish. At 24 h after treatment, cells were lifted by trypsinization, washed, and counted by flow cytometry, measuring green fluorescence (GFP+: shaded; untreated cells: unshaded). In (A) and (B) 3PO was assembled and delivered to sub-confluent cells, as described [58]. (C) Capsid protein complexes are nontoxic relative to Ad5 on HeLa cells in culture. 1: untreated cells; 2: Ad5-GFP; 3: Protamine + DNA; 4: 3PO; 5: 3POF and 6: Fiber alone.
The ability of the penton base to support gene expression as well as gene silencing from plasmid and siRNA payloads, respectively [23,58], further validated the use of this protein for cell penetration and delivery of foreign molecules. Further modification of PBK10 by C-terminal fusion to the receptor-binding domain of heregulin-α (forming the protein, HerPBK10) enabled retargeting of gene transfer to cultured cells displaying the human EGFR (Figure 2) [47]. HerPBK10 augmented the luciferase gene delivery of protamine-condensed plasmid by 34-fold (over complexes lacking HerPBK10) on HER2+ MDA-MB-453 cells [47]. Specific targeting was validated by competitive inhibition with free heregulin ligand, and differential delivery to HER2-positive (MDA-MB-453) and HER2-negative (MDA-MB-231) breast cancer cell lines [47]. The ability of HerPBK10 to target HER2-positive tumor cells is based on the enhanced ligand–receptor affinity on HER2-positive cells. Heregulin specifically interacts with the HER3 or HER4 subunits, which can both heterodimerize with HER2, to form the functional receptor [62]. However, the affinity of HER3 or HER4 for the ligand is considerably increased when HER2 is amplified, which typifies HER2-positive tumors [63,64]. This feature presents a possible advantage to using HerPBK10 for targeted cell penetration of therapeutic molecules as an alternative to signal-blocking molecules directed at HER2.
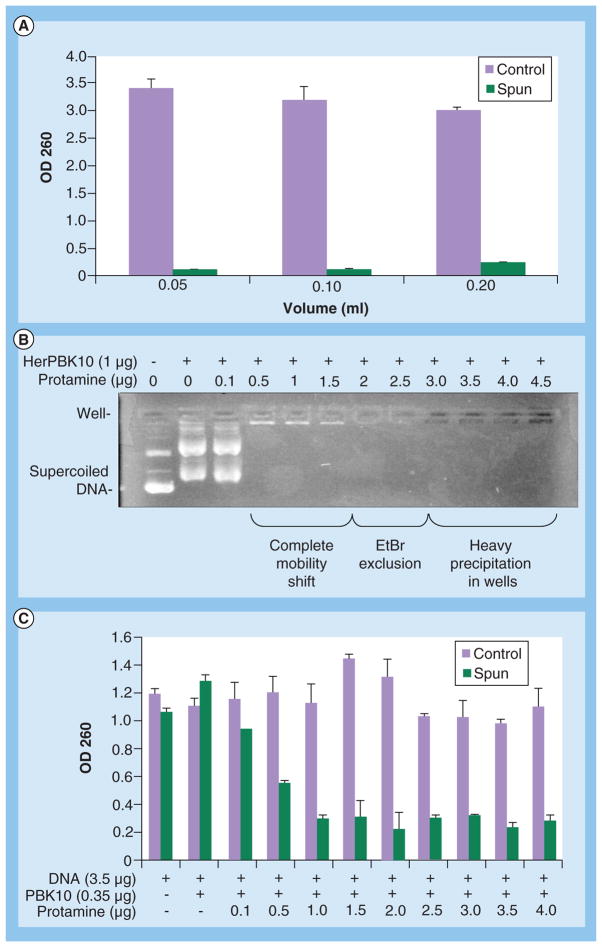
A notable problem with these gene delivery studies is the packaging of the nucleic acid macromolecule itself, which requires considerable DNA condensation to collapse the plasmid into a virus-sized particle [65]. Packaging and condensation of large nucleic acid molecules, including plasmid DNA, not only enables protection of the payload from serum and intracellular nucleases, but also helps systemic delivery in vivo by promoting facile passage through capillaries and fenestrations, and shielding from immune molecules [65,66]. While protamine was incorporated in PBK10- and HerPBK10-based gene-delivery complexes to accomplish this [47,58], the tight binding of this protein to DNA has the potential to abrogate decondensation and gene expression after gene delivery. Indeed, we have observed that at high enough concentration, protamine promoted insoluble precipitates (Figure 4) that correlated with reduced gene transfer (Figure 3A), and have prevented facile development for in vivo application.
Figure 4. Protamine-mediated precipitation of 3PO.
(A) 3PO was prepared by mixing 10 μg of DNA, 1 μg PBK10, and 10 μg protamine in a final volume of either 200, 100 or 50 μl. Mixtures were incubated at room temperature for 15 min, then spun in a microcentrifuge (15K RPM), while control samples were not. Supernatant absorbances were then measured at OD 260 to detect soluble DNA. (B) Increasing protamine excludes EtBr and forms precipitates. Plasmid DNA (0.35 μg, pEGFP) was incubated with indicated reagents for 15 min at room temperature, then electrophoresed on 0.8% agarose gel that was subsequently stained with EtBr to identify the DNA. (C) 3PO was prepared by mixing 0.35 μg PBK10, 3.5 μg DNA + increasing amounts of protamine in 100 μl final volume. After 15 min room temperature incubation, samples were spun in a micro-centrifuge (15K RPM), while control samples were not. Supernatant absorbances were then measured at 260 nm to detect soluble DNA.
EtBr: Ethidium bromide.
On the other hand, using HerPBK10 for drug delivery has proven to be a more successful application compared with gene delivery. In one approach, we have exploited the nucleic acid binding domain of HerPBK10 to deliver the chemotherapeutic agent, doxorubicin (Dox) via DNA intercalation. The combination of HerPBK10 with a small double-stranded nucleic acid and Dox-enabled spontaneous, noncovalent assembly of the complex, HerDox [67]. This particle (~10–20-nm diameter) resisted drug release in mouse blood and human serum, but exhibited release after cell entry. HerDox showed tumor-preferential accumulation after systemic delivery in a xenograft mouse model of human HER2-positive cancer (human MDA-MB-435 tumor cells subcutaneously implanted in the flanks of immune-deficient NU/NU mice) while sparing nontumor tissue, including the heart and liver, in contrast with systemic treatment with free, untargeted Dox. Importantly, multimodality imaging, including in situ confocal fluorescence imaging, verified that HerDox exhibited penetration into tumor cells and delivery of Dox to the tumor cell nuclei [68], in contrast with mere accumulation at the tumor interstitium. The dose of systemic HerDox that completely ablated tumor growth (0.004 mg/kg/day for 7 sequential days) equated more than ten-times the dose of untargeted Dox (>0.04 mg/kg/day for 7 days) [67]. This extremely low dose speaks to the advantage of targeted tumor penetration, which not only enabled tumor-preferential accumulation of the drug, but also allowed very low doses to be effective, thus, having both a therapeutic and safety advantage, compared with the untargeted drug.
We have also used HerPBK10 to deliver sulfonated corrole compounds to tumor cells in vitro [60] and in vivo [59]. Corroles are macrocyclic compounds with structural similarity to porphyrins, and can be synthesized as complexes bound to different types of metal ions [69]. Studies using human serum albumin have shown that the amphiphilic 2,17-bis-sulfonato-5,10,15(trispentaf luorophenyl)corrole binds stably in protein pockets with high affinity and nearly undetectable dissociation [70]. Likewise, we have shown that sulfonated corroles can form serum-stable complexes with HerPBK10 [59,60]. The gallium-metallated corrole compound, S2Ga, is highly fluorescent upon light stimulation at 424 nm and also highly cytotoxic when allowed to penetrate into cells. Sulfonated corroles are unable to penetrate through cell membranes without the aid of a membrane disruption agent, hence free soluble corroles have little cytotoxicity in the absence of a membrane-penetrating carrier. Our laboratory’s studies have shown that S2Ga binds HerPBK10 at a 25–30:1 corrole:protein ratio [60], forming serum-stable, round particles of 10–20 nm in diameter [71]. The resulting complex, HerGa, could target HER2-positive tumors in vivo (in immune-deficient mice bearing human xenografts of HER2-positive MDA-MB-435 tumor cells) after systemic delivery (via tail vein injection) and induce tumor cell death while avoiding nontumor tissue [59]. Systemic HerGa at 0.008 mg/kg/day for 7 days not only ablated tumor growth, but also caused some tumor shrinkage, which could be matched by Dox when delivered intratumorally at 2.5 mg/kg/day for 7 days. Tumor targeting of HerGa could be detectable via the fluorescence emitted by the corrole upon light stimulation in vivo, and contrasted with the untargeted corrole molecule, which exhibited widespread circulation in vivo and near exclusion from tumors. These findings were verified by fluorescence imaging of extracted tumors and tissues after systemic delivery. Mechanistic studies confirmed that membrane penetration is required for effective cytotoxicity, as HerPBK10 constructs lacking the penton base domain were unable to facilitate corrole-mediated cell death [72]. The cell death mechanism occurred through corrole-mediated elevation of superoxide, leading to oxidative damage of the mitochondrial membrane and cytoskeleton. Illumination at specific wavelengths, including at near IR wavelength, augmented this damage through the induction of singlet oxygen [71,73]. Competitive inhibition by free ligand and intracellular trafficking studies using confocal microscopy confirmed that targeting occurred specifically through HER binding and receptor-mediated internalization. Moreover, HerPBK10 enabled targeting to HER2-positive cells in a mixed culture of HER2-positive and HER2-negative cells [59].
The dual fluorescence and cytotoxicity of the gallium corrole imparts multifunctionality to the HerGa particle, enabling the potential for both tumor detection and treatment within the same molecule. While fluorescence detection has its limitations in the clinic (due to the limited tissue penetration of the excitation light), it may be possible to use corrole fluorescence to delineate tumor margins during surgical procedures. Moreover, the fluorescence lifetime of HerGa may offer diagnostic possibilities along with its detection and therapeutic potential. For example, we have observed that the fluorescence lifetime of HerGa can differ depending on whether HerGa is located in tumor or nontumor (i.e., liver) tissue in a mouse xenograft model of human MDA-MB-435 tumor cells subcutaneously implanted in nude mice [74]. Since even targeted therapies cannot avoid some delivery to the liver, this finding suggests that the fluorescence lifetime of HerGa can be used to distinguish tumor from nontumor tissue in vivo during tumor detection. The fluorescence lifetime of HerGa also exhibits a linear correlation with pH, as increasing pH yields a linear increase in fluorescence lifetime [72]. Fluorescence lifetime changes are observable during HerGa uptake into cells, showing an initial decrease during the first 30 min of uptake followed by a slight increase that levels off by approximately 1 h after uptake [72]. Based on its correlation with pH, this pattern could be indicative of a slightly acidic (pH 6.5) environment 30 min after uptake, followed by entry into a more neutral environment at 1 h. Given the well-studied intracellular trafficking of whole adenovirus and soluble recombinant penton base, these findings correspond well with entry into early endosomes within the first 30 min after uptake followed by cytosolic entry at 1 h [3,23,41]. Hence, HerGa has the potential to act as a probe, broadcasting microenvironmental pH.
The augmented cytotoxicity by HerGa when photoexcited presents additional possibilities with regard to treatment regimens. Specifically, we observed in vitro that illumination of HerGa-treated cells at the maximum absorbance wavelength of the corrole (424 nm) as well as at red light wavelength (590–630 nm) notably enhanced cytotoxicity, while the illumination itself (without HerGa) did not cause cell death under the same conditions [73]. Importantly, cell death augmentation by the latter wavelength supports the possibility of applying photoexcitation in vivo, since longer wavelengths penetrate tissue better. In addition, we have observed that HerGa exhibits retention in tumors up to 30 days after tumor accumulation [71]. Taken together, these findings raise the possibility of using photoexcitation as a follow-up treatment to destroy any residual tumor cells surviving initial HerGa-treatment. The prolonged tumor retention of HerGa allows a generous time window in which to apply light as a follow-up treatment.
The prevalence of preexisting antibodies against the Ad capsid in the general population and their ability to prevent transduction by Ad gene therapy vectors raises concern over the efficacy of capsid-derived vectors. Several studies have confirmed that the majority of pre-existing neutralizing antibodies are directed against the hexon and secondarily against the fiber knob [75]. HerPBK10 did not induce any detectable neutralizing antibodies when dosed repeatedly in immune competent (C57BL6) mice [59]. A dose of protein equating the therapeutic efficacy of HerGa and a tenfold higher dose were administered alongside a dose of whole Ad that is known to cause considerable neutralizing antibody induction. The postimmune serum from Ad-treated mice, which contained high levels of neutralizing antibody, was tested for blocking cell binding of HerPBK10. While the antiserum could partially recognize HerPBK10, it did not prevent interaction of HerPBK10 to its target cells in vitro.
Fiber
Given the accepted status of CAR as a noninternalizing primary receptor for the fiber protein, the cell uptake and nuclear targeting of recombinant soluble fiber as well as fiber-derived peptides reported by the following studies raised unexpected and intriguing possibilities for gene and drug transfer. Our studies have shown that uptake of soluble full-length Ad5 fiber into HeLa cells was actin-mediated and temperature-independent [14]. It was observed that recombinant fiber protein could assemble with protamine-condensed plasmid DNA and facilitate gene transfer. Full-length but not shaft-deleted (knob only) fiber exhibited uptake. Inhibition by heparin suggested that uptake occurred through binding to HS-GAG, which can undergo endocytosis after binding. A likely motif implicated in this phenomenon is a KKTK near the tail domain of the shaft. This domain is recognized by heparin as well as HS-GAG. Xie et al. have shown that soluble fiber and even the knob alone is capable of internalizing in the lacrimal cells of the eye through interactions with CAR and HS-GAG [17]. This interaction was associated with macropinocytotic cell entry. As Ad5 infection of both lacrimal acinar cells and hepatocytes require fiber and CAR-dependent interactions, the knob has been explored as a means to deliver foreign molecules into these cells. Specifically, Sun et al. demonstrated that knob expressed as a fusion to a diblock copolymer of elastin-like polypeptide could form approximately 40-nm particles due to the self-assembly of the diblock elastin-like polypeptide at physiological temperatures [76]. The knob retained trimerization capability, and the particle exhibited entry into hepatocytes and trafficking to lysosomes mainly via CAR binding and uptake. While use of this particle for therapeutic delivery remains to be demonstrated, these findings suggest that knob-directed targeting could be of use for transfer of cargo to hepatocytes and lacrimal acini.
Hexon
Soluble Ad5 hexon has shown nuclear homing ability when delivered into cells in vitro by polyethylenimine or microinjection, and improved gene transfer when added to polyethylenimine–plasmid complexes [77]. This activity required interaction with nuclear pore complexes, similar to the whole virus [78] and supported higher gene transfer levels compared with classic nuclear localization sequence (NLS) peptides. Interestingly, the hexon does not contain a typical NLS, yet in these particular studies, seemed to utilize similar cellular machinery as NLS proteins for nuclear transport. Nuclear delivery can be a rate-limiting step for gene therapy as well as the delivery of any therapeutic molecule thats mechanism of action takes place in the nucleus. For such therapeutic payloads, membrane penetration is not enough, as cytosolic barriers, including cytosolic nucleases and molecular crowding in the cytosolic mileu [79,80], can reduce efficient delivery to the intended subcellular destination. Hence, the augmented nuclear delivery by the hexon has the potential to enhance therapeutic efficacy of gene and drug molecules.
Future perspective
Of the three capsid proteins that have been explored for therapeutic delivery, recombinant penton base proteins have been tested the most. Penton base-derived complexes, HerDox and HerGa, in particular, are likely to enter larger scale preclinical trials in the coming years due to recent successful testing of drug delivery, therapeutic efficacy, safety and low-to-no immune stimulation in animal models. The use of an US FDA-approved drug in HerDox, and the recent demonstration that HerDox has greater therapeutic efficacy and safety compared with the untargeted drug in an animal model of human HER2-positive cancer should facilitate its development toward the clinic. The need for multifunctional particles in the clinic that are capable of detection, diagnosis, and intervention may be addressed by HerGa, whose fluorescence could be used to delineate tumor margins during surgery or allow tumor detection by endoscopy while mediating corrole-induced tumor killing. Continued development of HerGa as an imaging platform could lead to advancement into more clinically relevant imaging modalities, such as MRI. Numerous different metallated corroles may be likely candidates for such an application. Future studies in more clinically relevant animal models will likely expedite the translation of these capsid protein particles from preclinical to clinical studies in the next decade.
Executive summary.
Adenovirus capsid roles in early infection
The outer shell of the adenovirus (Ad) capsid comprises three major types of proteins: hexon, penton base and fiber.
The early stages of Ad infection entail initial cell binding mediated by interaction of the fiber with primary cell surface receptors, followed by secondary binding of the penton base to cell-surface integrins, triggering integrin-receptor-mediated endocytosis, subsequent endosomal escape and intracellular trafficking to the nucleus.
The hexon comprises the majority of the viral capsid and can interact with cell-surface glycosaminoglycans during the early stages of Ad infection of certain cells.
Capsid proteins as soluble delivery agents
Due to its multiple functions in viral infection, including cell surface binding, endocytosis and endosomal penetration, the penton base has been considered as a possible reagent for gene and drug delivery.
Several studies have demonstrated that soluble recombinant penton base and penton base dodecahedrons recapitulate the cell entry and intracellular trafficking of whole Ad.
The penton base has been modified to deliver nucleic acids and drugs to tumor cells in vitro and in vivo.
Soluble fiber can penetrate cells through a novel cell entry pathway and deliver a foreign payload.
The Ad hexon can enhance nuclear delivery of DNA through its nuclear homing capability.
The hexon–heparan sulfate proteoglycans interaction is bridged by coagulation factor X.
Acknowledgments
The author thanks C Rey, M Medina-Kauwe and D Revetto for their continued support.
Glossary
- Capsid
Outer shell of a virus, composed of proteins
- Serotype
Type of virus based on its serologically recognized antigens
- CryoEM
Transmission electron microscopy of samples at freezing temperatures
- Recombinant
Artificially created through molecular genetic manipulation
- Protamine
Nucleic acid binding protein with a highly positive charge
- Fluorescence lifetime
Time it takes for a fluorescence signal to decay
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was supported by grants from the NIH/NCI (R01CA102126, R01CA140995, R01CA129822, and R21 CA116014), the DoD (W81XWH-06–1–0549), the SG. Komen Breast Cancer Foundation (BCTR02–1194), and the Donna and Jesse Garber award. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Van Oostrum J, Smith PR, Mohraz M, Burnett RM. The structure of the adenovirus capsid. III. Hexon packing determined from electron micrographs of capsid fragments. J Mol Biol. 1987;198(1):73–89. doi: 10.1016/0022-2836(87)90459-1. [DOI] [PubMed] [Google Scholar]
- 2.Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21(4):704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medina-Kauwe LK. Endocytosis of adenovirus and adenovirus capsid proteins. Adv Drug Deliv Rev. 2003;55(11):1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Boudin ML, Boulanger P. Assembly of adenovirus penton base and fiber. Virology. 1982;116(2):589–604. doi: 10.1016/0042-6822(82)90151-9. [DOI] [PubMed] [Google Scholar]
- 5.Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol. 2000;74(22):10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Raaij MJ, Mitraki A, Lavigne G, Cusack S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature. 1999;401(6756):935–938. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 8.Roelvink PW, Lizonova A, Lee JG, et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72(10):7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia D, Henry LJ, Gerard RD, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994;2(12):1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- 10.Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286(5444):1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 11.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98(26):15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina-Kauwe LK, Chen X. Using GFPligand fusions to measure receptor-mediated endocytosis in living cells. In: Litwack G, editor. Vitamins and Hormones. Elsevier Science; San Diego, CA, USA: 2002. pp. 81–95. [DOI] [PubMed] [Google Scholar]
- 13.Medina-Kauwe LK, Leung V, Wu L, Kedes L. Assessing the binding and endocytosis activity of cellular receptors using GFP-ligand fusions. BioTechniques. 2000;29:602–609. doi: 10.2144/00293rr03. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Rentsendorj A, Agadjanian H, Chen X, et al. The Ad5 fiber mediates nonviral gene transfer in the absence of the whole virus, utilizing a novel cell entry pathway. Gene Ther. 2005;12(3):225–237. doi: 10.1038/sj.gt.3302402. First demonstration that the fiber protein crosses cell membranes and can deliver a payload into cells. [DOI] [PubMed] [Google Scholar]
- 15.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9(11):1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 16.Lenaerts L, Van Dam W, Persoons L, Naesens L. Interaction between mouse adenovirus type 1 and cell surface heparan sulfate proteoglycans. PLoS ONE. 2012;7(2):e31454. doi: 10.1371/journal.pone.0031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Chiang L, Contreras J, et al. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J Virol. 2006;80(23):11833–11851. doi: 10.1128/JVI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayo-Puxan N, Cascallo M, Gros A, Huch M, Fillat C, Alemany R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J Gene Virol. 2006;87(Pt 9):2487–2495. doi: 10.1099/vir.0.81889-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Li ZY, Liu Y, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17(1):96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: bridging the resolution gap between x-ray crystallography and electron microscopy. EMBO J. 1993;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton. Mol Cell. 2005;17(1):121–135. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Chiu CY, Mathias P, Nemerow GR, Stewart PL. Structure of adenovirus complexed with its internalization receptor, alphavbeta5 integrin. J Virol. 1999;73(8):6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rentsendorj A, Xie J, Macveigh M, et al. Typical and atypical trafficking pathways of Ad5 penton base recombinant protein: implications for gene transfer. Gene Ther. 2006;13(10):821–836. doi: 10.1038/sj.gt.3302729. [DOI] [PubMed] [Google Scholar]
- 24.Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108(8):2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 25.Kalyuzhniy O, Di Paolo NC, Silvestry M, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105(14):5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddington SN, Mcvey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Meier O, Boucke K, Hammer SV, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158(6):1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 29.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74(15):7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 31.Seth P. Adenovirus-dependent release of choline from plasma membrane vesicles at an acidic pH is mediated by the penton base protein. J Virol. 1994;68(2):1204–1206. doi: 10.1128/jvi.68.2.1204-1206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth P, Willingham MC, Pastan I. Binding of adenovirus and its external proteins to Triton X-114. Dependence on pH. J Biol Chem. 1985;260(27):14431–14434. [PubMed] [Google Scholar]
- 33.Wang K, Guan T, Cheresh DA, Nemerow GR. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin beta5. J Virol. 2000;74(6):2731–2739. doi: 10.1128/jvi.74.6.2731-2739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotten M, Weber JM. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213(2):494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- 36.Greber UF, Webster P, Weber J, Helenius A. The role of the adenovirus protease on virus entry into cells. EMBO J. 1996;15(8):1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J Virol. 2001;75(3):1387–1400. doi: 10.1128/JVI.75.3.1387-1400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazawa N, Leopold PL, Hackett NR, et al. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J Virol. 1999;73(7):6056–6065. doi: 10.1128/jvi.73.7.6056-6065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seth P, Fitzgerald D, Ginsberg H, Willingham M, Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005;79(4):1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪▪.Hong SS, Gay B, Karayan L, Dabauvalle MC, Boulanger P. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology. 1999;262(1):163–177. doi: 10.1006/viro.1999.9864. Provides evidence that the penton base penetrates endosomes and enters the nucleus. [DOI] [PubMed] [Google Scholar]
- 42.Prchla E, Plank C, Wagner E, Blaas D, Fuchs R. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J Cell Biol. 1995;131(1):111–123. doi: 10.1083/jcb.131.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelkar S, De BP, Gao G, Wilson JM, Crystal RG, Leopold PL. A common mechanism for cytoplasmic dynein-dependent microtubule binding shared among adeno-associated virus and adenovirus serotypes. J Virol. 2006;80(15):7781–7785. doi: 10.1128/JVI.00481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelkar SA, Pfister KK, Crystal RG, et al. Cytoplasmic dynein mediates adenovirus binding to microtubules. J Virol. 2004;78(18):10122–10132. doi: 10.1128/JVI.78.18.10122-10132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leopold PL, Kreitzer G, Miyazawa N, et al. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11(1):151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 46.Karayan L, Hong SS, Gay B, Tournier J, D’angeac AD, Boulanger P. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J Virol. 1997;71(11):8678–8689. doi: 10.1128/jvi.71.11.8678-8689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina-Kauwe LK, Maguire M, Kasahara N, Kedes L. Non-viral gene delivery to human breast cancer cells by targeted Ad5 penton proteins. Gene Ther. 2001;8:1753–1761. doi: 10.1038/sj.gt.3301583. [DOI] [PubMed] [Google Scholar]
- 48▪▪.Fender P, Ruigrok RW, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nature Biotechnol. 1997;15(1):52–56. doi: 10.1038/nbt0197-52. First characterization of penton dodecahedrons and their potential use for molecular delivery. [DOI] [PubMed] [Google Scholar]
- 49.Szolajska E, Burmeister WP, Zochowska M, et al. The structural basis for the integrity of Adenovirus Ad3 dodecahedron. PLoS ONE. 2012;7(9):e46075. doi: 10.1371/journal.pone.0046075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vives RR, Lortat-Jacob H, Chroboczek J, Fender P. Heparan sulfate proteoglycan mediates the selective attachment and internalization of serotype 3 human adenovirus dodecahedron. Virology. 2004;321(2):332–340. doi: 10.1016/j.virol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Fender P, Schoehn G, Perron-Sierra F, Tucker GC, Lortat-Jacob H. Adenovirus dodecahedron cell attachment and entry are mediated by heparan sulfate and integrins and vary along the cell cycle. Virology. 2008;371(1):155–164. doi: 10.1016/j.virol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 52.Gout E, Schoehn G, Fenel D, Lortat-Jacob H, Fender P. The adenovirus type 3 dodecahedron’s RGD loop comprises an HSPG binding site that influences integrin binding. J Biomed Biotechnol. 2010;541939 doi: 10.1155/2010/541939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuschiotti P, Schoehn G, Fender P, et al. Structure of the dodecahedral penton particle from human adenovirus type 3. J Mol Biol. 2006;356(2):510–520. doi: 10.1016/j.jmb.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Schoehn G, Fender P, Chroboczek J, Hewat EA. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 1996;15(24):6841–6846. [PMC free article] [PubMed] [Google Scholar]
- 55.Galinier R, Gout E, Lortat-Jacob H, Wood J, Chroboczek J. Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry. 2002;41(48):14299–14305. doi: 10.1021/bi020125b. [DOI] [PubMed] [Google Scholar]
- 56.Garcel A, Gout E, Timmins J, Chroboczek J, Fender P. Protein transduction into human cells by adenovirus dodecahedron using WW domains as universal adaptors. J Gene Med. 2006;8(4):524–531. doi: 10.1002/jgm.862. [DOI] [PubMed] [Google Scholar]
- 57.Zochowska M, Paca A, Schoehn G, et al. Adenovirus dodecahedron, as a drug delivery vector. PLoS ONE. 2009;4(5):e5569. doi: 10.1371/journal.pone.0005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪▪.Medina-Kauwe LK, Kasahara N, Kedes L. 3PO, a novel non-viral gene delivery system using engineered Ad5 penton proteins. Gene Ther. 2001;8:795–803. doi: 10.1038/sj.gt.3301448. First report of the engineering of the Ad5 penton base into a nonviral gene-delivery vector. [DOI] [PubMed] [Google Scholar]
- 59.Agadjanian H, Ma J, Rentsendorj A, et al. Tumor detection and elimination by a targeted gallium corrole. Proc Natl Acad Sci USA. 2009;106(15):6105–6110. doi: 10.1073/pnas.0901531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agadjanian H, Weaver JJ, Mahammed A, et al. Specific delivery of corroles to cells via noncovalent conjugates with viral proteins. Pharm Res. 2006;23(2):367–377. doi: 10.1007/s11095-005-9225-1. [DOI] [PubMed] [Google Scholar]
- 61.Rentsendorj A, Agadjanian H, Chen H, et al. The Ad5 fiber mediates non-viral gene transfer in the absence of the whole virus, utilizing a novel cell entry pathway. Gene Ther. 2005;12(3):225–237. doi: 10.1038/sj.gt.3302402. [DOI] [PubMed] [Google Scholar]
- 62.Sepp-Lorenzino L, Eberhard I, Ma Z, et al. Signal transduction pathways induced by heregulin in MDA-MB-453 breast cancer cells. Oncogene. 1996;12(8):1679–1687. [PubMed] [Google Scholar]
- 63.Goldman R, Levy RB, Peles E, Yarden Y. Heterodimerization of the ErbB-1 and ErbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry. 1990;29(50):11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- 64.Sliwkowski MX, Schaefer G, Akita RW, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269(20):14661–14665. [PubMed] [Google Scholar]
- 65.Medina-Kauwe LK. Non-viral mediated gene delivery for therapeutic applications. In: Lowenstein P, Castro M, editors. Gene Therapy for Neurological Disorders. Informa Healthcare; London, UK: 2006. pp. 115–140. [Google Scholar]
- 66.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9(1):E18–E29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪▪.Agadjanian H, Chu D, Hwang JY, et al. Chemotherapy targeting by DNA capture in viral protein particles. Nanomedicine. 2012;7(3):335–352. doi: 10.2217/nnm.11.104. First report of the use of penton base-derived protein for chemotherapy delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang JY, Park J, Kang BJ, et al. Multimodality imaging in vivo for preclinical assessment of tumor-targeted doxorubicin nanoparticles. PLoS ONE. 2012;7(4):e34463. doi: 10.1371/journal.pone.0034463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross Z, Galili N, Saltsman I. The first direct synthesis of corroles from pyrrole. Angew Chem Int Ed Engl. 1999;38:1427–1429. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1427::AID-ANIE1427>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 70.Mahammed A, Gray HB, Weaver JJ, Sorasaenee K, Gross Z. Amphiphilic corroles bind tightly to human serum albumin. Bioconjug Chem. 2004;15:738–746. doi: 10.1021/bc034179p. [DOI] [PubMed] [Google Scholar]
- 71.Hwang JY, Lubow J, Chu D, et al. Photoexcitation of tumor-targeted corrole induces singlet oxygen-mediated augmentation of cytotoxicity. J Control Release. 2012;163(3):368–373. doi: 10.1016/j.jconrel.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang JY, Lubow J, Chu D, et al. A mechanistic study of tumor-targeted corrole toxicity. Mol Pharm. 2011;8(6):2233–2243. doi: 10.1021/mp200094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang JY, Lubow J, Gray HB, et al. Investigating photoexcitation-induced mitochondrial damage by chemotherapeutic corroles using multimode optical imaging. J Biomed Opt. 2012 doi: 10.1117/1.JBO.17.1.015003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hwang JY, Wachsmann-Hogiu S, Ramanujan VK, et al. A multimode optical imaging system for preclinical applications in vivo: technology development, multiscale imaging, and chemotherapy assessment. Mol Imaging Biol. 2011;14(4):431–432. doi: 10.1007/s11307-011-0517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradley RR, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J Virol. 2012;86(1):625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun G, Hsueh PY, Janib SM, Hamm-Alvarez S, Mackay JA. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J Control Release. 2011;155(2):218–226. doi: 10.1016/j.jconrel.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪.Carlisle RC, Bettinger T, Ogris M, Hale S, Mautner V, Seymour LW. Adenovirus hexon protein enhances nuclear delivery and increases transgene expression of polyethylenimine/plasmid DNA vectors. Mol Ther. 2001;4(5):473–483. doi: 10.1006/mthe.2001.0472. First report on the use of the hexon for improving nuclear delivery. [DOI] [PubMed] [Google Scholar]
- 78.Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16(19):5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pollard H, Toumaniantz G, Amos JL, et al. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J Gene Med. 2001;3(2):153–164. doi: 10.1002/jgm.160. [DOI] [PubMed] [Google Scholar]
- 80.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275(3):1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]