Abstract
Rationale
Varenicline, an approved smoking cessation pharmacotherapy, also shows promise as a potential treatment for alcohol dependence. However, varenicline has not been tested in heavy drinkers, and it remains to be determined whether varenicline could reduce alcohol craving and consumption in smokers who are trying to quit smoking.
Objectives
We conducted a preliminary study to examine the effect of varenicline on drinking behavior and the effects of extended varenicline pretreatment on smoking.
Methods
Thirty heavy drinking smokers received smoking cessation counseling and were randomly assigned to receive either an extended 4-week pretreatment with varenicline 2 mg daily or the usual 1-week pretreatment. Those in the extended pretreatment group received active medication for 8 weeks (i.e., 4 weeks of active pre-treatment followed by 4 weeks of active treatment), and participants in the usual pretreatment group received active medication after a placebo lead in (i.e., 3 weeks of placebo followed by active medication for 5 weeks).
Results
Participants who received varenicline during the first 3 weeks reported significantly greater reductions in alcohol craving and numerically fewer heavy drinking days compared to those who received placebo, and these differences persisted during the open-label phase. Extended pretreatment was associated with numerically greater reductions in cigarette smoking over the entire study period. There were no differences, however, in smoking abstinence rates following the smoking quit date between the two groups.
Conclusions
Findings from this preliminary study suggest that varenicline may be a promising strategy for concurrently reducing heavy drinking and promoting smoking changes in heavy drinkers.
Keywords: Smoking cessation, Varenicline, Heavy drinking, Alcohol craving
Introduction
Combined heavy alcohol use and cigarette smoking is common (Grant et al. 2004), represents a significant health burden (Durazzo et al. 2007; Kuper et al. 2000; Lowenfels et al. 1994; Vaillant et al. 1991; Znaor et al. 2003), and is associated with poor treatment outcomes. Heavy drinkers are less likely to initiate a smoking quit attempt (Osler et al. 1999; Zimmerman et al. 1990) and to achieve both short-and long-term abstinence from smoking when they try to quit (Cooney et al. 2004; Hughes 1995; Leeman et al. 2008). Likewise, smoking and higher nicotine dependence levels at the start of alcoholism treatment are associated with greater alcohol urge, an increased likelihood of relapse to drinking, and a greater number of drinks consumed upon relapse (Abrams et al. 1992; Hintz and Mann 2007). Among individuals who have achieved long-term abstinence from alcohol, continued smoking is a predictor of alcohol relapse (Dawson 2000). Thus, interventions that could concurrently reduce smoking and heavy drinking in this high risk group would be very important.
Current FDA approved pharmacotherapies for alcohol dependence (i.e., acamprosate, naltrexone, and disulfiram) have small to moderate effects on drinking (Kranzler and Van Kirk 2001; Pettinati and Rabinowitz 2006) and may be less beneficial for smokers (Baltieri et al. 2009; Mason and Lehert 2009). Varenicline, an FDA-approved smoking cessation aid, shows promise as a potential treatment for alcohol dependence in smokers. Nicotinic acetylcholine receptors (nAChRs) are known to play a role in the rewarding effects of both nicotine and alcohol. Varenicline, a partial agonist of α4β2, α3β2, and α6 nAChRs, and an agonist of α3β4 and α7 nAChRs (Coe et al. 2005; Davis and de Fiebre 2006; Obach et al. 2006; Rollema et al. 2007), results in higher rates of abstinence from smoking than bupropion or placebo (Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006). Varenicline, however, has not been tested for smoking cessation in heavy drinkers. In fact, alcohol-dependent smokers were excluded from the varenicline clinical trials conducted to date.
Animal and human laboratory research data suggest that varenicline may be useful for treating alcoholism. Varenicline administration reduces alcohol seeking and consumption in rats (Steensland et al. 2007) and mice (Kamens et al. 2010). Varenicline also modulates the effects of ethanol on dopamine release (Ericson et al. 2009). Varenicline administered acutely reduced dopamine levels in response to ethanol and semi-chronic pretreatment with varenicline abolished the additive effect of co-administration of nicotine and ethanol on accumbal dopamine levels, suggesting that varenicline can reduce the reinforcing effects of ethanol (Ericson et al. 2009). Research on specific nAChR subtypes has found that varenicline reduces drinking in β2 knockout (KO) and α7 KO mice, suggesting that receptors containing these subunits are not solely required for varenicline’s effects on drinking (Kamens et al. 2010). In contrast, varenicline’s effects appear to be dependent on activation of receptors containing the α4 subunit (Hendrickson et al. 2010). Specifically, varenicline did not reduce drinking in α4 knockout mice but dramatically decreased drinking in animals expressing α4 nAChRs that are hypersensitive to agonist (Hendrickson et al. 2010). In a human laboratory self-administration paradigm with heavy drinking smokers, 1-week exposure to varenicline resulted in a greater reduction in alcohol consumption and craving compared to placebo (McKee et al. 2009). Taken together, these results suggest that it would be worthwhile to test whether varenicline reduces alcohol craving and consumption in heavy drinkers who are trying to quit smoking.
The typical dose schedule for varenicline involves a 1-week pretreatment phase to ensure that smokers achieve a steady state medication level when smoking abstinence is initiated (Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006). This pretreatment period may also promote smoking cessation success by reducing smoking reinforcement and dependence on inhaled nicotine during the pretreatment period (Rose et al. 2006). The possibility that varenicline may also improve quit rates through an extinction mechanism derives from the observation that 1-week point prevalence smoking abstinence rates increase over the first 4 weeks following the quit date as compared to placebo (Gonzales et al. 2006). Thus, extended pretreatment with varenicline may yield better smoking cessation outcomes than the standard 1 week lead in period. Extended pretreatment may further promote smoking abstinence if varenicline pretreatment also reduces alcohol consumption (a common smoking relapse precipitant) prior to the tobacco quit attempt.
Therefore, we conducted a pilot study of active varenicline (titrated to 2 mg/daily) over 8 weeks compared to active varenicline for 5 weeks with a 3-week placebo pretreatment phase in 30 heavy drinking smokers. We hypothesized that varenicline would result in a lower percentage of heavy drinking days and lower alcohol craving compared to placebo and that it would be well tolerated in a sample of heavy drinkers. We also anticipated that extended pretreatment (i.e., 4 weeks prior to the smoking quit date) with varenicline would result in a greater likelihood of continuous abstinence from smoking than usual pretreatment (i.e., 1 week).
Materials and methods
Design
Thirty heavy drinkers seeking smoking cessation treatment were randomly assigned to one of two conditions: extended (4-week) pretreatment with varenicline prior to the smoking quit date or usual (1-week) pretreatment. The extended pretreatment group received active medication for 8 weeks (i.e., 4 weeks of active pre-treatment followed by 4 weeks of active treatment), and the usual pretreatment group received active medication after a placebo lead-in (i.e., 3 weeks of placebo followed by active medication for 5 weeks). The primary outcome for the 3-week placebo-controlled phase was percent heavy drinking days. During this period, participants prepared to make a smoking cessation attempt the day after their week 4 appointment but did not receive counseling about their drinking until the week 4 appointment. The primary outcome for the post-smoking cessation quit date phase was continuous abstinence from smoking over the last 4 weeks of treatment. Given that a high rate of smoking abstinence is achieved with varenicline during the first month after the quit date (Gonzales et al. 2006; Jorenby et al. 2006) and our primary focus was on the effect of varenicline on alcohol seeking and consumption, we provided varenicline treatment for 4 weeks following the smoking quit date instead of the typical 11 weeks provided for standard smoking cessation treatment for the purposes of this pilot study.
Participants
Eligibility requirements included being 18–75 years of age, smoking ≥5 cigarettes/day ≥ 3 days/week, having fewer than 3 months of smoking abstinence within the past year, and exceeding maximum weekly drinking limits every week within the past 4 weeks (i.e., >7 drinks/week for women, >14 drinks/week for men) and maximum daily drinking limits on at least one occasion within the past 4 weeks (i.e., >3 drinks/day for women, >4 drinks/day for men) (National Institute on Alcohol Abuse and Alcoholism 2004).
Only one person per household could enroll. Women were excluded if they were pregnant, nursing, or not using a reliable form of birth control. Smokers were excluded for current, serious neurological, psychiatric, or medical illness (as assessed by study physician); current drug dependence other than nicotine; current serious alcohol dependence assessed by the study nurse practitioner and defined as (a) a history of seizures, delirium, or hallucinations during alcohol withdrawal, (b) a Clinical Institute Withdrawal Assessment scale (Sullivan et al. 1989) score of ≥8, (c) report drinking to avoid withdrawal symptoms, or (d) a history of alcohol withdrawal treatment; current use of smokeless tobacco, pipes, cigars, nicotine replacement products, or marijuana; use of clonidine, varenicline, bupropion, or nortriptyline within the past month; or a positive urine drug test for any illegal drug.
Procedures
This trial was approved by the Institutional Review Board of Yale University School of Medicine. Participants were recruited through newspaper advertisements, posters/flyers placed around the local community, notices on our website, and other internet-based advertisements (e.g., http://www.google.com). Prospective participants were screened by telephone and at two subsequent intake appointments, one of which included a physical examination with laboratory testing. Following screening, 30 eligible participants were randomized to one of the two treatment conditions (i.e., extended pretreatment or usual pretreatment) with randomization stratified by gender. Random sequence was provided by the statistician (RW) to the pharmacist who assigned participants. Participants were randomized between December 15, 2008 and April 7, 2010, and the last follow-up appointment was completed on May 5, 2010.
One to two weeks following the intake, eligible participants started treatment. Prior to enrollment, participants selected a quit date with the study nurse practitioner to commence 28 days after starting treatment. For the first 3 weeks of the pretreatment phase, participants were randomly assigned to receive either varenicline titrated to 2 mg/daily or placebo. Both participants and research staff were blind to participant assignment. In order to maintain the blind, all medications were encapsulated and dispensed in blister packs for the first 4 weeks and then switched to bottles for the open label phase of the study. The titration schedule (with induction beginning in the first week for those in the extended pretreatment group or the fourth week for those in the usual pretreatment group) for varenicline was as follows: varenicline 0.5 mg once per day for days 1–3, varenicline 0.5 mg twice per day for days 4–7, then varenicline 1 mg twice per day thereafter (Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006). In the final week of the pretreatment period, participants in the usual pretreatment condition began varenicline using the titration schedule described above. Participants in the extended pretreatment condition continued on 2 mg daily.
During the pretreatment phase, participants attended weekly appointments to receive medication refills and counseling to prepare for their upcoming smoking quit attempt and complete study assessments. Participants were assigned to one of three counselors, all with extensive smoking cessation counseling experience (e.g., Toll et al. 2008, 2010). Provision of brief psychoeducational smoking cessation counseling was standardized; counselors adhered to a manual. Counseling was adapted from the American Lung Association Freedom from Smoking® program and emphasized enhancement of motivation and confidence to quit smoking, creation, and implementation of a quit plan, utilization of stimulus control techniques, promotion of skills related to coping with urges and withdrawal symptoms, and application of relapse prevention strategies. Consistent with Clinical Practice Guidelines for smoking cessation (Fiore et al. 2008), counselors provided brief advice for participants to reduce their alcohol use to facilitate quitting smoking. In the present study, this advice occurred at the last pretreatment appointment (i.e., the day before the smoking quit date). Specifically, participants were advised to consider abstaining from alcohol use for the first 2 weeks of quitting smoking or to at a minimum moderate their drinking and were provided with information about drinking strategies for moderating consumption. A handout summarizing this information was provided to all participants at the session.
After the smoking quit date, participants returned weekly to receive smoking counseling and medication refills and to complete assessments. Smoking counseling reinforced advice to abstain from/moderate drinking to promote smoking cessation. Participants were paid $10 for attending each appointment.
Measures
Intake measures used to screen potential participants for eligibility and characterize the enrolled sample included a demographic questionnaire, a smoking history questionnaire, substance use, eating, panic, psychosis, and mood disorder sections of the Structured Clinical Interview for DSM-IVAxis I Disorders (First et al. 1996) and the Clinical Institute Withdrawal Assessment for Alcohol (revised) (Sullivan et al. 1989).
Drinking outcomes
At each appointment, frequency, and quantity of alcohol use was obtained using the Timeline Followback (TLFB) interview (Sobell and Sobell 2003), alcohol craving was measured using the Obsessive Compulsive Drinking Scale (Anton et al. 1995), and subjective ratings of alcohol stimulating and sedating effects were measured using a version of the Subjective High Assessment Scale adapted for this study (Martin et al. 1993).
Smoking outcomes
Frequency and quantity of smoking was measured at each appointment using the TLFB interview (Sobell and Sobell 2003), and abstinence was biochemically confirmed with breath carbon monoxide (CO) levels.
Safety and tolerability
Varenicline safety and tolerability was monitored at each appointment using: (1) the Columbia Suicide Severity Rating Scale (Posner et al. 2007), an interview assessing past and current suicidal ideation, intent, and attempts and (2) a modified version of the Systematic Assessment for Treatment Emergent Effects (Johnson et al. 2005; Levine and Schooler 1986), an interview assessing physical or health problems and changes in physical appearance or activity level in an open-ended format as well as specific symptoms commonly associated with varenicline on a scale from 0 (not present) to 3 (severe). Varenicline safety and tolerability in heavy drinkers was evaluated through a count of adverse effects and severity ratings of these effects on both measures.
Statistical analyses
Baseline characteristics were analyzed using chi-square tests for categorical variables and general linear models (GLM) for continuous variables. The intention of this study was to develop effect size estimates to be used for a larger trial and to assess the feasibility of use of varenicline with this population. Thus, we recruited 30 participants for these purposes. Group differences in percentage of heavy drinking days with baseline as a covariate and changes in alcohol craving during the placebo-controlled phase of the study (i.e., up to week 3) were also examined by GLM. Percentage of days abstinent, drinks per drinking day, and changes in subjective ratings of alcohol were also examined as secondary outcomes using GLM. Logistic regression analyses were performed to evaluate differences in continuous smoking abstinence over the last 4 weeks of treatment and 7-day point prevalent abstinence over the last week of treatment. Abstinence from smoking was defined as self-reported abstinence (no smoking, not even a puff) during the specified post-quit treatment period, verified by an exhaled CO level of ≤10 ppm (SRNT Subcommittee on Biochemical Verification 2002). Consistent with the convention in the field of smoking cessation clinical trials, participants who dropped out or missed multiple appointments were considered to be smoking. Data for a single missed appointment were coded abstinent when participants reported not smoking, and a CO≤10 ppm was obtained at the appointments before and after the missed session.
Mixed models were employed to analyze number of cigarettes smoked over all 8 weeks of treatment with baseline as a covariate. Adverse events reported at a severity greater than baseline were compared during the 3-week placebo-controlled phase and over the course of the entire 8-week study using Fisher’s exact tests for individual adverse events, including suicidal ideation and behavior on the Columbia Suicide Severity Rating Scale. No corrections for multiple testing were employed. An effect size estimate for percentage of heavy drinking days was computed using Cohen’s d (Cohen 1988).
Results
Participant characteristics
One hundred five heavy drinking smokers completed a study phone screen. Of the 99 volunteers who met initial phone screen eligibility, 59 attended an intake appointment. Among those who attended the intake, four chose not to enroll and 25 were deemed ineligible, producing a final sample of 30 study participants. Reasons for study exclusion included (1) current serious health problems or medical conditions (n=11), (2) not meeting smoking or drinking study criteria (n=8), (3) other drug use (n=4), and (4) current depression requiring treatment (n=2). The final randomized sample of 30 provided further information about their smoking and drinking following the initial receipt of study medication. Twenty-five completed treatment and 27 provided data at the end of treatment (week 8).
The intent to treat sample (16 men, 14 women) was primarily Caucasian (90%), had a mean age of 43.17± 8.12 years, and smoked an average of 21.10±8.12 cigarettes per day for a mean of 19.25±11.84 years. The majority of participants met current criteria for alcohol dependence (77%; n=23), and the remainder met lifetime alcohol dependence criteria (23%; n=7). All participants reported smoking 7 days/week. Approximately a third of the sample (33%) reported prior use of varenicline for smoking cessation. The two conditions were equivalent at baseline on key demographic, smoking, and drinking variables (see Table 1).
Table 1.
Summary of participant characteristics by condition assignment
| Characteristics | Condition
|
|
|---|---|---|
| Varenicline pretreatment (n=15) | Placebo pretreatment (n=15) | |
| Age (M±SD) | 42.87 ± 8.52 | 43.47 ± 7.99 |
| Gender [n (%)] | ||
| Male | 8 (53) | 8 (53) |
| Female | 7 (47) | 7 (47) |
| Race/ethnicity [n (%)] | ||
| Racial/ethnic minority | 1 (7) | 2 (13) |
| Caucasian | 14 (93) | 13 (87) |
| Education [n (%)] | ||
| High school degree or less | 3 (20) | 4 (27) |
| Some college | 10 (67) | 6 (40) |
| College degree | 2 (13) | 5 (33) |
| Cigarettes per day (M±SD) | 22.17 ± 7.31 | 18.88 ± 6.85 |
| Years smoking at this level (M±SD) | 16.13 ± 10.82 | 22.85 ± 12.36 |
| Prior varenicline use [n (%)] | ||
| Yes | 4 (27) | 6 (40) |
| No | 11 (73) | 9 (60) |
| Current alcohol dependence [n (%)] | ||
| Yes | 12 (80) | 11 (73) |
| No | 3 (20) | 4 (27) |
| % heavy drinking days (M±SD) | 42.67 ± 26.28 | 48.44 ± 31.99 |
| % abstinent days (M±SD) | 31.78 ± 23.36 | 21.11 ± 29.35 |
| OCDSa total alcohol craving (M±SD) | 7.95 ± 3.71 | 8.16 ± 3.43 |
OCDS obsessive compulsive drinking scale
Drinking outcomes
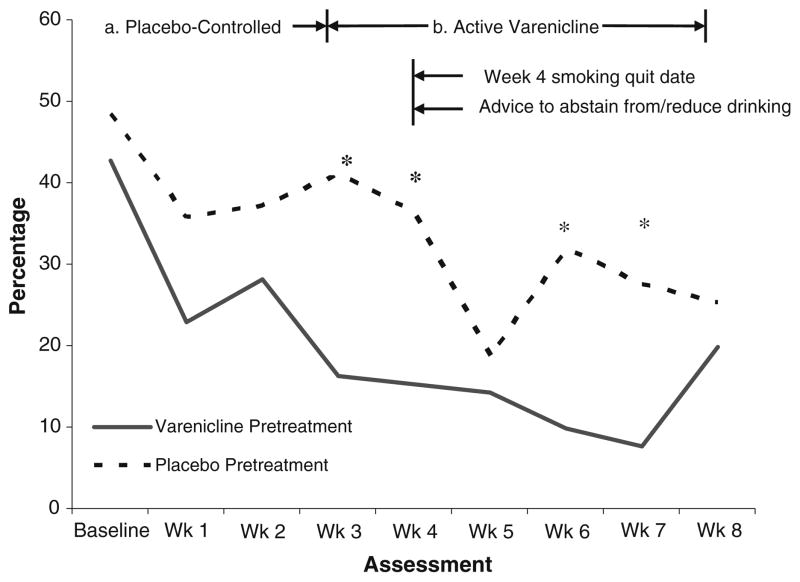
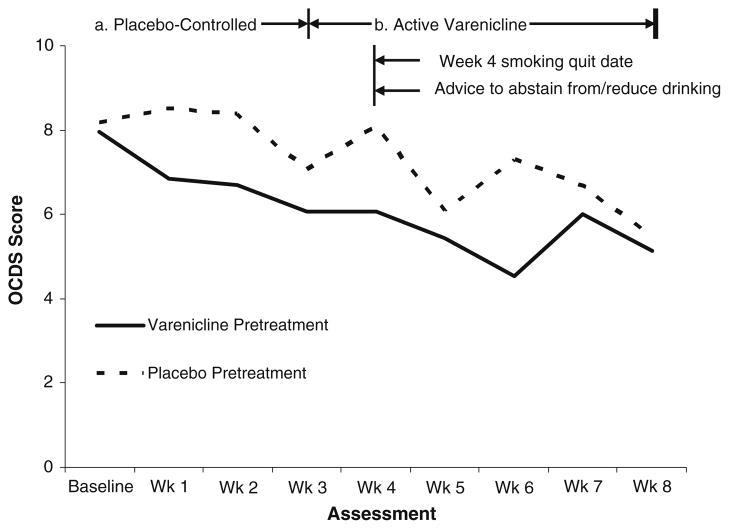
During the placebo-controlled pretreatment phase, varenicline-treated participants reported a tendency toward fewer heavy drinking days (22.7%±17.5%) compared with placebo-treated participants (38.0%±31.2%) [F (1, 27)=3.91, p=0.06, Cohen’s d=0.60], controlling for baseline heavy drinking days (see Fig. 1). Varenicline was also associated with a significantly greater reduction in alcohol craving over this period than placebo [M change=1.59, SD=1.92 vs. M change=0.29, SD=2.59; F (1, 19)=5.84, p=0.03] (see Fig. 2) as well as significantly greater increases in subjective ratings of alcohol sedating effects [M change=2.92, SD=8.92 vs. M change=−7.09, SD=7.34; F (1, 22)=8.80, p=0.01]
Fig. 1.
Percentage of heavy drinking days over entire study period by condition (*p<0.05)
Fig. 2.
Obsessive–compulsive drinking scale craving scores over entire study period by condition (significant decrease in craving from baseline to week 3 and baseline to week 8 in varenicline pretreatment condition, p<0.05)
After the placebo-controlled phase, participants in the extended varenicline pretreatment condition reported a significantly lower percentage of heavy drinking days at several time points (i.e., weeks 4, 6, and 7) (see Fig. 1) as well as lower alcohol craving (see Fig. 2). The reduction in craving from baseline to the end of treatment (week 8) was greater among participants in the extended varenicline pretreatment condition than the usual pretreatment condition (i.e., placebo) [M change=2.43, SD=3.16 vs. M change=1.57, SD=3.57; F (1, 133)=4.69, p=0.03]. Likewise, the reduction in heavy drinking from baseline to the end of treatment was numerically greater among participants in the extended varenicline pretreatment condition [M change=32.10, SD=30.96 vs. M change=17.08, SD= 19.11; F (1, 162)=1.94, p=0.17, Cohen’s d=0.58]. Similarly, the increase in subjective ratings of alcohol sedating effects from baseline to the end of treatment was numerically greater among extended varenicline-treated participants [M change=4.55, SD=16.73 vs. M change=−11.33, SD=18.87; F (1, 18)=3.98, p=0.06].
During the placebo-controlled phase and entire treatment period, there were no differential effects of condition on changes in percent abstinent days, number of drinks per drinking occasion, or ratings of alcohol stimulating effects (p’s>0.30).
Smoking outcomes
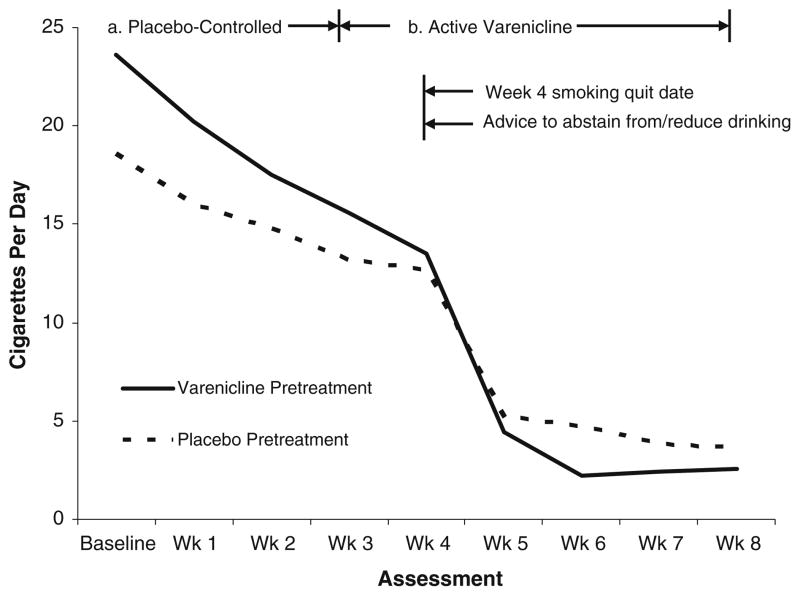
There were no differences in rates of continuous smoking abstinence over the last 4 weeks of treatment (p=0.37) or 7-day point prevalence smoking abstinence over the last week of treatment (p=0.27) between the two conditions. Across both conditions, 43.33% (13/40) of participants were abstinent from smoking at the end of treatment on the 7-day point prevalence measure. There was a non-significant trend for a condition by time interaction for cigarettes smoked over the entire study period (F (1, 196)=3.85, p= 0.05). Specifically, participants in the extended varenicline pretreatment condition exhibited a tendency to reduce their number of cigarettes smoked more rapidly over time compared with participants in the usual pretreatment condition (see Fig. 3).
Fig. 3.
Number of cigarettes smoked over entire study period by condition (significant reduction in smoking over time in varenicline pretreatment condition, p<0.05)
Safety and tolerability
No participant reported current suicidal ideation or behavior on the Columbia Suicide Severity Rating Scale during the study. There were no serious adverse events. During the placebo-controlled pretreatment phase, participants who received varenicline reported a higher incidence of mild nausea on the SAFTEE than participants who received placebo (67% vs. 20%; p=0.03, Fisher’s exact test). The two conditions, however, did not significantly differ on ratings of nausea over the entire study period. After the placebo-controlled pretreatment phase, more participants in the usual pretreatment condition reported moderate constipation compared to participants in the extended pretreatment condition (20% vs. 0%; p=0.22, Fisher’s exact test), with this difference occurring during the week after the quit date. The frequency of other symptoms was equivalent between conditions during both periods. Adverse effects led to a recommendation for a dose reduction for one participant assigned to the usual pretreatment condition; that participant then discontinued the medication when the reported effects persisted despite a dose reduction.
Adherence did not significantly differ by condition (p> 0.10]. Based on medication taken and appointments attended while participants were actively enrolled, adherence during the pretreatment phase was as follows: 70.89% doses, 91.67% session attendance (extended varenicline pretreatment) and 73.39% doses, 90% session attendance (usual pretreatment). During the post-quit phase, adherence was as follows: 70.97% doses, 80% session attendance (extended varenicline pretreatment) and 60.22% doses, 77.33% session attendance (usual pretreatment).
Discussion
To our knowledge, this is the first empirical report testing the efficacy of varenicline for reducing heavy drinking in smokers attempting to quit smoking as well as the efficacy of extended varenicline pretreatment for promoting smoking cessation in this population. We successfully retained a high percentage of participants during the extended pretreatment phase as well as over the entire study period. Moreover, medication adherence was high, and the adverse effects of varenicline were similar to those reported by non-heavy drinking smokers (Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006).
Consistent with prior human laboratory research (McKee et al. 2009), varenicline resulted in greater reductions in alcohol craving and a tendency toward greater reductions in alcohol consumption than placebo. Although the overall difference between groups on percentage of heaving drinking days during the placebo-controlled phase was non-significant (p=0.06), the effect size was in the medium to large range in size (Cohen’s d=0.6, Cohen 1988) and favored varenicline. Thus, it is possible that significance would be achieved with a larger sample. Moreover, significant group differences in percent heavy days drinking were observed for 50% of the individual weekly assessments across the entire study (i.e., weeks 3, 4, 6, and 7). The behavioral mechanism by which varenicline may affect alcohol craving and consumption remains to be determined. Nevertheless, our finding that varenicline increased the sedating effects of alcohol relative to placebo suggests that varenicline may reduce alcohol reinforcement. Consistent with this effect, varenicline, compared to placebo, was associated with smaller increases in positive subjective effects of alcohol in a recent human laboratory study of varenicline (McKee et al. 2009) and increases in the sedating effects of ethanol assessed in mice (Kamens et al. 2010).
Of note, the improvements in drinking with extended varenicline pretreatment were apparent prior to and following advice to reduce or abstain from alcohol consumption, suggesting that varenicline may be useful in helping hazardous drinking smokers prepare to change their alcohol consumption. Johnson and colleagues (2003, 2007) pioneered an approach in which actively drinking participants began topiramate and received brief counseling emphasizing medication adherence in order to promote gradual improvements in drinking rather than requiring alcohol abstinence prior to enrollment. Using this model, topiramate lead to significant reductions in drinking compared to placebo by the end of treatment although the percentage of individuals abstaining from alcohol was relatively low. Similar reductions in drinking among heavy drinkers not counseled to change their drinking have been observed with naltrexone (O’Malley 2008). A next iteration of this model could combine a period of pretreatment emphasizing medication adherence followed by a period emphasizing active efforts to change drinking. Pretreatment with varenicline may lend itself to this approach.
Extended pretreatment with varenicline did not reduce heavy drinking at all time points potentially due to a range of factors. During the initial weeks of pretreatment (i.e., weeks 1–2), some participants may have initially persisted in habitual drinking despite reductions in alcohol craving. In the post-quit period (i.e., week 5), brief advice about alcohol provided to all participants the day before quitting smoking may have also initially equalized drinking. Larger samples and longer treatment studies are needed to understand the effects of varenicline over time.
Although extended pretreatment with varenicline was associated with a tendency toward greater reductions in the number of cigarettes over time, this did not translate into improved smoking abstinence. The short duration of varenicline treatment post-cessation may have limited larger effects of extended pretreatment on smoking abstinence. Regardless, the 7-day point prevalence abstinence rate of 43% across the two groups measured at 4 weeks post-quit is quite encouraging and consistent with the rate observed with varenicline in studies of non-heavy drinking smokers at this same time point (i.e., approximately 43–48%; Jorenby et al. 2006; Nides et al. 2006).
Combined heavy alcohol use and cigarette smoking represents a serious public health concern (Pelucchi et al. 2006). Heavy alcohol use reduces smoking cessation success (Kahler et al. 2009, 2010; Leeman et al. 2007), and smoking is a risk factor for drinking relapse (Abrams et al. 1992; Hintz and Mann 2007). There is an urgent need to identify effective interventions that could concurrently reduce smoking and drinking. Together with emerging evidence from preclinical studies and laboratory based research, the results of this preliminary clinical trial suggest that varenicline warrants further investigation in a larger double-blind, placebo-controlled trial in a heavy drinking smoking sample.
Acknowledgments
We would like to thank Susan Neveu, Jessica Hopkins, and Erica Peters for assistance with recruitment, retention, and counseling of participants. We would also like to thank Elaine LaVelle for assistance with data management. Dr. Stephanie O’Malley is a member American College of Neuropsychopharmacogy work-group, the Alcohol Clinical Trial Initiative, sponsored by Eli Lilly, Janssen, Johnson & Johnson, Schering Plough, Lundbeck, Glaxo Smith Kline and Alkermes; partner, Applied Behavioral Research; contract, Nabi Biopharmaceuticals; Advisory Board, Gilead Pharmaceuticals; consultant, GlaxoSmithKline, Brown University; Scientific Panel of Advisors, Hazelden.
This research was supported in part by NIH grants P50-AA15632 (SS0), K12-DA000167 (BAT), K05-AA014715 (SSO), and P50-AA012870 (SSO), T32-AA015496 (LMF), and the State of Connecticut, Department of Mental Health and Addictions Services. The treatment of participants in this study complies with the ethical standards of the American Psychological Association.
Footnotes
All other authors report no conflict of interest.
Contributor Information
Lisa M. Fucito, Email: lisa.fucito@yale.edu, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA
Benjamin A. Toll, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA. Yale Cancer Center, New Haven, CT 06519, USA. Smilow Cancer Hospital at Yale–New Haven, New Haven, CT 06519, USA
Ran Wu, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA.
Denise M. Romano, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA
Ece Tek, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA.
Stephanie S. O’Malley, Email: stephanie.omalley@yale.edu, Department of Psychiatry, CMHC-S200, Yale University School of Medicine, 34 Park Street, New Haven, CT 06519, USA. Yale Cancer Center, New Haven, CT 06519, USA
References
- Abrams DB, Rohsenow DJ, Niaura RS, Pedraza M, Longabaugh R, Beattie MC, Binkoff JA, Noel NE, Monti PM. Smoking and treatment outcome for alcoholics: effects on coping skills, urge to drink, and drinking rates. Behav Ther. 1992;23:283–297. [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Daró FR, Ribeiro PL, De Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2009;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cooney JL, Cooney NL, Patten CA, George TP. Comorbidity of nicotine dependence with affective, psychotic and substance use disorders. In: Kranzler HR, Tinsley JA, editors. Dual diagnosis and psychiatric treatment: substance abuse and comorbid disorders. 2. Marcel Dekker; New York: 2004. pp. 211–259. [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;9:179–185. [PMC free article] [PubMed] [Google Scholar]
- Dawson D. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Durazzo T, Cardenas V, Studholme C, Weiner M, Meyerhoff D. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaen C, Baker T, et al. Clinical practice guideline. USDHHS, Public Health Service; Rockville: 2008. Treating tobacco use and dependence, 2008 update. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders, research version, patient edition. Biometrics Research, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha-4-beta-2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of 4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz T, Mann K. Long-term behavior in treated alcoholism: evidence for beneficial carry-over effects of abstinence from smoking on alcohol use and vice versa. Addict Behav. 2007;32:3093–3100. doi: 10.1016/j.addbeh.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice (NIAAA research monograph no 95-3931) Government Printing Office; Washington: 1995. [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ait-Daoud N, Roache J. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol. 2005;66:157–167. doi: 10.15288/jsas.2005.s15.157. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha-4-beta-2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kahler C, Borland R, Hyland A, McKee S, Thompson M, Cummings K. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2009;100:214–220. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler C, Borland R, Hyland A, McKee S, Thompson M, Cummings K. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2010;110:101–107. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology. 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. doi: 10.1111/j.1530-0277.2001. tb02356.x. [DOI] [PubMed] [Google Scholar]
- Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- Leeman RF, Huffman CJ, O’Malley SS. Alcohol history and smoking cessation in nicotine replacement therapy, bupropion sustained release, and varenicline trials: a review. Alcohol Alcohol. 2007;42:196–206. doi: 10.1093/alcalc/agm022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman R, McKee S, Toll B, et al. Risk factors for treatment failure in smokers: relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine Tob Res. 2008;10:1793–1809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Lowenfels A, Maisonneuve P, Cavallini G, et al. Prognosis of chronic pancreatitis: an international multicenter study. International Pancreatitis Study Group. Am J Gastroenterol. 1994;89:1467–1471. [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mason B, Lehert P. Effects of nicotine and illicit substance use on alcoholism treatment outcomes and acamprosate efficacy. J Addict Med. 2009;3:164–171. doi: 10.1097/ADM.0b013e3181917d53. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Newsletter. 2004. NIAAA council approves definition of binge drinking. [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- O’Malley SS. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Osler M, Prescott E, Godtfredsen N, Hein H, Schnohr P. Gender and determinants of smoking cessation: a longitudinal study. Prev Med. 1999;29:57–62. doi: 10.1006/pmed.1999.0510. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health. 2006;29:193–198. [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Rabinowitz AR. Choosing the right medication for the treatment of alcoholism. Curr Psychiatry Rep. 2006;8:383–388. doi: 10.1007/s11920-006-0040-0. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Wilson VB, editors. Assessing alcohol problems: a guide for clinicians and researchers. 2. National Institute on Alcohol Abuse and Alcoholism; Bethesda: 2003. pp. 75–99. [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bardett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. PNAS. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-AR) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O’Malley SS. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addict Behav. 2008;33:173–179. doi: 10.1016/j.addbeh.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, White M, Wu R, Meandzija B, Jatlow P, Makuch R, O’Malley SS. Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomized trial. Drug Alcohol Depend. 2010;111:200–206. doi: 10.1016/j.drugalcdep.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant G, Schnurr P, Baron J, Gerber P. A prospective study of the effects of cigarette smoking and alcohol abuse on mortality. J Gen Intern Med. 1991;6:299–304. doi: 10.1007/BF02597425. [DOI] [PubMed] [Google Scholar]
- Zimmerman R, Warheit G, Ulbrich P, Auth J. The relationship between alcohol use and attempts and success at smoking cessation. Addict Behav. 1990;15:197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]
- Znaor A, Brennan P, Gajalakshmi V, Mathew A, Shanta V, Varghese C, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681–686. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]