Abstract
Background
Although the Diabetes Prevention Program (DPP) lifestyle intervention reduced type 2 diabetes incidence by 58% among high-risk adults at academic centers, it requires translation into typical primary care settings. Using baseline data from the Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care (E-LITE) randomized controlled trial, we evaluated the potential of its two DPP-based interventions to reach their target populations and be adopted into routine use.
Methods
Overweight/obese adults with increased cardiometabolic risk enrolled from one primary care clinic. Using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) model, we assessed reach with data on patient identification, participation, and representativeness, and adoption with data on intervention feasibility and potential for organizational diffusion.
Results
The target population was identified by searching electronic health records. Contact was attempted for 2391 patients who completed initial screening by phone (56% uptake) or online (44%). Most (88%) of those screened ineligible were not within the target population; 12% were excluded because of research requirements. Conservatively estimated participation rate was 44%. Participants (n=241) included 54% men and had a mean (SD) age of 52.9 years (10.6) and body mass index of 32 kg/m2 (5.4). Regarding adoption, all clinic physicians agreed to participate. The feasibility of intervention implementation and dissemination was enhanced by leveraging existing intervention, training, and primary care resources.
Conclusions
E-LITE's lifestyle interventions had fair-to-good potential for primary care reach and adoption. Our trial evidence and structured reporting may inform real-world implementation of translational trials by health networks, physicians, and payers.
Keywords: Behavior therapy/methods; Metabolic syndrome X/prevention and control; Obesity/therapy, overweight/therapy; Prediabetic state/therapy; Primary health care/methods; Translational medical research/methods
1. Introduction
Sixty-eight percent of American adults are overweight or obese, and 34% are obese [1]. Many have comorbidities (e.g., metabolic syndrome) that further increase their risk for morbidity and mortality. While the obesity epidemic threatens to overwhelm scarce healthcare resources, emerging evidence suggests that its dire health consequences may be blunted or even reversed by lifestyle interventions that are efficacious, safe, and cost effective, as long as such interventions are appropriately disseminated.
The highly successful Diabetes Prevention Program (DPP), tested in a rigorous efficacy trial, convincingly demonstrated that an intensive lifestyle intervention led to sustained, clinically significant weight loss and reduced the risk for incident type 2 diabetes mellitus (diabetes) by 58% among high-risk adults [2–5]. The DPP intervention was delivered primarily in individual face-to-face meetings, which is resource intensive. To achieve widespread dissemination and uptake in primary care practice, the DPP intervention needs to retain (or improve upon) its demonstrated efficacy but be delivered in more cost-effective formats. These may include formats that are group-based, take advantage of information technologies, or both.
A recently-published randomized controlled trial (RCT) reported promising one-year outcomes for a group-based DPP translation conducted in the community [6]. An earlier, cluster-randomized trial showed that a DPP-based group intervention delivered in the YMCA resulted in significant and sustained weight loss among group participants compared to controls [7]. The remainder of published studies of group-based DPP translations have largely used nonrandomized designs, with many [7–13], but not all [14–17], conducted in primary care settings. Alternative delivery modalities (e.g., DVD) have also begun to emerge, although their effectiveness is less well documented [18]. Taken together these studies suggest that group- or DVD-based DPP translations are feasible and likely to be effective, and already are being disseminated. However, rigorous evaluations of scalable DPP translational interventions remain necessary.
During this intermediate stage of experimentation between introduction of the original DPP intervention and widespread dissemination of effective and scalable translations, it is worthwhile to scrutinize emerging translations with the following questions: does this specific DPP-based intervention have the potential to achieve the levels of reach and adoption necessary for its widespread dissemination, and if so what is it about the intervention that indicates this potential? A corollary question for trialists and other researchers is: how should characteristics of reach and adoption be reported in order to assist providers, healthcare networks, and payers to understand whether the trial and its intervention is pertinent to their patient and health delivery needs?
We seek to assist stakeholders and researchers to answer these important questions through structured reporting of intervention characteristics that pertain to applicability in real-world settings. In this report we used recruitment and baseline assessment data from a 3-arm RCT, E-LITE (Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care), a recently completed randomized controlled trial (RCT), to assess the potential reach and adoption in primary care settings [19,20] of its two translations of the original DPP lifestyle intervention. Participants in both active E-LITE intervention groups sustained significantly greater weight loss at 15 months compared to the usual care group, with approximately 50% in each achieving 5% or greater weight loss from baseline (an accepted cut-point indicative of clinically meaningful weight loss), results which are in press [21]. Thus, we hope the current assessment will serve as a model for how emerging successful DPP translational interventions and other translational trials can report reach and adoption characteristics. Such reporting may promote their appropriate dissemination, for example, by enabling comparisons across variants of the original DPP intervention or trials in general.
2. Research design and methods
2.1. RCT characteristics
2.1.1. Study setting and design
The E-LITE study protocol has been previously reported [22]. Briefly, the trial was designed to examine the effectiveness of two lifestyle interventions adapted from the DPP-based Group Lifestyle Balance™ program for weight management and cardiometabolic risk factor reduction in primary care practice, with the a) group-based delivery and b) DVD-based delivery, compared to each other and also to the third study arm of c) usual care control. Although the core DPP content was delivered using different modalities in the two active intervention arms, both received similar technology-mediated self-management support and long-term follow-up, as further described under “RCT Interventions” below.
2.1.2. Eligibility
Patients were eligible for enrollment if they were overweight or obese adults (body mass index (BMI) 25 kg/m2 or greater) with elevated cardiometabolic risk (pre-diabetes, metabolic syndrome, or both) routinely seen at a single primary care clinic that is part of a community-based multi-specialty practice group within a large California Bay Area health network. Patients were ineligible if they had existing diabetes, cardiovascular disease, or other serious medical condition (e.g., kidney disease, cancer), were using a weight loss drug or planning bariatric surgery, were transferring care out of the clinic site, did not speak English or had no computer access, or were participating in another research study or lifestyle management program.
2.1.3. Recruitment
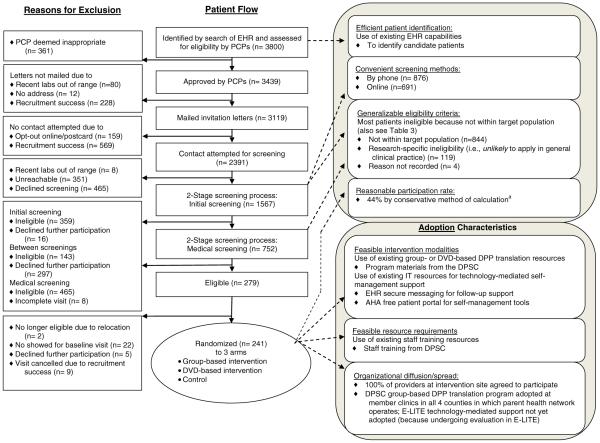
We identified overweight or obese, at-risk adults receiving care from the 21 active primary care providers (PCPs) at the study clinic site using the electronic health records (EHR) system (Fig. 1). PCPs reviewed lists of these potentially eligible patients and excluded those whom they deemed inappropriate for study inclusion. Approved patients were mailed invitation letters signed by or for their PCPs and, if they did not indicate a desire for no contact, were contacted for screening. Because of early recruitment success, not all PCP-approved patients were mailed an invitation letter or contacted for screening.
Fig. 1.
The screening process involved two stages. First, patients were offered the option of completing initial screening online on their own or being phoned for screening by research staff. The initial screening process did not require clinical evaluation, asking instead about logistical constraints (e.g., transfer of care elsewhere), known exclusionary medical conditions or treatments, and willingness to consider participation and undergo further screening. Those not excluded at the initial screening then underwent medical screening (e.g., BMI measurements, laboratory testing) to confirm clinical eligibility (overweight/obesity and pre-diabetes or metabolic syndrome). Eligible patients were then invited for final written consent at a baseline evaluation and those who met all eligibility criteria were randomized. Target sample size was 240.
2.1.4. Randomization and blinding
Patients were randomly assigned on a 1:1:1 basis to one of the three study arms using randomization and blinding procedures previously described [22].
2.1.5. Interventions and outcome measurements
Throughout the trial, all participants continued to receive standard care from their PCPs, including any behavioral obesity management provided in that context. Participants in the two active intervention arms completed a 3-month intensive intervention phase and a 12-month maintenance phase. The intensive interventions followed the University of Pittsburgh Diabetes Prevention Support Center (DPSC)'s standardized, packaged Group Lifestyle Balance™ translations of the original DPP using group- or individual, at-home DVD-based delivery. During the 3-month intensive phase, 12 weekly classes for the group-based participants were given by a dietitian (lead lifestyle coach) and exercise physiologist and covered diverse topics related to healthy eating, exercise, and behavioral self-management. To assure intervention fidelity, the DPSC provides training and certification of instructors who will be delivering the intervention to patients. The E-LITE dietitian lifestyle coach completed this DPSC instructor certification. During group sessions, the DPSC group-based DPP intervention manual [11,23] was followed, with the only exceptions being two additional activities added to all group sessions: a food-tasting at check-in and a 30- to 45-minute physical activity demonstration and practice at the session end. Participants in the DVD-based arm attended a single group orientation during which they received the DPSC DVD, which covered the same topics as the group sessions and which they were instructed to view and follow on a weekly basis on their own. During the intensive and maintenance phases, participants in both active intervention groups received technology-mediated self-management support and counseling in two forms. First, participants were encouraged to use the secure email messaging system available through their EHR patient portal to communicate with the study lifestyle coach regarding self-management activities, barriers to progress, or other aspects of behavioral weight management. Second, they were trained to use the award-winning American Heart Association (AHA) free online patient Web portal Heart360® that gave them access to interactive self-management tools that enable easy tracking of weight, physical activity, and cardiometabolic risk factors (www.heart360.org).
The primary outcome measure for E-LITE was BMI at 15 month follow-up. Secondary outcome measures are described in detail elsewhere [22] and included other clinical measures (e.g., fasting plasma lipid and glucose levels, blood pressure), as well as measures of quality of life, mental health, adherence, and healthcare utilization.
2.1.6. Statistical analyses
The primary analysis compared BMI between each active intervention arm and the usual care control arm. Statistical analyses have been reported previously in greater detail [22].
2.2. Assessment of intervention potential for reach and adoption using baseline data
The RE-AIM Framework (reach, effectiveness, adoption, implementation, and maintenance) was designed to assess an intervention's potential for sustainable implementation and public health impact in real-world settings—and thereby to enhance the translation of research into practice [19,24].
Independent efforts to broadly disseminate the DPSC's group and DVD programs have already been underway [11,18]. To help inform the furtherance of these efforts, we used recruitment process and baseline data from the E-LITE trial to assess the reach and adoption potential of its two active interventions. Other RE-AIM dimensions (effectiveness, implementation, and maintenance) will be evaluated separately with outcome data from E-LITE. We applied a grading scale (good, fair, poor) for the applicability of reach and adoption components to real-world practice and provided descriptive summaries for these determinations.
We assessed reach components of participation number, rate, and representativeness. We used the conservative estimation of participation rate proposed by Glasgow et al. [25]:
We also evaluated how E-LITE reached the target population by assessing its methods for identifying and recruiting the target population [19,20].
We assessed adoption components of setting representativeness and organizational spread. We also explored potential barriers to or support for organizational spread by evaluating the feasibility of intervention modalities and resource requirements [19,20].
3. Results
We successfully randomized 241 patients (Fig. 1). Compared with participants in the original DPP trial [2], E-LITE participants were more likely to be male (54%), have educational attainment beyond high school (97%), and self-identify as white (78%) or Asian (17%), rather than black (0.4%) or Latino/Hispanic (4%). The educational and race/ethnicity profiles represented the general adult patient population at the enrolling primary care clinic site. Otherwise, E-LITE and DPP participants had similar baseline age and clinical characteristics (Table 2).
Table 2.
Characteristics of Potential E-LITE Participants During Sequential Stages of Recruitment and at Randomization in E-LITE vs. DPP.
| E-LITE |
Original DPP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mailed letter (n = 3119) | Initial screena (n = 1567) |
Medical screen (n = 752) | Eligible (n = 279) | Randomized (n = 241) |
Randomized (n = 3234) Overall | |||||||
| By phone (876) | Online (691) | P valueb | Overall | By phone initial screen (117) | Online initial screen (124) | P valueb | Overall | |||||
| Age, yr (SD) | 48.4 (13.1) | 49.9 (12.7) | 51.6 (12.1) | 0.010 | 50.7 (12.4) | 51.4 (11.3) | 53.5 (10.7) | 51.9 (9.8) | 53.9 (11.3) | 0.13 | 52.9 (10.6) | 50.6 (10.7) |
| Female, % | 45.5 | 44.3 | 52.2 | 0.002 | 47.8 | 47.8 | 45.0 | 39.3 | 53.2 | 0.03 | 46.0 | 67.7 |
| Race/ethnicity, % | 0.003 | 0.40 | ||||||||||
| White | 71.2d | 69.1 | 77.4 | 72.9 | 75.1 | 75.6 | 76.9 | 79.0 | 78.0 | 54.7 | ||
| Asianc | 18.5d | 22.3 | 14.5 | 18.7 | 17.7 | 19.3 | 16.2c | 17.7c | 17.0 | 4.4 | ||
| Latino/Hispanic | 7.6d | 6.4 | 5.8 | 6.1 | 5.8 | 4.4 | 6.0 | 2.4 | 4.2 | 15.7 | ||
| Black | 1.7d | 1.5 | 1.6 | 1.6 | 1.0 | 0.4 | 0 | 0.8 | 0.4 | 19.9 | ||
| Other | 1.0d | 0.6 | 0.7 | 0.7 | 0.4 | 0.4 | 0.9 | 0 | 0.4 | 5.3 | ||
| Educational attainment 13 + yr, % | 97.2 | 74.3 | ||||||||||
| Clinical characteristics (SD) | ||||||||||||
| BMI, kg/m2 | 32.0 (5.4) | 34.0 (6.7) | ||||||||||
| Pre-diabetes, % | 54.5 | 100 | ||||||||||
| Metabolic syndrome, % | 86.7 | 52.9 | ||||||||||
| Waist circumference, cm | 41.9 (4.7) | 41.4 (5.7) | ||||||||||
| Fasting plasma glucose, mg/dL | 99.9 (9.5) | 106.5 (8.3) | ||||||||||
| Triglycerides, mg/dL | 171.1 (69.2) | 159.1 (85.8) | ||||||||||
| HDL, mg/dL | 46.1 (12.4) | 43.9 (10.5) | ||||||||||
| Systolic BP, mm Hg | 118.8 (11.7) | 123.7 (14.7) | ||||||||||
| Diastolic BP, mm Hg | 73.6 (8.3) | 78.3 (9.3) | ||||||||||
E-LITE = Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care. Yr = year. SD = standard deviation. BMI = body mass index. BP = blood pressure. HDL = high density lipoprotein cholesterol.
Missing race/ethnicity data on 95 (6.1%) of all patients who underwent initial screening: 14 (2.0%) who completed screening online, 81 (9.3%) who completed by phone.
P value calculations are for comparisons of by phone vs. online groups.
Two randomized participants were of Pacific Islander race–one who initially screened online and the other by phone. The remaining patients in this row were of Asian race.
For race/ethnicity data at the “mailed letter” recruitment stage, n= 1499 because the remainder of the original 3151 patients declined to answer/left blank the race/ethnicity question on the questionnaire used by the health system at the time of their entrance into the health network.
3.1. Reach characteristics
3.1.1. Methods of potential participant identification
Use of the EHR to define an initial pool of the target population was efficient, with good applicability to sites with similar technologies.
3.1.2. Target population size
For the single study site, the original EHR search identified 3800 potential participants of whom 3439 were deemed eligible by their PCPs indicating that within one clinic there were many potential participants to be targeted for intervention. (Fig. 1)
3.1.3. Target population risk factors
Certain risk factors (BMI, blood pressure measurements) were determined to have good applicability to real-world practice because they were low-cost and routinely performed. Other risk factors were found to have fair applicability because they were either more costly to assess (laboratory measurements of plasma cholesterol or glucose levels) or not done routinely in current practice (waist circumference measurements) but remained readily obtainable in general clinical practice.
3.1.4. Recruitment strategies
Patients who were approved for contact by their PCPs were mailed a hardcopy invitation letter signed by or for the PCP. The letter described two methods for initial screening: by phone with a research assistant (traditional method) or online on their own (technology enhanced method). Of those who chose to accept screening (n=1567), 876 (55.9%) completed screening by phone and 691 (44.1%) did so online. (Table 2) Of patients ultimately randomized, 124 of 241 (54%) completed the initial screening online on their own. Patients who chose to perform the initial screen online were significantly more likely to be older, female, and self-identified as white, although absolute age or percent differences between groups were not pronounced. (Table 2) Mailed invitation letters were classified as having good real-world applicability for participant first contact because they were cheap and efficient. Similarly, the performance of initial screening online was deemed to have fair-to-good applicability because online contact with patients was also cheap and efficient and is becoming more common, particularly in practices with EHRs. Screening by phone was deemed to have fair applicability because it was more accessible for some patients but also more costly – based on personnel requirements – than online screening.
3.1.5. Eligibility and ineligibility determinations
Twenty-two percent (279 of 1246) of patients who agreed to undergo full screening (to the point of confirmed eligibility or ineligibility determination) were found eligible. Among ineligible patients (other than those with no reason recorded, n=4), 119 of 963 (12%) were excluded for research-specific reasons that were not likely to be applicable in general clinical practice (e.g., cardiovascular disease or diabetes already present), but the majority (844, 88%) were excluded because they were not within the population expected to be targeted in the real world (e.g., were not overweight/obese). Ineligibility criteria were thus deemed to have fair applicability for real-world translation. (Table 3)
Table 3.
Reasons for ineligibility and whether these are likely to apply in clinical practice.
| Reason for ineligibility is… | Count (n = 963)a | Percent |
|---|---|---|
| …Likely to apply in clinical practice (i.e., patient is not within target population) | (844) | (87.6) |
| Unable to confirm high risk for cardiometabolic disease | 516 | 53.6 |
| Unable to confirm pre-diabetes or metabolic syndrome | 467 | 48.5 |
| BMI<25 | 49 | 5.1 |
| Logistical constraints | 247 | 25.6 |
| Care elsewhere/moving elsewhere | 113 | 11.7 |
| Unable to commit to time/effort required | 101 | 10.5 |
| No computer access | 14 | 1.5 |
| Planning bariatric surgery soon/enrolled in other program | 10 | 1.0 |
| Not English proficient | 9 | 0.9 |
| Medical conditions | 81 | 8.4 |
| Pregnant or plan for pregnancy in near future | 40 | 4.2 |
| Recovering from/in treatment for cancer | 19 | 2.0 |
| Severe psychiatric disturbance | 16 | 1.7 |
| Systolic≥160 and/or diastolic≥100 | 5 | 0.5 |
| Living in a long-term care facility | 1 | 0.1 |
| …Unlikely to apply in clinical practice (i.e., reason is research-specific) | (119) | (12.4) |
| Logistical constraints | 13 | 1.3 |
| Household member involved in study | 13 | 1.3 |
| Medical conditions | 106 | 11.0 |
| On weight loss or diabetes medication | 33 | 3.4 |
| Cardiovascular disease and/or diabetes already present | 27 | 2.8 |
| Change in certain classes of medication in prior 3 monthsb | 27 | 2.8 |
| BMI>=40c | 10 | 1.0 |
| TG>=400 | 5 | 0.5 |
| eGFR<60 | 4 | 0.4 |
BMI = body mass index (kg/m2). TG = triglyceride level (mg/dL). eGFR = estimated glomerular filtration rate.
Of the 967 potential participants who were determined to be ineligible, four had no reason recorded for ineligibility.
Class of medication includes antidepressant, antihypertensive, or antihyperlipidemic agents.
Removed as an ineligibility criterion in 8/2009.
3.1.6. Participation rate
Overall, PCPs reviewed 3800 patients and approved 3439 (91%) to be screened for participation. (Fig. 1) Primarily due to rapid recruitment success, invitation letters were sent to only 3119 approved patients, and for 728 of these, further contact was not attempted. Of the 2391 for whom contact was attempted for initial screening, 351 (15%) were unreachable, 783 (33%) declined to participate, 359 (15%) failed initial screening, 143 (6%) failed between screenings (e.g., labs newly out of range), and 465 (19%) failed the subsequent medical screening. Participation rate using the conservative formula was 44% [25], (Table 1) a rate assessed to have fair real-world applicability.
Table 1.
Reach and adoption categories and characteristics of E-LITE: applicability for translation into real-world practice.
| Category | Characteristic in E-LITE | Applicability for translation into real-world practice (good, fair, or poor) |
|---|---|---|
| Reach (identification, recruitment, and representativeness of participants) | ||
| Methods of identification | Use of EHR to identify candidate patients | • Fair to good–applies well to clinics with EHR capabilities, which are growing in number |
| Target population size | 3880 patients identified in initial search of clinic EHR with PCPs approving 3520 for further evaluation | • Good–obesity/overweight is highly prevalent within a single clinic |
| Risk factors | Patients with obesity/overweight and also cardiometabolic risk factors (pre-diabetes and/or metabolic syndrome) (Table 2) | • Good–for low-cost measurements (e.g., BMI) |
| • Fair–for lab-based criteria (e.g., cholesterol panel) and some clinical measurements, because are more costly or are not routinely performed but also are readily available | ||
| Recruitment strategies | Mailed letter as first invitation contact 1st stage of screening performed either online or by phone Of those patients ultimately randomized over half completed the initial screening online | • Good–for letters, because cheap and easy to do |
| • Fair to good–for online contact, as this kind of contact with patients is growing and increasingly applicable in the real world | ||
| • Fair–for phone calls, because more costly/labor intensive | ||
| Eligibility and ineligibility | 22% of those screened were determined to be eligiblea (Fig. 1) 88% of ineligibility determinations were due to reasons likely to apply in the real world. (Table 3) | • Fair–acceptable distribution of ineligibility reasons that have face validity and are easy for patients/physicians to determine |
| Participation rate | Conservative estimate = 44%b (Fig. 1) More liberal estimate = 98%b | • Fair–reasonable when compared to RCTs in which conservative participation rates were calculated |
| Participants vs. non-participants | Age, sex, and race/ethnicity data demonstrate no compelling differences in demographics, with the possible exception of older and white patients being overrepresented (Table 2) | • Fair–no strong selection bias by demographics |
| Adoption (research interface with potential program settings) | ||
| Setting | Single private, urban primary care clinic with all PCPs agreeing to participate | • Fair to good–applies well to private, urban clinics but less well to others |
| Intervention modalities | Group classes and DVDs with parallel content; EHR secure messaging system; AHA online portal of self-management tools | • Good–for group classes, which are commonly used in many clinic settings |
| • Fair–for DVDs, EHR messaging, and use of online portal, because these require personal IT access and skills. However, use of DVDs and online interactions/tools with patients is growing in frequency and is increasingly applicable in real world settings | ||
| Resource requirements | Research staff with intervention training and materials provided by the DPSC | • Fair–applicable to clinics that can allocate resources for personnel trainingc/materials but not applicable to other clinics |
| Organizational diffusion/spread | 100% of providers at intervention site agreed to participate Modified version of group-based intervention has been adopted by the health network of which the study clinic is a part | • Fair to good–applicable at local study clinic and modified group-based intervention adopted early by health network, but latter lacks certain components of intervention (i.e., search of clinic EHR, online screening, EHR secure messaging, use of AHA portal) so is unclear how these intervention components will apply |
E-LITE = Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care. EHR = electronic health record. PCP = primary care provider. DVD = digital versatile disc. AHA = American Heart Association. DPSC = Diabetes Prevention Support Center. BMI = body mass index (kg/m2).
Based on eligibility determinations for those patients who underwent full screening processes: (eligible)/(ineligible+eligible).
The conservative estimate of participation rate uses the Glasgow et al. approach [25]: participation rate=(number of participants who were randomized)/(number of participants for whom contact was attempted and eligibility was assumed or confirmed). The more liberal estimate is the percent of patients who were eligible who then became participants.
Personnel training currently costs $275 for the 2-day training program provided on-site at the Pittsburgh DPP Support Center.
3.1.7. Characteristics of participants versus non-participants
While participants who were randomized were more likely than non-participants to be older and self-identified as white, other demographic differences across various stages in the recruitment process were not pronounced, (Table 2) indicating fair applicability for real-world translation.
3.2. Adoption characteristics
3.2.1. Setting
Regarding adoption, the E-LITE clinic site was determined to have good applicability for private practice, urban primary care clinics, but to be less applicable to other contexts (e.g., county public health clinics, rural health clinics). (Table 2)
3.2.2. Intervention modalities
The intervention modality of group classes was deemed to have good real-world applicability because of its use at a diversity of clinics. Use of technology-based interventions – namely, DVDs for the DVD-based intervention group and the EHR secure messaging system and AHA free patient self-management web portal for both active intervention groups – was determined to be most applicable to clinics with patient populations who are computer and internet users, resulting in a determination of fair real-world applicability.
3.2.3. Resource requirements
Group class training and content were supported by the DPSC, which offered (and continues to offer) staff certification, manuals, and handouts on how to conduct these. Similarly, the DVDs with content parallel to the group classes' were produced and supported by the DPSC. Certification training is currently offered for $275 per person for on-site training in Pittsburgh, PA (http://www.diabetesprevention.pitt.edu/docs/registration.pdf). Manuals and handouts can be downloaded from the Support Center website (http://www.diabetesprevention.pitt.edu/glbmaterials.aspx) for free as long as they will be used for non-commercial purposes. These resource requirements were assigned fair applicability, because clinics must also have the financial capability to purchase and assign personnel for certification training and to reproduce manual and handout materials.
3.2.4. Organizational spread
All 21 providers actively involved in patient care at the lone study clinic site during the recruitment period agreed to participate in the study. The parent health network adopted the Pittsburgh group-based DPP translation program – but without the E-LITE modifications to session content (i.e., food tasting and guided physical activity) or technology-mediated support – within all four California Bay Area counties in which it operates. Within each county, the health network has one or more “centers” that offer primary care to large numbers of patients (n=12 total). There are 5 additional “offices” that are smaller sites for primary care provision. In each county, at least one of the “centers” offers the group-based DPP translation program, which equates to 5 of 12 (42%) centers or 5 of 17 (29%) total primary care sites (i.e., including the smaller “offices”) offering the program within the health network. Yet, for a self-paid fee, patients who receive care at any primary care site are eligible to enroll in the program even if it is not being conducted at their personal clinic site. Together, the 17 primary care sites have more than 66,000 obese adult patients. The health network has staggered roll-out of the program to the 5 initial sites in order to assure that existing resources could adequately support it. Thus, organizational spread was determined to have fair-to-good applicability.
4. Discussion
The E-LITE interventions have fair-to-good real-world applicability on the dimensions of reach and adoption. While prior studies have explicitly applied the RE-AIM Framework [19,24] to translational RCT interventions, few studies of behavioral weight management interventions in primary care settings have done so and even fewer have reported RE-AIM characteristics in a structured fashion useful for for researchers and potential end users of the intervention being tested [26]. Regarding the reach component of participation rate, three internet-based diabetes self-management trials among primary care practices reported participation rates (using the same conservative formula as applied in the present paper) of 37%, 38%, and 50% [25,27], respectively, similar to E-LITE's 44% participation. A recent translational weight loss trial conducted among county health departments reported an average participation rate of 1.2%, although this was based on an estimated target population that was defined to include all low-income obese women living in the participating counties; thus it encompassed a much broader target population than the one in E-LITE [26]. We were unable to identify papers that applied the RE-AIM Framework to other DPP-based translational weight loss interventions. Yet structured reporting on RE-AIM dimensions within reports of translational interventions may promote better understanding of intervention characteristics in the same way that other reporting initiatives have for study structure and conduct [28–30]. In turn, better understanding may assist comparisons between interventions and promote appropriate (versus inappropriate) dissemination if stakeholders are better able to identify and understand the pertinence of a given intervention to their particular patient populations and organizations [26].
Innovations that may be worthy of particular attention in E-LITE are its uses of information technology. Use of an online screening tool to reach potential participants is an emerging approach in the scientific literature and likely to be even less common in routine clinical practice. We identified three nutrition-related trials that described use of patient-completed online screening for enrollment. Two nutrition trials noted an increase in recruitment success and decrease in staff time allocated to screening with use of online screening compared to telephone screening [31,32]. A third trial noted variability in use of online screening according to user age, income, and race/ethnicity, but that a diverse participant population was ultimately enrolled by this means [33]. Similarly, the E-LITE patients who chose to screen online rather than by phone were significantly older and more likely to be female and self-identified as white than their phone-screening counterparts. Yet the absolute age or percentage differences between the two groups were generally modest. Regarding adoption characteristics, a recent AHA review on “New and emergent weight management strategies for busy ambulatory settings” emphasized the potential importance of internet strategies to promote weight loss but did not identify any studies that incorporated EHR systems into weight management interventions [34]. The literature is also limited on credible, public internet-based self-management portals like the AHA's Heart360®. Evaluation of EHR secure messaging and internet self-management tools in E-LITE provides information on the effectiveness of these emerging intervention modalities [21].
Regarding adoption characteristics and leveraged use of lifestyle intervention materials from the DPSC, we identified one RCT and one cluster-RCT that used locally developed, group-based “adaptations” of the DPP intervention (not ones developed by the DPSC). These were delivered at a community diabetes care center and community YMCA gyms, respectively [6,7]. The former demonstrated greater reductions in the primary outcomes of waist circumference and plasma glucose and insulin levels among intervention group participants versus controls at 12 months [6]. The latter found a greater mean decrease in percent body weight among intervention group participants than controls (6.0 versus 2.0) at 6 months [7]. In an additional two studies, DPSC-affiliated researchers performed non-randomized trials of the DPSC group-based and DVD-based materials in primary care settings [11,18]. At 3 month follow-up, participants in these studies achieved significant mean decreases in percent body weight (range 3.5 to 6.6). Percent weight loss was comparable among those who received the group-based intervention (3.5% and 6.6%) [11,18] and the DVD-based interventions (5.6%) [18]. These results are similar to those achieved and sustained in E-LITE at 15 month, rather than 3 month, follow-up [21].
The implications of our analysis of reach and adoption characteristics may vary for different stakeholders. Health professionals (e.g., physician and nurse champions of obesity management) may be most interested in methods of patient identification and their recruitment and representativeness. For instance, the ability to leverage EHR and online resources to identify, screen, and provide self-management support to patients may allow primary care clinics to systematically focus on their subpopulation of obese patients. In addition, they may want to know what proportion of patients were excluded for reasons likely to apply at their clinics (e.g., BMI not in range of overweight/obese) or whether those patients who participated in the program resemble their clinic patients. For their part, healthcare organizations may be particularly interested in staff training requirements and in adoption characteristics—whether the program has a good chance of being disseminated throughout the organization or whether they have the ability to replicate the E-LITE intervention modalities (e.g., group classes, secure messaging). Finally, payers may be most concerned with having a target population that is clearly defined and sizable enough to warrant covering or subsidizing the intervention as a new benefit.
Our evaluation has multiple limitations. First, the E-LITE participants derive from a clinic located within the Silicon Valley region of the San Francisco Bay Area, an area known for its information technology expertise. This means that E-LITE participants are likely, on average, to have more information technology exposure and knowledge than the average American adult, although we also recognize that the majority of American households now contain at least one computer. Second, we had incomplete race/ethnicity data on patients who were mailed invitation letters, although PAMF was the first large ambulatory care organization to begin to collect self-reported race/ethnicity data in an outpatient setting and has more detailed data than most outpatient centers [35]. Third, our evaluation of adoption is limited to assessment of one clinic site for physician adoption and one health network for organizational diffusion. Finally, we recognize that our determinations of real-world applicability (“good, fair, or poor” in Table 1) are not based on quantitative data that would validate their scaling; at the same time, we provide descriptive information to explain the determinations, so that others can follow our reasoning and also come to their own determinations.
5. Conclusions
We found that E-LITE translations of DPP-based weight loss interventions into primary care practice have fair-to-good real-world applicability, with innovative strengths being leveraged use of information technologies and existing DPP Support Center resources. We hope that our experience with this analysis will encourage other researchers to report the reach, adoption, and additional RE-AIM characteristics of their DPP-related and other translational interventions in a structured fashion. Such reporting may help health professionals, healthcare organizations, and payers make informed judgments about which translational interventions best fit their practice and patient needs. It may also spur their greater confidence and investment in ground-level diffusion of evidence – that is, dissemination beyond trials – which must be the goal for widespread propagation and public health impact of translational research.
Acknowledgments
We are indebted to the following individuals for their contributions to the design and/or conduct of the study: Sandra R. Wilson, PhD (E-LITE Co-Investigator); Amy L. Muzaffar, MD (Study Physician); Lan Xiao, PhD (Biostatistician); Andrea Blonstein, MBA, RD, and Rachel Press, BA (Lifestyle Coaches); Veronica Luna, BS (Project Coordinator); Alicia Geurts, BS, Elizabeth Jameiro, MD, and Debbie Miller, BS (Research Assistants). We thank Lan Xiao and Veronica Luna for input on this article. We also wish to thank the E-LITE Data and Safety Monitoring Board. We also would like to acknowledge the Diabetes Prevention Support Center (DPSC) of the University of Pittsburgh for training and support in the Group Lifestyle Balance program; the current program is derivative of this material. Finally, special thanks go to the E-LITE participants and their families that made this study possible.
Role of funding source The E-LITE study was supported by grant R34DK080878 from the National Institute of Diabetes and Digestive and Kidney Diseases, a Scientist Development Grant award (0830362N) from the American Heart Association, and internal funding from the Palo Alto Medical Foundation Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the American Heart Association. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript. Trial registration is: ClinicalTrials.gov NCT00842426.
Abbreviations
- DPP
Diabetes Prevention Program
- RE-AIM model
Reach, Effectiveness, Adoption, Implementation, Maintenance model
- DVD
digital versatile disc
- E-LITE trial
Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care trial
- RCT
randomized controlled trial
- DPSC
Diabetes Prevention Support Center
- AHA
American Heart Association
- BMI
body mass index
- PCP
primary care provider
- EHR
electronic health record
Footnotes
Conflict of interest statements Dr. Stafford reports that he has provided consulting services to Mylan Pharmaceuticals in the past. The remaining authors declare that they have no competing interests.
References
- [1].Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- [2].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34:1451–7. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero David G. Translating the diabetes prevention program into the community: the DEPLOY pilot study. Am J Prev Med. 2008;35:357–63. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kramer MK, Miller R, Venditti E, Orchard TO. Group lifestyle intervention for diabetes prevention in those with metabolic syndrome in primary care practice. Diabetes Care. 2006;55(Suppl.):A517. [Google Scholar]
- [9].Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract. 2008;14:29–32. doi: 10.1097/01.PHH.0000303410.66485.91. [DOI] [PubMed] [Google Scholar]
- [10].Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31:684–9. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- [11].Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the diabetes prevention program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–11. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- [12].McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: translating an effective lifestyle intervention into clinical practice. Diabetes Educ. 2009;35(199–204):208. doi: 10.1177/0145721709332815. [DOI] [PubMed] [Google Scholar]
- [13].Amundson HA, Butcher MK, Gohdes D, Hall TO, Harwell TS, Helgerson SD, et al. Translating the diabetes prevention program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ. 2009;35(209–4):216. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- [14].Aldana SG, Barlow M, Smith R, Yanowitz FG, Adams T, Loveday L, et al. The Diabetes Prevention Program: a worksite experience. AAOHN J. 2005;53:499–505. [PubMed] [Google Scholar]
- [15].McBride PE, Einerson JA, Grant H, Sargent C, Underbakke G, Vitcenda M, et al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. J Nutr Health Aging. 2008;12:745S–9S. doi: 10.1007/BF03028624. [DOI] [PubMed] [Google Scholar]
- [16].Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ. 2011;37:659–68. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- [17].Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102:336–42. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kramer M, Kriska AM, Venditti EM, Semler LN, Miller RG, McDonald T, et al. A novel approach to diabetes prevention: evaluation of the group lifestyle balance program delivered via DVD. Diabetes Res Clin Pract. 2010;90:e60–3. doi: 10.1016/j.diabres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- [19].Glasgow RE, Lichtenstein E, Marcus AC. Why don't we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–7. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Akers JD, Estabrooks PA, Davy BM. Translational research: bridging the gap between long-term weight loss maintenance research and practice. J Am Diet Assoc. 2010;110:1511–22. doi: 10.1016/j.jada.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the diabetes prevention program lifestyle intervention into primary care: a randomized trial. Arch Intern Med. doi: 10.1001/2013.jamainternmed.987. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract. 2009;10:71. doi: 10.1186/1471-2296-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].University of Pittsburgh, Group Lifestyle Balance Manual of Operations [Accessed 9-19-11]; Available at http://www.diabetesprevention.pitt.edu/docs/Group%20Lifestyle%20Balance%20Manual%20of%20Operations091511.pdf; 2010.
- [24].Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Glasgow RE, Strycker LA, Kurz D, Faber A, Bell H, Dickman JM, et al. Recruitment for an internet-based diabetes self-management program: scientific and ethical implications. Ann Behav Med. 2010;40:40–8. doi: 10.1007/s12160-010-9189-1. [DOI] [PubMed] [Google Scholar]
- [26].Samuel-Hodge CD, Garcia BA, Johnston LF, Kraschnewski JL, Gustafson AA, Norwood AF, et al. Rationale, design, and sample characteristics of a practical randomized trial to assess a weight loss intervention for low-income women: the Weight-Wise II Program. Contemp Clin Trials. 2012;33:93–103. doi: 10.1016/j.cct.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [27].Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30:67–73. doi: 10.1016/j.amepre.2005.08.037. [DOI] [PubMed] [Google Scholar]
- [28].Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- [29].Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- [30].Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smith KS, Eubanks D, Petrik A, Stevens VJ. Using web-based screening to enhance efficiency of HMO clinical trial recruitment in women aged forty and older. Clin Trials. 2007;4:102–5. doi: 10.1177/1740774506075863. [DOI] [PubMed] [Google Scholar]
- [32].Arab L, Hahn H, Henry J, Chacko S, Winter A, Cambou MC. Using the web for recruitment, screen, tracking, data management, and quality control in a dietary assessment clinical validation trial. Contemp Clin Trials. 2010;31:138–46. doi: 10.1016/j.cct.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stopponi MA, Alexander GL, McClure JB, Carroll NM, Divine GW, Calvi JH, et al. Recruitment to a randomized web-based nutritional intervention trial: characteristics of participants compared to non-participants. J Med Internet Res. 2009;11:e38. doi: 10.2196/jmir.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rao G, Burke LE, Spring BJ, Ewing LJ, Turk M, Lichtenstein AH, et al. New and emerging weight management strategies for busy ambulatory settings: a scientific statement from the American Heart Association. Circulation. 2011;124 doi: 10.1161/CIR.0b013e31822b9543. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [35].Ulmer C, McFadden B, Nerenz D, editors. Race, ethnicity, and language data: standardization for health care quality improvement. Institute of Medicine; 2009. [PubMed] [Google Scholar]