Abstract
One important behavioral role for head movements is to assist in the redirection of gaze. However, primates also frequently make head movements that do not involve changes in the line of sight. Virtually nothing is known about the neural basis of these head-only movements. In the present study, single-unit extracellular activity was recorded from the superior colliculus while monkeys performed behavioral tasks that permit the temporal dissociation of gaze shifts and head movements. We sought to determine whether superior colliculus contains neurons that modulate their activity in association with head movements in the absence of gaze shifts and whether classic gaze-related burst neurons also discharge for head-only movements. For 26% of the neurons in our sample, significant changes in average firing rate could be attributed to head-only movements. Most of these increased their firing rate immediately prior to the onset of a head movement and continued to discharge at elevated frequency until the offset of the movement. Others discharged at a tonic rate when the head was stable and decreased their activity, or paused, during head movements. For many putative head cells, average firing rate was found to be predictive of head displacement. Some neurons exhibited significant changes in activity associated with gaze, eye-only, and head-only movements, although none of the gaze-related burst neurons significantly modulated its activity in association with head-only movements. These results suggest the possibility that the superior colliculus plays a role in the control of head movements independent of gaze shifts.
INTRODUCTION
Over the last several decades, much neurophysiological work has been done to clarify the role of the superior colliculus (SC) in the generation of saccadic eye movements. This structure is also known to be involved in coordinated eye-head gaze shifts. For example, numerous studies have shown that micro-stimulation of SC evokes movements of both the eyes and head in both cats (Guillaume and Pelisson 2001, 2006; Munoz et al. 1991; Pare et al. 1994; Roucoux et al. 1980) and monkeys (Freedman et al. 1996; Klier et al. 2001). In addition, the activity of single SC neurons, particularly of the classic gaze-related burst cells (sometimes referred to as “burst neurons” in this report), is well correlated with gaze amplitude (Freedman and Sparks 1997a; Munoz et al. 1991). Data from a variety of species have led to the idea that the role of this structure in the control of gaze is part of a more general role in the control of orienting movements. Stein and Clamann (1981) stimulated the SC in cat and found a topographic map of pinna movements in register with the map of saccadic eye movements. Stimulation of the optic tectum in goldfish evokes not only eye movements but also movements of the tail (Herrero et al. 1998). In rodents, stimulation of SC evokes a whole-body circling response (Tehovnik 1989; Tehovnik and Yeomans 1986). In the bat, stimulation of SC not only evokes movements of the pinnae and head but also sonar vocalizations (Valentine et al. 2002). In the primate SC, a number of studies have reported neural activity that is correlated with arm movements (Kutz et al. 1997; Stuphorn et al. 1999, 2000; Werner 1993; Werner et al. 1997). Stimulation of the cat SC during ongoing reach movements induces perturbations in mid-flight (Courjon et al. 2004).
Data also exist to support the notion that the SC has access to head movement control circuitry. Anatomical studies have demonstrated tecto- and tectoreticulospinal projections (Cowie et al. 1994; Huerta and Harting 1982; May and Porter 1992; Robinson et al. 1994). In terms of electrophysiological evidence, Corneil et al. (2002) reported that when SC was stimulated at current levels below the threshold to evoke gaze shifts, head movements were sometimes evoked in the absence of a gaze shift [i.e., vestibuloocular reflex (VOR) gain of one]. These authors also reported that subthreshold stimulation evokes neck muscle electromyographic activity (Corneil et al. 2002, 2007). The onset of this activity is time-locked to the visual response of SC during the performance of a gap task (Corneil et al. 2004). Petit and Beauchamp (2003) used fMRI to study head movements in humans. These authors reported that head-only movements were associated with activation of a number of areas, including frontal eye fields, supplementary eye fields, intraparietal sulcus, precuneus, MT, basal ganglia, thalamus, and SC, suggesting that head-only movements may be subserved by many of the same areas that control saccades. Although these studies do not convincingly establish a role for SC in the control of head movements independent of gaze shifts, they do at least suggest the possibility.
Humans and monkeys do sometimes make head movements without gaze shifts. Humans do this frequently, such as when shaking the head to indicate “no” or nodding the head to indicate “yes” while speaking to another person. Monkeys may make head movements without gaze shifts as part of displays of aggression and/or when biting off a chunk of food while fixating on a distant target. When the eyes are at somewhat eccentric orbital positions, such head movements could be used to more favorably orient the pinnae toward the current target of visual fixation and restore the eyes to a more optimal, central orbital position. It is possible that SC might play a role in the generation of such movements.
Although none of the available data demonstrates unambiguously that SC is involved in the generation of head movements independent of gaze shifts, this possibility is worth investigating. The issue, unfortunately, is difficult to address using standard behavioral tasks that elicit coordinated eye-head gaze shifts. The problem is that the eyes and head are temporally coupled, which makes it difficult to demonstrate that single-unit activity is truly related to head movements and not eye or gaze. This problem is particularly difficult to address because the relationship between eye and head movements during gaze shifts is highly lawful and consistent (Freedman and Sparks 1997b).
With these considerations in mind, we have devised a series of behavioral tasks that dissociate movements of the eyes and head. Monkeys were trained to make eye movements without head movements, head movements without gaze shifts, and coordinated eye-head gaze shifts. Using these tasks, we sought to determine whether the SC contains neurons that discharge in association with head movements in the absence of gaze and whether saccade-related burst neurons also fire for head movements.
Portions of these results have been published previously in preliminary form (Gandhi and Walton 2006).
METHODS
Two male macaque monkeys (Macaca mulatta) were used. Approval for this study was granted by the Institutional Animal Care and Use Committee for the University of Pittsburgh, and all procedures were in compliance with guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Each monkey initially underwent sterile surgery to implant a Teflon-coated coil of wire underneath the conjunctiva of one eye to monitor gaze position using the magnetic search coil technique (Fuchs and Robinson 1966; Judge et al. 1980) and to affix a stainless steel post to the skull to permit head restraint. This head post was also used as a base to which a miniature laser module (Edmund Optics, Model No. P57-100) was mounted during experiments (Gandhi and Sparks 2001). This laser was used to provide the animal with visual feedback regarding his head position during some of the behavioral tasks (see following text). A second coil of wire was then implanted in the bone cement to monitor head position. After these procedures, the animals were trained for 4–6 mo on the tasks described in the following text. After the animals had attained proficiency on these tasks, a second surgery was performed to prepare for neurophysiological recording. A single stainless steel chamber was positioned over a 15-mm hole trephined in the skull. In each animal, the chamber was roughly centered on the midline and tilted 38° with respect to the coronal plane, such that the electrode penetrations were approximately orthogonal to the SC surface.
Single-unit recording
Tungsten microelectrodes (Microprobe) were used to record extracellular activity from the intermediate and deep layers of SC. On-line, the SC was identified by the presence of distinctive bursting of background activity associated with flashes of room lights and gaze shifts, stimulation-evoked gaze shifts, and recordings of typical gaze-related burst neurons and buildup cells.
Behavioral tasks and visual display
Data acquisition was accomplished through the use of custom software written in LabView running the Real Time module (Bryant and Gandhi 2005). Targets were presented on a cylindrical, tri-state light-emitting diode (LED) board spanning 96° horizontally and 80° vertically. LEDs were spaced 2° apart.
Head-movement-related activity was primarily assessed using a series of eye-head dissociation tasks (Fig. 1). These began with the appearance of a red LED to which the monkey was asked to align the laser spot within 3 s. Next, a green LED was illuminated at a second location, and the animal was required to look to the new target without allowing the laser spot to move >5–7° away from the red LED (hereafter referred to as an “eye-only movement”). For some trial-types (Fig. 1A), the red target was extinguished and the green target changed to red. Because the animal was required to continue looking at this target after the color change, completion of this stage required a head movement without a change in gaze (hereafter referred to as a “head-only” movement). Fixation of both head and eye was then required for a variable period (generally 800-1,200 ms). For the head-only task, the animal was then rewarded. On other trials, referred to as head-only-gaze trials, the animal was required to perform an additional gaze shift to earn the reward. In this case, the red LED was extinguished simultaneously with the appearance of a yellow target at a new location. Reward was contingent upon looking to the location of this target and maintenance of fixation for a variable period. The monkey was permitted to use any combination of eye and head movements to fixate the yellow target. This step was implemented several months into the project as an additional control to address the possibility that apparent head-only activity could, in fact, be related to anticipation of a reward (Ikeda and Hikosaka 2003). Randomly interleaving trials with and without the additional gaze step prevented the animal from knowing when the reward was coming as he was making head-only movements. An additional advantage of this trial type is that it ensured that the eyes were centered in the orbits at the time of the gaze shift. For the gaze dissociation task, instead of the green target turning red and the original red target disappearing (the signal to initiate a head-only movement), the green and red targets and the laser were extinguished simultaneously with the appearance of a yellow target at a new location. Reward was then contingent on the animal successfully looking to the yellow target and maintaining fixation for 800-1,200 ms. Note that the eye-in-head and head-in-space positions were aligned when the yellow target appeared after a head-only movement but were dissociated when the yellow target appeared instead of the cue to initiate a head-only movement.
FIG. 1.
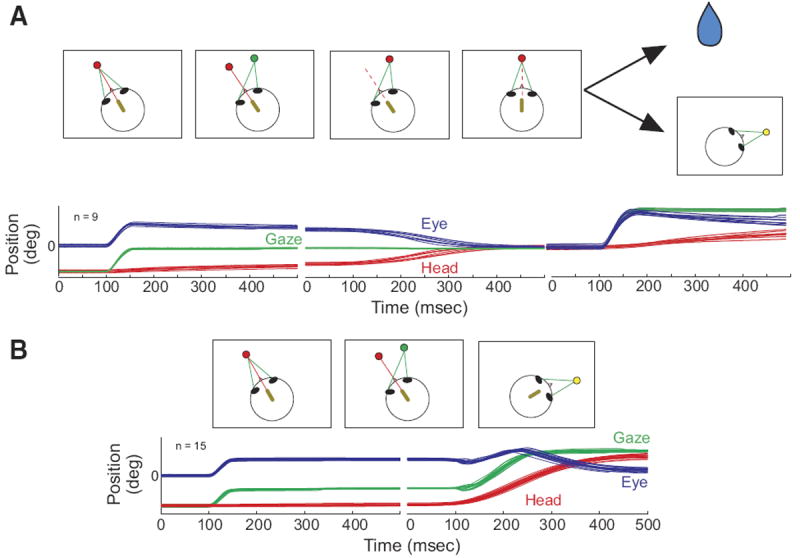
Schematic representation of the “head-only” and “head-only-gaze” tasks (A) and the gaze task (B). In A, the arrows depict 2 different possibilities, a reward (depicted by the drop of water) or the appearance of a yellow target at a new location, resulting in the “head only” and “head-only-gaze” tasks, respectively. A miniature laser (yellow oval), mounted on the head, produces a red spot on a light-emitting diode (LED) board. Green lines indicate the animal’s line of sight (gaze). The dashed red line indicates that the laser may be either on or off at this stage of the task. Below the schematic representations of the tasks, eye (blue), head (red), and gaze (green) position traces are shown as a function of time. In each trace, 100 ms are shown before the onset of the movement.
Standard oculomotor tasks performed with the head unrestrained (schematics not shown) were often incorporated with the eye-head dissociation tasks. On the target step task, a green LED was first illuminated, and the monkey was required to fixate it within 500 ms. After a variable fixation interval (800-1,200 ms), this target was extinguished at the same time that a second green LED was illuminated at a new location. In this task, the monkey was rewarded for directing his gaze to each target when it was visible. The delay task was similar except that the first and second targets overlapped by a variable period (typically 400-1,000 ms). In this case, the monkey had to maintain fixation on the first target until it was extinguished. In the gap task, the first green target was extinguished 200 ms before the illumination of the second target. Reward was contingent upon the animal looking to the second target when it appeared. For the target step, delay, and gap tasks there were no constraints placed on head position. The animal was free to make any combination of eye and head movements as long as he looked to each target at the proper time. These standard oculomotor tasks were always run in equal proportions and were, collectively, not more than 40% of the total trials. However, for two cells, the gap trials were briefly run in blocks at 100%.
In early recordings, the head-only and gaze dissociation tasks were each run on 30% of the trials with the standard oculomotor tasks comprising the other 40% of trials. When the head-only-gaze task was introduced, it was run on 50% of trials with the gaze dissociation and standard oculomotor tasks run on 30 and 20% of trials, respectively.
For all trial types, a list of possible horizontal target locations was randomly combined with a list of possible vertical locations to yield a wide range of directions and amplitudes. For gaze movements, most target locations fell within the expected movement field for burst neurons at the site. However, a small number of trials involved target locations that were expected to be outside the movement field. To limit anticipatory movements, 20–30% of target locations were ipsilateral. For head-only movements, targets were chosen from across the ipsilateral and contralateral visual fields. This was done to avoid making a priori assumptions about the tuning characteristics of putative head cells. One consequence of this strategy is that we were able to collect data for movements of many different amplitudes and directions but few repeated movements of the same vector. Note that the locations of eye-only targets were dictated by the requirements of the head-only trials because, by necessity, eye- and head-only movements used the same target location for each trial.
Data analysis
Gaze shifts and eye-only movements were identified using velocity threshold criteria of 50 and 30°/s for onset and offset, respectively. Because head movements are longer, slower, and more variable than gaze shifts, a more complex algorithm (Chen and Walton 2004) was used to identify head onset and offset. Briefly, onset and offset were determined when a certain percentage of A/D points within a sliding window were found to be above (onset) or below (offset) a 6°/s velocity threshold. For onset, the optimal criterion was empirically determined to be 72/100 A/D points. If, at any point during that window, three consecutive points were found to be below the 6°/s threshold, the “count” was reset. For example, consider a 100-ms window in which the first 5 A/D points were above threshold, the next 24 points were below threshold, and the final 71 points were above threshold. In this case, the criteria would not be satisfied because the first five points would not count. When the 72/100 criterion was satisfied, the onset was defined as the first point within that window that was above threshold. For offset, a similar sliding window was used, except that the optimal criterion was found to be 16/22 A/D points below threshold. Chen and Walton (2004) have found that this algorithm, in conjunction with appropriate filtering, detects head movements as small as 1 or 2° without false positives.
To determine if a neuron exhibited activity associated with head-only movements, two intervals were selected for comparison. The head-only movement interval was defined as the period beginning 10 ms before the onset of the movement and ending at movement offset. Average firing rate during this period was compared with the average firing rate during a 400 ms baseline interval beginning 600 ms before head onset. Examination of raw traces showed that during this period, the head, eye, and gaze positions were generally stable. If, on a given trial, the eye or head was already moving during this period, the trial was not considered for this analysis. Activity associated with gaze shifts and eye-only movements was assessed using a similar approach. In this case, the 400-ms baseline window started 700 ms before the onset of the gaze shift. This was done to exclude prelude activity that would occasionally be included in the baseline period if the same window were used for all movement types. The eye-only and gaze intervals were defined as the period beginning 8 ms before the onset of the movement and ending at movement offset. For each movement (head-only, eye-only, or gaze), the cell’s modulation was computed by subtracting the mean baseline firing rate from the mean firing rate during the movement. Paired groups t-tests were used to determine if a cell significantly modulated its activity in association with head-only movements, eye-only movements, and/or gaze shifts. For these statistical comparisons, only contraversive movements were used. Due to the animals’ tendency to make small head movements that were unrelated to the task, statistical comparisons involving head-only movements were only conducted on movements >5° in amplitude.
For some analyses, it was necessary to measure the interval between a sudden change in firing rate and the onset or offset of a head movement. Burst onset was defined as the point in time at which instantaneous spike frequency first exceeded a threshold, and remained above this threshold for a minimum of 100 ms. For most cells, a threshold of 20 spike/s was used. For some cells, however, this was inappropriate due to a higher baseline firing rate. For these cells, a different threshold was empirically set such that burst onsets were never detected in the absence of gaze or head movements. Burst lead was then computed as the number of milliseconds between the onset of increased discharge and the onset of the head movement. Positive values indicate that the increase in firing rate preceded the head movement. Analyses involving burst lead and burst time were only performed on cells that exhibited at least a 30-spike/s increase in average firing rate associated with the head movement for the 10% of trials with the largest modulation. Pause onset was defined as the point in time at which instantaneous spike frequency dropped below 10 spike/s and remained below this threshold for a minimum of 100 ms. For a trial to be considered, the instantaneous firing rate over the previous 200 ms had to be consistently in excess of 30 spike/s. Pause lead was defined as the number of milliseconds between pause onset and head onset. Pause offset was defined as the point in time at which instantaneous spike frequency rose to a threshold of 20 spike/s for ≥50 ms.
An inverse distance interpolation algorithm (Sigmaplot 2004, Systat Software) was used to construct filled contour plots with neuronal activity (number of spikes or average firing rate) plotted as a function of horizontal and vertical amplitude (head or gaze). These plots were used to gain insight into the general tuning characteristics of each cell.
RESULTS
Sufficient data were obtained from 210 neurons to characterize their discharge properties with respect to head movements. Neurons were identified as possible head cells if they significantly modulated their activity in association with head-only movements, and this modulation could not be more parsimoniously explained by variables other than head movements (n = 56; see “additional controls” in the following text). In the subsections that follow, the basic discharge characteristics of head cells are first described followed by an evaluation of their tuning characteristics during head-only movements, eye-only movements, gaze shifts, and the head component of gaze shifts. The next section examines the discharge characteristics of high-frequency gaze-related burst neurons in association with head-only movements. Next, the modulation of all cells during gaze shifts, eye-only movements, and head-only movements are summarized and considered as a function of their locations within the SC. Finally, potential alternative explanations for the present results are considered.
Modulation of collicular activity during head-only movements: basic characteristics
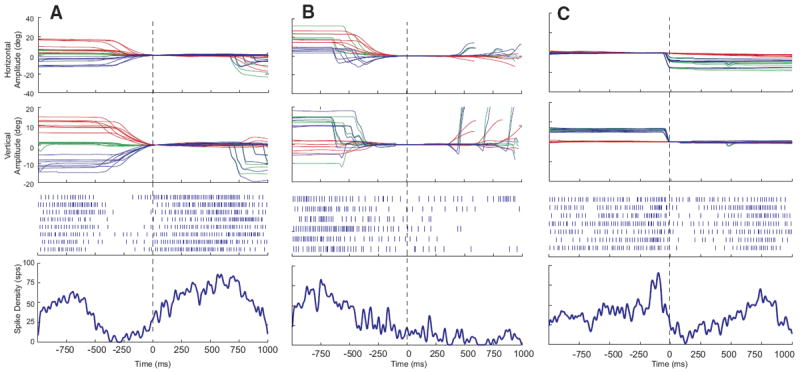
The majority (n = 39; 70%) of these 56 neurons were characterized by increased discharge rate (typically 50–200 spike/s) during head movements and a lower firing rate (anywhere from 0 to 100 spike/s) when the head was stable. After the end of the head movement, the activity returned toward baseline. Many were completely quiescent when the head was not moving. Raster plots, averaged spike density functions, and corresponding movement waveforms are shown for one example neuron during head-only movements (Fig. 2A), coordinated eye-head movements (Fig. 2B), and eye-only movements (Fig. 2C). Head, eye, and gaze positions are plotted as a function of time by red, blue, and green traces, respectively. Note that the increased discharge precedes head onset in A and B. Also in these panels, the cell can be seen to discharge at a very low rate during the low-velocity head drifts near the beginning of the plot, then activity ceases when the head is completely stable. Finally, the firing rate dramatically increases in association with the higher velocity, visually guided head movement. After the offset of these head movements, firing rate declines but does not always return to baseline because the head often drifted at subthreshold velocities after the end of the movement. For head-only movements (A), these often occurred as the monkey made small adjustments to head position after the end of the primary head movement to more precisely align the laser spot with the red LED.
FIG. 2.
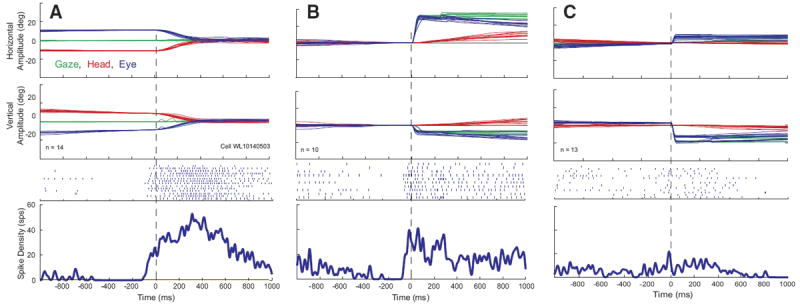
Example data for 1 cell that increased its firing rate in association with head movements. Eye, head, and gaze position traces, raster plots, and averaged spike density functions are shown for head-only movements (A), gaze shifts (B), and eye-only movements (C). Data are aligned on movement onset, indicated by the vertical dashed lines. Note that the cell begins to discharge before the onset of the head movement in both A and B. Same color conventions used for Fig. 1.
For some of these cells, upward activity modulation was sometimes observed in association with the gaze shift itself, in addition to the activity accompanying the head movement, although the gaze-related modulation was much weaker than the high-frequency bursts that characterize gaze-related burst neurons. This can be seen from the spike density function in Fig. 2B. Activity was also observed in association with eye-only movements (C), but this result is difficult to interpret because it is likely that some head cells discharge in association with such movements.
Aligning the head-only data on head movement offset (Fig. 3A) or eye-only offset (Fig. 3C) had little effect other than a shift of the averaged spike density function because similar vector movements generally had highly similar durations. In Fig. 2B, data are synchronized on gaze onset so that the reader can see the weak bursts that often accompanied gaze shifts. In Fig. 3B, data are synchronized on the offset of the head movement associated with the gaze shift so that the reader can see what this cell does after the end of the head movement. Surprisingly, the spike density function shows no obvious decline after the offset of the movement. This is due to two factors. First, it should be remembered that there were no constraints on head position when the animals were making eye-head gaze shifts or after these movements. Thus low-velocity head drifts inevitably occurred on some trials after the coordinated eye-head gaze shifts were over. These drifts were not necessarily in the same direction as the measured head movement and usually occurred after a brief (50–200 ms) period of no movement. Second, the animals were rewarded 800-1,200 ms after the animal fixated the target. Thus the position traces in B include large gaze shifts the animals performed after the end of the trial. A close inspection of the rasters for individual trials in B reveals some trials in which discharge continued beyond the measured head offset and then decreased and others in which the firing rate declined for several hundred milliseconds and then increased again. The former correspond to trials in which head drifts occurred after the offset of the measured head movement and the latter represent increased discharge associated with the large gaze shifts visible on the right side of the plot.
FIG. 3.
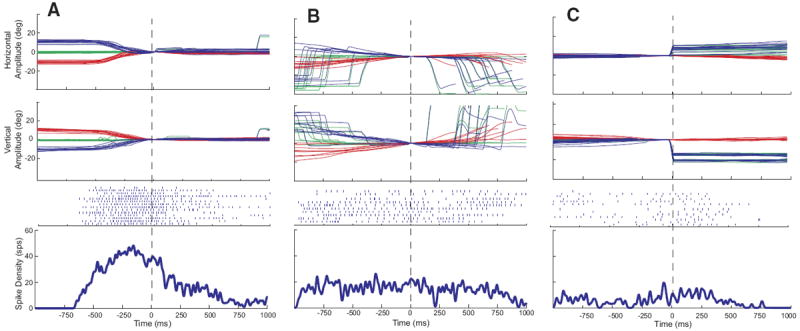
Example data for the same cell shown in Fig. 2. Data are aligned on head movement offset (A and B) or eye-only offset (C). Same format as Fig. 2.
We also isolated neurons that were characterized by the following properties: tonic discharge when the head was not moving, an abrupt decrease in firing rate—often a complete cessation of discharge—that was temporally associated with head-only movements, and resumption (or increase) in firing rate around the time of head-only movement offset. Low-velocity head drifts were generally associated with decreased firing rate or even cessation of discharge. Data are shown for one example neuron in Fig. 4. In A, the firing rate can be seen to increase as the low-velocity head drifts slow down and end prior to the visually guided head-only movement. Then the firing rate abruptly decreases shortly before the visually guided head-only movement begins. The averaged spike density function gives the impression that resumption was very gradual but a close inspection of the rasters for individual trials shows that the cell often returned to baseline firing rate over a period of 50–100 ms (looked at over the longer time scales of head movements, this seems “abrupt,” and this is what is meant by this term in this manuscript). These “head pauser” cells (n = 17; 30% of 56 neurons) also paused in association with gaze shifts (Fig. 4B) and sometimes eye-only movements (C). However, only one such cell was recorded in the rostral portion of SC and satisfied all of the criteria introduced by Munoz and Wurtz (1993) for classification as a fixation cell.
FIG. 4.
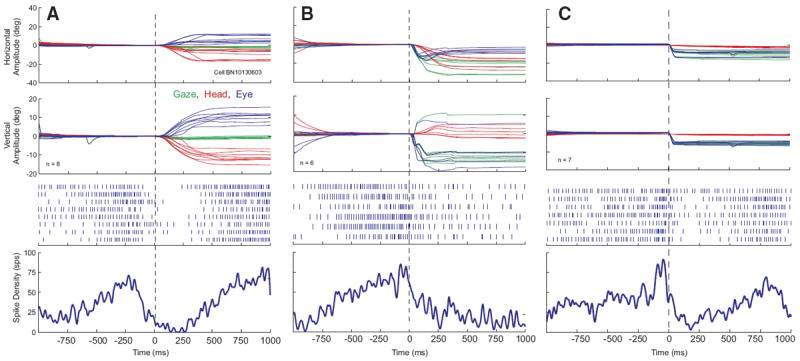
Example data for 1 cell that decreased its firing rate in association with head movements. Data are aligned on movement onset. Same format as Fig. 2. Note that, prior to the head movement, the firing rate abruptly decreased to near 0.
As with Fig. 3, aligning the data on movement offset had little effect on the averaged spike density functions for head-only (Fig. 5A) and eye-only data (C). It is important to note, however, that there was only a loose temporal association between head movement offset and pause end (A). This may be due, in part, to subthreshold drifts that occurred shortly after the end of the measured head movement. In A, these drifts are particularly apparent in the vertical head position traces. In B, data are aligned on head movement offset. The firing rate remained at a low rate after the end of the measured head movement, even when the head was stable.
FIG. 5.
Example data, aligned on movement offset. Same format as Fig. 3. At approximately the time of head offset, the firing rate abruptly returned to baseline. On gaze trials, the neuron did not always return to baseline after the end of the head movement associated with the gaze shift.
Spatial tuning of putative head cells
Most putative head cells modulated their discharge for a wide range of movements across the contralateral hemifield; few had circumscribed movement fields within the limit of head movements we were able to measure. Figure 6A shows a movement field plot for one typical cell. Although the trial-to-trial variability is high, the average firing rate for this cell is clearly higher for down-right head-only movements than for movements in other directions. To reveal structure that may be obscured by this variability, raw movement field plots were converted into filled contour plots using an inverse-distance based smoothing algorithm in Sigmaplot. Panel B shows an example, computed from the raw data points shown in A.
FIG. 6.
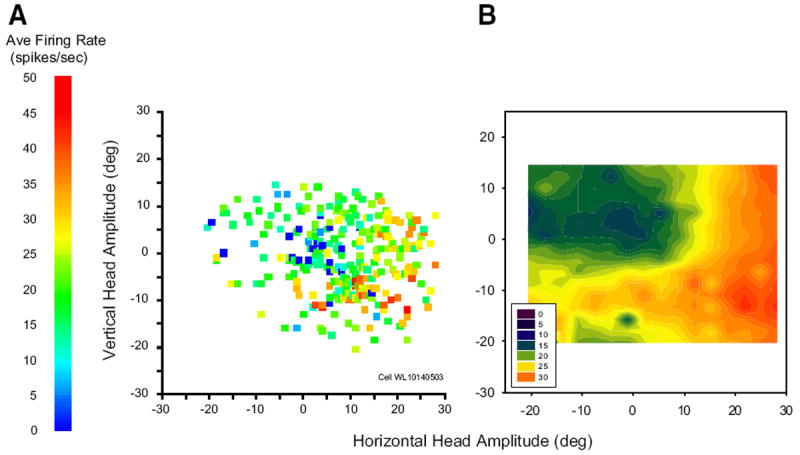
Movement field plots for an example head cell. Each data point represents a single movement. A: average firing rate during the movement is plotted as a function of horizontal and vertical head-only amplitude. B: filled contour plot constructed from the raw data points shown in A. For this example cell, vectorial head displacement seems to be predictive of firing rate.
Cells that modulated their activity in association with head-only movements displayed a variety of tuning profiles. Most increased their firing rate monotonically with amplitude, although this relation often saturated for larger head movements. However, varying degrees of directional biases were also observed, ranging from weak to quite strong. This can be appreciated from Fig. 7, which shows filled contour plots for three cells that displayed robust increases in discharge associated with head-only movements. This heterogeneity meant that there was no single curve fitting approach that was appropriate for every cell. Some cells (for example, see A) were best described by fitting a quadratic equation relating firing rate modulation to vectorial amplitude, regardless of direction. The quadratic fits were used because this relation tended to saturate for large head movements in many cells. Other neurons (B) were better described by fitting a plane to the horizontal amplitude, vertical amplitude, and firing rate modulation data. All 56 putative head cells were analyzed using both of these approaches. The results were not strongly affected by whether the modulation was positive or negative for head movements. The quadratic fits produced significant correlations for 25/56 cells (44.6%). Next, planar fits were performed with and without an interaction term, as follows: FRmod = y0 + aX + bY and FRmod = y0 + aX + bY + cXY. where FRmod is the predicted modulation of the cells firing rate, X is the horizontal head displacement, and Y is the vertical head displacement. The planar model significantly accounted for the firing rate modulation for 26/56 cells (46.4%). Eight cells showed significant correlations for the quadratic fits but not for the planar fits. Average R2 values were 0.08 ± 0.07 (SD) and 0.10 ± 0.10 for the quadratic and planar fits, respectively. For the plane fits, average coefficients for horizontal and vertical amplitudes were 0.15 ± 0.50 and 0.05 ± 0.06, respectively. Including an interaction term improved the fit somewhat, yielding an average R2 of 0.12 ± 0.10 but only 25/56 were significant. Average coefficients for horizontal amplitude, vertical amplitude, and the interaction term were 0.06 ± 0.35, −0.16 ± 0.78, and 0.001 ± 0.06, respectively.
FIG. 7.
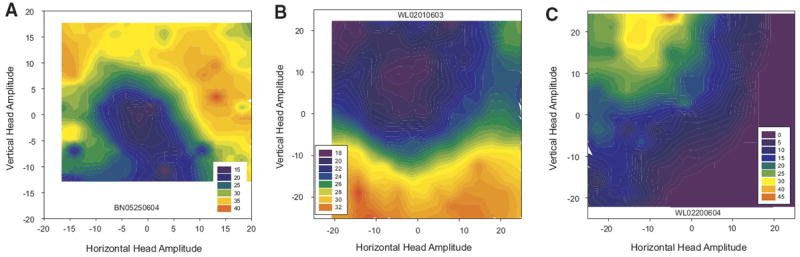
Filled contour plot movement fields for 3 example head cells. Putative head cells had highly heterogeneous tuning profiles with some more sensitive to amplitude than direction (A), some more sensitive to direction (B), and some showing movement fields similar to those for gaze cells in superior colliculus (C).
These analyses were also conducted using the mean firing rate during the head movement instead of the average modulation. This approach resulted in slightly worse fits for the quadratic equation (R2 mean ± 0.07 ± 0.06) but improved the planar fits. Average R2 values were 0.13 ± 0.11 and 0.15 ± 0.12 with and without the interaction term, respectively. Average coefficients for horizontal and vertical amplitudes were 0.05 ± 0.34 and −0.06 ± 0.65, respectively, without the interaction term and 0.06 ± 0.35, −0.08 ± 0.84, and 0.001 ± 0.06, respectively, when the interaction term was used. Using this approach, the distribution of average firing rate was statistically accounted for in 34/56 neurons by the planar model without the interaction term, in 33/56 by the planar model with the interaction term, and in 25/56 by the quadratic fit.
For some cells, the timing of discharge was predictive of head displacement. To assess this relationship, burst lead was computed as the interval (in ms) between the onset of activity and the onset of the head movement. This analysis could only be performed on cells that strongly increased their activity in association with head movements and that displayed a fairly abrupt onset of activity on a majority of trials (see METHODS for more detail). Ten cells met these criteria and, of these, burst lead was significantly related to head displacement for 8/10 (80%). Two of these cells showed more complex effects. Figure 8 shows the relationship between burst lead and head displacement for these two cells. The data were fit with a four-parameter Gaussian
FIG. 8.
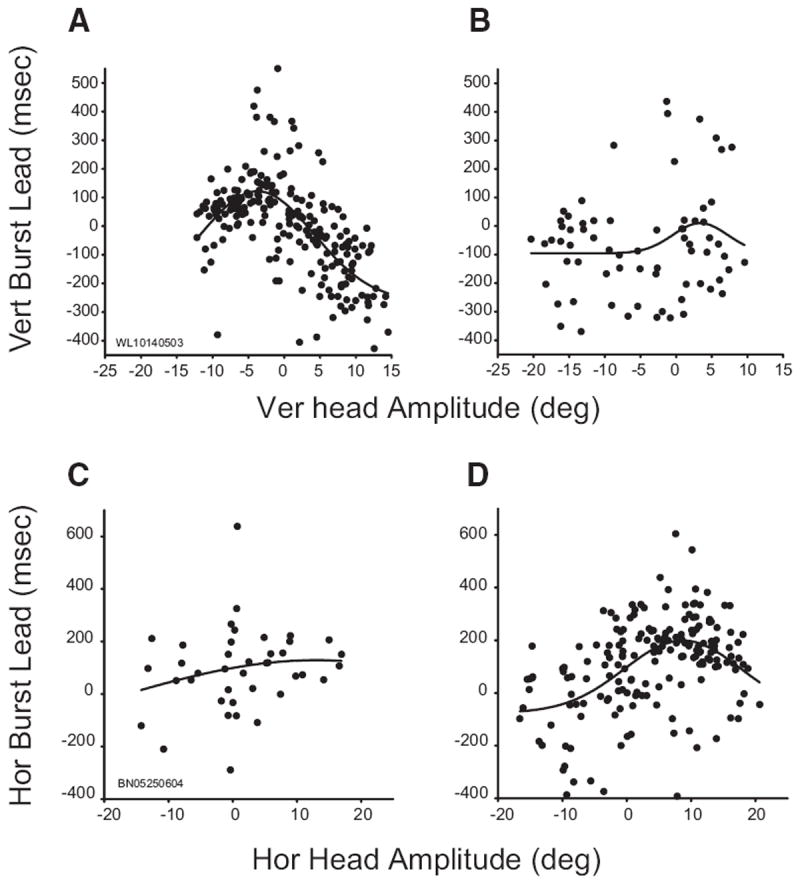
Relationship between burst timing and head displacement. Data in all panels are fitted with 4 parameter Gaussian functions. Top and bottom: data from 2 different cells. Left and right: data from trials in which the vertical initial head position was 10° up or 20° up, respectively. A and B: vertical burst lead (see text) was highly predictive of vertical head displacement for this example neuron when the head started at 10° up (A) but not when it started at 20° up (B). C and D: for this cell, horizontal burst lead was highly predictive of horizontal head displacement when the initial head position was 20° up (D) but not when it was 10° up (C).
For the cell shown in the top row (which is the same cell shown in Figs. 2 and 3), burst lead was strongly predictive of the amplitude of the vertical component (R2 = 0.44) when the initial vertical head position was 10° up (A). Burst onset led head onset for horizontal movements and those that were slightly down. However, for upward movements, burst onset generally lagged head onset. This relationship, however, disappeared when the same analysis was repeated for trials in which the vertical initial head position was 20° up (R2 = 0.05; B). Similarly, for the cell shown in the bottom row, burst lead was predictive of horizontal head displacement (R2 = 0.24) when the vertical initial head position was 20° up (D), yet no relationship was found when the head started at 10° up (R2 = 0.04; C).
For putative head cells that decreased their firing rate for head-only movements (n = 17), analyses involving pause duration or pause lead were only performed on cells with ≥15 trials with clearly defined pauses (see METHODS). Seven putative head cells met these criteria. Pause duration was significantly correlated with head movement duration for 2/7 cells. No relationship was found between the timing of the resumption of activity and head displacement or the time of head offset. This negative result may have been due to slow, subthreshold drifts after the measured end of the movement. These cells often discharged at a substantially reduced frequency or were quiescent during these slow drifts. For 4/7 cells, pause lead was significantly correlated with vertical and/or vectorial head displacement. Figure 9 shows this relationship for these cells. Note that, for three of the four cells, the correlation was negative with the cell pausing earlier for small head movements than for large ones.
FIG. 9.
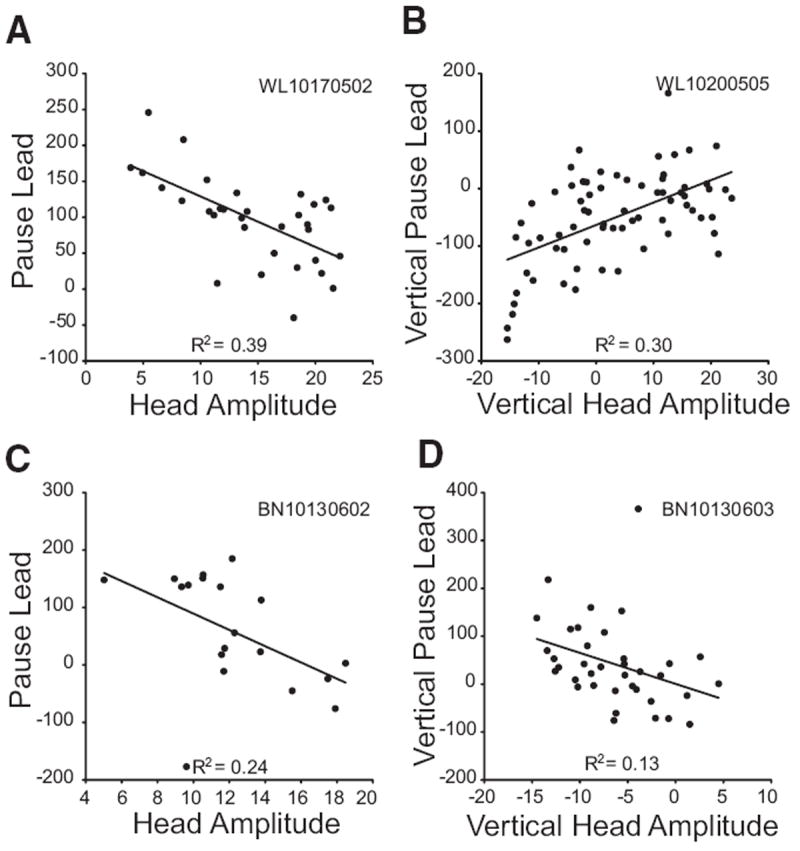
The relationship between pause lead (see text) and head displacement is shown for 4 cells that paused in association with head-only movements. Left: pause lead is correlated with vectorial head displacement. Right: vertical pause lead is correlated with vertical head displacement.
Comparison of movement fields
Next, we compared each putative head cell’s movement field response during head-only movements, gaze shifts, and the head component of gaze shifts. Figure 10 illustrates this comparison for one neuron, and it also demonstrates the difficulties encountered in performing a robust quantitative analysis. The head-only movement field (A) shows, despite the considerable trial-to-trial variability, clearly higher activity for downward head-only movements than for movements in other directions. B shows a movement field for head movements associated with gaze shifts. Activity may appear weaker than that shown in A, but this is difficult to interpret because these movements were primarily horizontal. C shows modulation associated with gaze shifts (i.e., the same movements used in B) and eye-only movements (not shown in B). Most of the gaze data recorded for this cell involved large-amplitude movements because burst neurons recorded on this track had movement fields centered near (40, −40). Eye-only data were therefore included in C to show how this cell responded for small-amplitude movements (that would normally be accomplished with eye-only movements anyway). This cell showed little modulation for gaze shifts, even when they were directed downward, and no evidence of a gaze movement field.
FIG. 10.
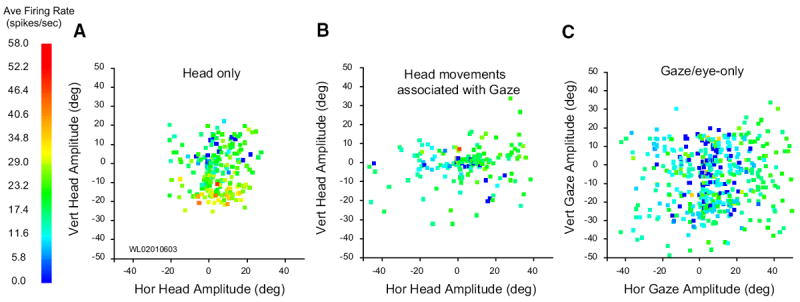
Movement fields for 1 putative head cell. A: average firing rate plotted as a function of horizontal and vertical head-only amplitude. Although much trial to trial variability in firing rate is apparent, the cell clearly discharges at a higher rate for downward head movements. B: average firing rate plotted as a function of horizontal and vertical amplitude of head movements associated with gaze shifts. When contributing to a gaze shift, head movements were mostly horizontal, even if the gaze shift had a substantial vertical component. Nonetheless, the cell was clearly active for head movements of all directions that accompany gaze shifts (i.e., there are very few blue points in this panel). C: average firing rate plotted as a function of horizontal and vertical gaze amplitude. Although the cell does discharge at a low rate during gaze shifts, there was no indication of organization or tuning. Data points shown in this panel include trials from small amplitude eye-only movements and larger amplitude gaze shifts.
We sought to perform a comparison between head-only movements and head movements associated with gaze shifts. Our monkeys were trained to make head-only movements in all directions, up to an amplitude of ~30°. Head movements associated with gaze shifts, however, tend to be mostly horizontal, even if the gaze shift itself has a strong vertical component (Fig. 10B) (Freedman and Sparks 1997b). We considered restricting the comparison to horizontal head movements but, given our current lack of understanding of the neurophysiological basis of eye-head coordination, we couldn’t assume that the head movement that accompanies a gaze shift is the same as the one requested by SC, a reflection of the neural uncertainty problem (Sparks 1999). For these reasons, we did not pursue any statistical comparisons of firing rate between the two different types of head movements.
We also attempted to perform direct statistical comparison of the movement fields associated with head-only movements and gaze shifts. If a topographic map existed for head-only movements, and, furthermore, if it was in spatial register with the topographic map known for gaze shifts, we could have compared the responses for movements of the same “hot-spot” vector. This approach, however, was quickly deemed futile as we found no topography for head movements and no hot-spot vector for head-only movements. In fact, the heterogenous head-only movement fields rarely resembled the circumscribed movement fields associated with gaze shifts. In the end, we opted for a generalized comparison of modulation in activity for head-only movements and gaze shifts across contralateral movements. This analysis is presented later, after the characterization of classic gaze-related burst neurons for head-only movements.
Do classic gaze-related burst neurons modulate in association with head movements?
The data presented so far indicate that the SC contains cells that modulate their response in association with head-only movements as well as during gaze shifts but that the discharge during gaze shifts is not the classic high-frequency burst that characterizes many SC neurons (quantitative details to be provided in the following text). Thus the next question to be addressed was whether neurons displaying high-frequency gaze-related bursts also discharged in association with head movements. In general, cells displaying high-frequency bursts associated with gaze shifts fired weakly—usually not at all—for head-only movements. Rasters and averaged spike density functions are shown in Fig. 11 for one example neuron, along with eye, head, and gaze position traces. On head unrestrained gap trials (B), this cell displayed clear prelude activity and a high-frequency burst of spikes associated with the gaze shift. The bottom inset shows the mean spike density for the same gap trials aligned on target onset and emphasizes the buildup response (arrow). On head-only trials (A), this cell discharged at low frequency, but this activity was nowhere near as robust as either the prelude or burst associated with gaze shifts. Many cells, particularly classic gaze-related burst neurons, did not discharge at all during head-only movements. This can be seen in Fig. 12, which shows movement field plots for one cell for gaze shifts (A) and head-only movements (B). This cell was recorded at a relatively rostral site in the right SC and preferred small gaze shifts (~8, −5). There was little or no discharge associated with head only movements. To emphasize this point, average firing rates for head-only movements are plotted on a different scale (Fig. 12B). Indeed, classic gaze-related burst neurons (i.e., no prelude activity) never significantly modulated their activity in association with head-only movements (see following text for more details).
FIG. 11.
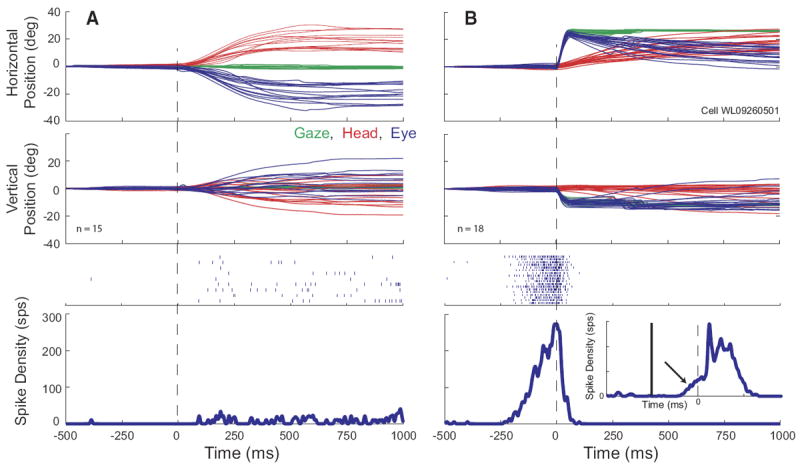
Example data for a high-frequency gaze related burst cell. Same format as Fig. 2. On gap trials (B), the cell displays a classic buildup of activity prior to the gaze shift. Bottom inset: averaged spike density function for the same trials, aligned on target onset (gap duration = 200 ms). The x and y scales for the inset are the same as for the outer panel. The offset of the fixation target and the onset of the gaze target are denoted by vertical solid and dashed lines, respectively. The arrow shows buildup activity. On head-only trials (A), the cell discharged at a very low rate, but did not show the robust burst of activity that was characteristic of gaze shifts into its response field.
FIG. 12.
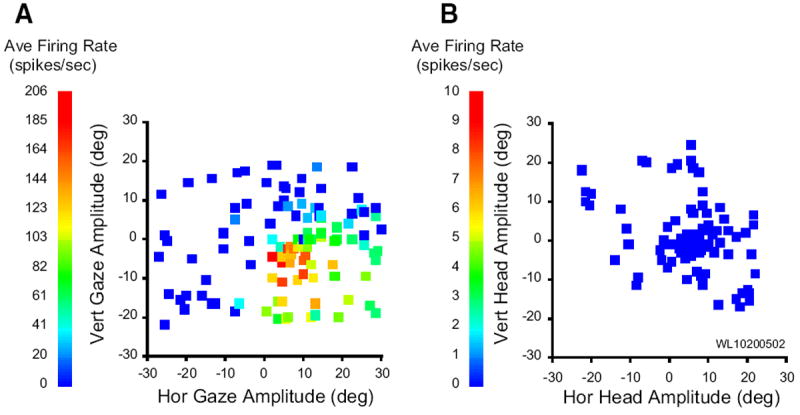
Movement field plots for a gaze related, high-frequency burst neuron. A: average firing rate during gaze shifts, plotted as a function of horizontal and vertical gaze amplitude. B: average firing rate during head-only movements, plotted as a function of horizontal and vertical head displacement. In general, this cell was completely quiescent during head-only trials. To emphasize this point, the average firing rate is plotted on a different scale in B.
Comparison of modulation for gaze and head-only movements
Two analyses were conducted across all neurons to compare the level of modulation for head-only movements and gaze shifts and between head- and eye-only movements. The objectives were to determine if the strength of modulation is different for these movement types and whether different or overlapping populations of SC neurons showed significant change in activity for eye-only/gaze movement and head-only movements.
In the first case, a modulation parameter was computed as the difference in activity during the appropriate movement interval and its baseline periods, yielding “gaze modulation” or “eye-only modulation” and/or “head-only modulation” values for each trial. A paired t-test was applied to the distribution of values in the two intervals for each type of movement directed into the contralateral field (see following text). This procedure determined whether a given neuron showed statistically significant modulation for just head-only movements, eye movements (eye-only or gaze) only, both types of movements, or neither. Figure 13A plots each neuron’s mean modulation during gaze shifts against its mean modulation during head-only movements. The outcome of the statistical test was used to classify each cell as exhibiting significant sensitivity to gaze (red circles), head (blue squares), both (green diamonds), or neither (black stars). As mentioned previously, classic gaze-related burst neurons never significantly modulated their discharge in association with head-only movements and therefore consistently fell close to the horizontal dashed lines in this figure. In contrast, it was common to find cells exhibiting gaze prelude activity and/or weak gaze-related bursts in the “both” category. Overall, the modulation associated with gaze shifts was greater than the modulation associated with head-only movements for neurons in the “gaze” (naturally) and “both” categories (2-tailed t-test; Table 1). Similar results were obtained when comparing head-only to eye-only movements (C).
FIG. 13.
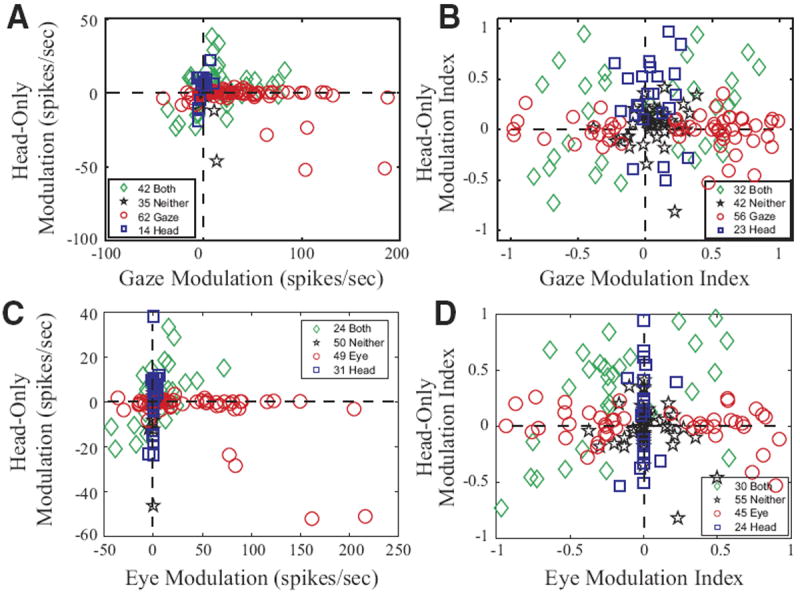
Comparison of modulation associated with gaze and eye-only movements to modulation associated with head-only movements. □, ○, and ◇ denote cells that significantly modulated their activity in association with movements of the head, gaze, or both, respectively. ☆, cells that did not significantly modulate for either. A and B compare data for gaze and head-only movements. C and D compare data for eye-only and head-only movements. A and C: change in firing rate during the movement in spike/s. B and D: modulation index (see text). Values of 0 indicate no modulation at all; values of 1 or −1 indicate that the cell fired exclusively during or before the movement, respectively.
TABLE 1.
Summary of modulation analyses
| Category | n | Gaze Modulation | Head Modulation | N | Gaze Modulation Index | Head Modulation Index |
|---|---|---|---|---|---|---|
| Blue squares (“head”) | 14 | 0.1 (4.7) | 4.4 (10.1) | 23 | 0.05 (0.15) | 0.29 (0.39) |
| Red circles (“gaze”) | 62 | 41.1 (46.4) | −3.1 (10.3) | 56 | 0.25 (0.56) | −0.01 (0.16) |
| Green diamonds (“both”) | 42 | 16.2 (28.2) | 3.7 (13.9) | 32 | 0.00 (0.53) | 0.15 (0.46) |
| Black stars (“neither”) | 35 | 6.6 (9.0) | −2.1 (8.2) | 42 | 0.07 (0.16) | 0.04 (0.20) |
Gaze modulation (red circles) is significantly different from head modulation (blue squares), P < 0.01 (2 tailed t-test). Gaze modulation (green diamonds) is significantly different from head modulation (green diamonds), P < 0.05 (2-tailed t-test). Gaze modulation (blue squares) is not significantly different from head modulation (blue squares), P > 0.1 (2-tailed t-test). Gaze modulation index (red circles) is NOT significantly different from head modulation index (blue squares), P > 0.7 (2-tailed t-test). Gaze modulation index (green diamonds) is NOT significantly different from head modulation index (green diamonds), P > 0.2 (2-tailed t-test). Gaze modulation index (blue squares) is significantly different from head modulation index (blue squares), P < 0.01 (2-tailed t-test). Values are means ± SD.
In the second analysis, a modulation index was computed as (mean firing rate during movement − baseline firing rate)/(mean firing rate during movement + baseline firing rate) for eye-only movements, head-only movements, and gaze shifts. Again, a paired t-test was performed as explained in the preceding text. This resulted in slightly different percentages of neurons in the four categories. Figure 13B compares the modulation indices of the neurons during gaze shifts and head-only movements, and D compares the modulation indices of neurons during eye-only and head-only movements.
In all panels of Fig. 13, the numbers of cells in the four categories do not add up to 210 because cells that significantly modulated their activity in association with head-only movements are shown only if they were considered to be good head cell candidates (see Additional controls). Furthermore, it must be emphasized that these analyses were not restricted to trials that fell within the movement field of the cell (if any). For eye-only and gaze, all contraversive trials were included; for head-only movements, all contraversive movements over 5° in amplitude were used. This was done because cells displaying head-movement-related activity usually were active for all contraversive head movements. For head-movement-related activity, averaging across the entire contralateral hemifield appeared to more accurately represent the apparent head movement activity of these cells. An undesirable consequence was that the modulation associated with eye-only and gaze is underestimated in this figure due to the inclusion of trials outside of the movement field. However, because saccade/gaze related activity in SC has been well described in many studies, we felt that it was more important to accurately represent neural activity potentially related to head movements. Restricting these analyses to the 10% of trials with the highest firing rate produced more accurate estimates of eye-only and gaze-related activity, but the estimates for some head cells were misleading because the mean was affected too strongly by outliers. Overall, however, the figures were highly similar using these two approaches (data not shown). This “top 10%” approach was also used to assess the gaze-related activity for the 56 cells that showed significant head-movement-related activity. When this was done, only 7/56 had gaze-related firing rates in excess of 100 spike/s for the 10% of trials with the highest gaze-related firing rates. All seven of these cells displayed prelude activity that began >100 ms before gaze onset, and all seven continued to discharge for ≥100 ms after gaze offset. Spike density functions computed for the peri-gaze period never exceeded 500 spike/s for any of these seven cells. Thus classic gaze-related burst neurons and head-movement related cells represented two distinct, nonoverlapping populations of cells.
We next examined whether the modulation was a function of the location of the neurons, in terms of both the SC map and the depth from the SC surface. Cells displaying head-movement related activity were found throughout the mediolateral and rostrocaudal extent of SC (Fig. 14A). Typically, they were found between 1 and 4 mm below the collicular surface with the greatest concentration between 1 and 2 mm (Fig. 14B). Thus these cells were found intermingled between gaze-related neurons. Indeed, we often isolated gaze-related burst neurons and head cells in the same penetration, <1 mm from each other.
FIG. 14.
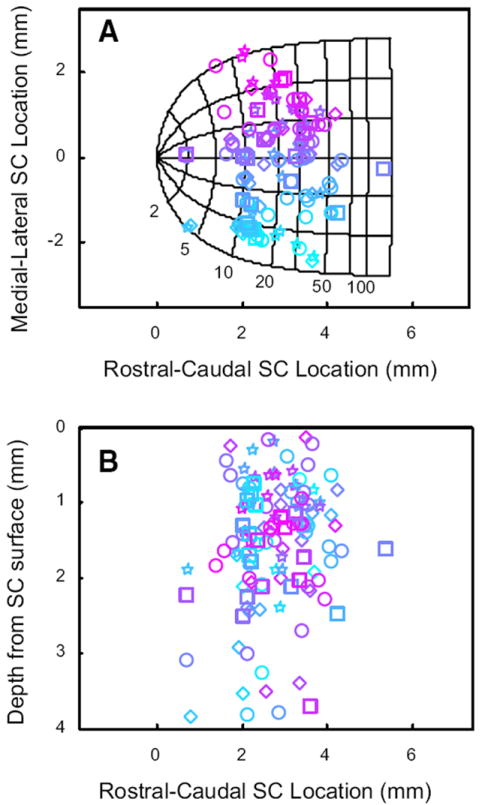
Cells with head-movement related activity were found intermingled with gaze-related neurons across the rostrocaudal and mediolateral extent of SC, and were usually found 0.5–2.5 mm below the surface of SC. A: locations of recordings on the SC map. Shape conventions are the same used for Fig. 13. Colors indicate the mediolateral location of each cell on the collicular map. B: estimated depth of each recording below the collicular surface. Color conventions are retained from A.
Additional controls
In our sample of neurons, we found that 87/210 (41%) significantly modulated their activity in association with head-only movements. However, 19/87 cells were rejected as head cell candidates despite significant modulation because the modulation associated with head-only movements was <10 spike/s for the 10% of trials with the largest modulation. For example, some cells that were usually silent during head only movements occasionally discharged three or four spikes during these movements. Such cells often exhibited a significant modulation only because they never discharged during the baseline period. It would be difficult to argue that such neurons are head cells. In addition, 12/87 cells were rejected as head cell candidates because the modulation was more parsimoniously explained as microsaccade or eye position effects (see following text for details). Thus 56 neurons were regarded as putative head cells.
It is possible that microsaccades may be more likely to occur during head-only movements than when the head is stationary. If this is the case, a cell that bursts in association with microsaccades would show a significant modulation for head-only movements in our analysis. To test for this possibility, saccade onset and offset velocity thresholds were modified (15°/s for onset and offset) to detect saccades of 0.5–2°. For all but five cells, modulation of firing rate was confirmed in the absence of microsaccades. These five cells clearly discharged in association with microsaccades and were therefore eliminated as potential head cells.
Before interpreting the present results as evidence that SC contains head-movement cells, it is necessary to verify that the modulation of activity in the present data are not due to cells carrying eye-in-head signals. During head-only movements, of course, the eyes counter-rotate in the orbits due to the VOR. A cell that encodes the positions of the eyes in the orbits would therefore significantly modulate its activity in association with head-only movements. To test this possibility, we performed regression analyses on the eye-in-head position during the baseline period when the eyes and head were stable. The slope of a linear regression line was found to be significantly different from zero for seven cells. These cells also were eliminated as potential head-movement cells.
One must consider the possibility that apparent head movement activity might, in fact, be a vestibular signal. During our head-only movements, the VOR gain was approximately one. Thus changes in firing rate would be expected for any vestibular cell. To test this possibility, for four cells that showed particularly robust modulation associated with head movements, the head was restrained and passive, whole-body rotations performed while the monkey fixated a target LED. Rotations were performed manually in the horizontal dimension at speeds approximating the speed of typical head-only movements. None of the four cells showed any modulation associated with whole-body rotations.
One must also consider the possibility that apparent head-movement related activity is a visual response. Potentially, visual responses might be observed in response to the laser spot sweeping across the visual field during head-only movements. To test this possibility, the laser spot was turned off during the head only movement in some or all head-only trials once the monkeys had extensive experience with the tasks (generally after 3 or 4 mo). The laser was turned back on when the head passed within 5° of the target. For all cells in which these laser-blank trials were used, the presence or absence of the laser spot was found to have no effect on firing rate.
DISCUSSION
We have found 56 cells in SC that can be regarded as likely head cells. Some (n = 39) increased their firing rate in association with head movements, others (n = 17) decreased or even paused. Cells exhibiting high-frequency bursts for gaze shifts generally showed little or no modulation associated with head-only movements. However, many cells with significant head-movement-related activity also exhibited lower-frequency bursts for gaze shifts. For many cells with significant head-movement activity, average firing rate was predictive of head displacement, and many showed biases for movements in a particular direction. Furthermore, burst lead was predictive of head displacement for 8/10 cells for which a detailed analysis was possible. For two of these cells, the presence of this relationship depended on the initial head position. The fact that the timing and level of activity of these cells is predictive of head-movement parameters supports the contention that these cells carry information related to head movements.
At least two previous studies have reported cells in the cat SC that may have been head cells. Straschill and Schick (1977) reported 15 neurons in cat SC that discharged before and during head movements. The firing rate of these neurons was correlated with head velocity. Peck (1990) described five neurons in cat SC that were characterized by bursts of spikes that were temporally associated with head movements. Neither of these studies, however, employed a head-only task; thus it is difficult to unequivocally state that the activity was related to head movements and not gaze shifts. To our knowledge, the present work represents the first direct evidence for the existence of head-only signals in the primate SC.
Potential alternative explanations
As mentioned in the preceding text, cells carrying vestibular signals would be expected to alter their firing rate in association with head-only movements. However, this is unlikely to be the case for the neurons in our sample for two reasons. First, increased discharge usually preceded the onset of the head movement. Second, passive whole body rotation performed on several putative head cells did not produce changes in firing rate.
One might suggest the possibility that increased activity during head movements might be a visual response to the moving laser spot. However, turning off the laser spot during head movements did not affect the activity of these cells during the movement. It should also be pointed out that the laser spot always swept through the visual hemifield opposite to the direction of the movement. During contraversive head-only movements, for example, the laser spot swept through the ipsilateral hemifield. Therefore if the present results were attributable to movement of the laser spot, the firing rate should have been higher for ipsiversive movements but the opposite pattern was found.
It is also conceivable that the activity could be associated with movement of the visual target across the retina during the head-only movement. This could occur in either of two ways. First, microsaccades might be more common during the head movement. Such microsaccades might tend to provoke increased activity from visual cells. Increased activity associated with microsaccades has, indeed, been reported in primary visual cortex and lateral geniculate nucleus (Martinez-Conde et al. 2002). In the present data, however, there were many trials in which head-only movements occurred in the absence of detectable microsaccades and robust discharge was often seen on these trials. Retinal slip would also occur during head movements if the VOR gain is not one. This drift could, potentially, increase the activity of visual cells. However, if this was the explanation for our results, increased activity should have been observed in response to passive, whole body rotation, but this was not the case. It should also be pointed out that many of these cells were recorded from SC sites encoding large gaze shifts. Visual receptive fields at such sites would not be expected to involve the parafoveal region of the visual field. Finally, retinal slip hypotheses would not explain why changes in discharge rate tended to precede the head movement.
One must also consider the possibility that apparent head-movement related activity is actually associated with covert saccade planning (Sparks 1999). During the head-only movement, however, the only target that is consistently visible is one that the animal is currently fixating. Nonetheless, it is not impossible that the animals might be planning a saccade back to the remembered location of the now-extinguished central target. However, this is unlikely to be the explanation for our results. The head cells we recorded generally displayed stronger activity associated with contraversive head movements than ipsiversive movements. If apparent head-movement related activity were the result of covert planning of saccades to the remembered location of the central target, the opposite pattern of activity should have been observed. In fact, the covert planning hypothesis predicts that “head movement fields” should have been found that closely resemble gaze movement fields, only reversed (i.e., in the “ipsilateral” hemifield). However, this was not the case. Furthermore, robust modulation of discharge was often observed during spontaneous head movements (including head-only movements) during the intertrial interval.
Cancellation of planned saccades is associated with sharp decreases in activity in many SC neurons (Pare and Hanes 2003). In our tasks, prior to the cue to initiate a head-only movement, the animal does not know whether he is going to be asked to make a head-only movement or a gaze shift. Conceivably, during this period, the monkey might be anticipating, and planning, a gaze shift that he then cancels when it becomes clear that it is a head-only trial. If this is the case, one might see neural activity during the dissociation period followed by a pause. Thus one must consider this as a possible alternative explanation for decreases in activity that temporally coincide with head movements. However, it must be pointed out that target direction and amplitude were randomized. Gaze targets were presented across a wide range of ipsi- and contralateral locations. In addition, the dissociation period was always more likely to be followed by a head-only movement than a gaze shift. For these reasons, we think it unlikely that the animals were planning gaze shifts during the dissociation period. It is also not clear why, if pauses in discharge were due to cancellation of planned saccades, the cells often abruptly started firing again around the time that the head movement ended. Finally, the fact that pause lead was often significantly correlated with head displacement suggests that decreases in activity were related to the head movement itself.
Another possibility that must be considered is that apparent head-movement-related activity is associated with microsaccades. It is conceivable that microsaccades might be more common during head-only movements and that SC neurons might participate in the generation of these movements. (Note that this is not the same as the above-mentioned possibility that microsaccades may lead to visual responses by inducing retinal slip. The question here is whether or not SC activity specifically related to microsaccades might give the false impression of head-movement activity.) Indeed, five neurons were rejected as head cell candidates for this reason. These cells were easily identified because robust correlations (r > 0.9) were found between burst onset and microsaccade onset time. For most of the cells in our sample, robust changes in discharge were often observed during head movements in the complete absence of detectable microsaccades. Microsaccades were also common during the dissociation period before head-only movements, and no activity was observed for such movements for most head cells. The microsaccade hypothesis also would not explain the relationships between neural activity and head-movement parameters.
It is also possible that SC cells with reward-related activity (Ikeda and Hikosaka 2003) might increase their firing rate during head-only movements if the animals come to expect a reward immediately after these movements. However, for most of our cells, one of our tasks required the animal to make a coordinated eye-head gaze shift after the head-only movement. Thus the animals should not have been expecting a reward at this stage of the task. Even when the head-only movement was not followed by a required gaze shift, the animal still had to maintain fixation for 800-1,200 ms to obtain the reward. In addition, one would not expect reward-related activity to return to baseline after the head-only movement as it consistently did for our head cells.
Finally, one must consider the possibility that apparent head-movement-related activity might be the result of a task-specific cognitive parameter rather than head movements per se. For example, it might be supposed that the head-only tasks are, in a sense, more demanding than standard eye-movement tasks. If this is the case, then changes in discharge might be associated with increased attention. This seems unlikely because changes in firing rate were also observed in association with coordinated eye-head gaze shifts, which, presumably, are not particularly difficult for the animal. Moreover, many of these cells were recorded in animals that had many months of experience with these tasks. Finally, for many of our putative head cells, robust changes in activity were observed in association with head movements during the intertrial interval when the monkeys were, of course, completely off task (data not shown).
Possible functional roles of head cells in SC
What role might SC cells play in head-movement control? In view of the broad tuning of neurons in our sample, one must be cautious about assigning an important functional role to these cells. Nonetheless, it is well known that populations of broadly tuned neurons can carry far more information than the activity of any single neuron. It should also be emphasized that, although the correlation coefficients reported in the present study would be quite low by the standards of most research involving the oculomotor system, they are comparable to those commonly found in the skeletal motor literature (e.g., Churchland et al. 2006; Werner et al. 1997).
There are many possible roles that head cells in SC might play. At least two studies, (Corneil et al. 2002; Cowie and Robinson 1994) have reported that low-frequency, long-duration stimulation evoked head-only movements from some SC sites. The authors of both of these studies suggested that SC may carry a parallel “head-only” drive. It is possible that the head-only movements that these authors reported might be mediated, at least in part, by cells of the same type reported in the present study. Thus it is possible that SC plays a role in the control of head movements independent of gaze shifts.
It is also possible that these cells might carry a corollary discharge signal from SC to other structures (Sommer and Wurtz 2004a,b). For example, accurate eye-head coordination may require information regarding the initial positions of the eyes in the orbits. Head-only movements clearly result in a change in eye-in-head position. It is not unreasonable to suppose that circuits controlling “head-only” movements might also relay information to the saccadic/gaze system to update a representation of eye position in head and that these circuits might involve SC.
With regard to potential functions, the head-pause cells warrant some discussion. It should be pointed out that there was often a negative correlation between pause lead and head displacement, a result that seems to argue against a simple “inhibitory gate” role analogous to the role that pontine omni-pause neurons play in the saccadic system. Given the more complex musculature of the head, however, this should not be surprising. It is also worth discussing the observation that the cells resumed discharge after head-only movements but often did not after the end of head movements associated with gaze shifts. This was true even when the head came to a complete stop. This also suggests that these cells should not be thought of as “head omnipause neurons.” It is possible that this difference between head-only and head-with-gaze movements is related to the fact that the animal was required to maintain a fairly stable head position after the former type of movement but not the latter.
The present data also may shed some light on the neural basis of head-movement control in general. Head-only movements and head movements associated with gaze shifts serve entirely different behavioral roles. With this in mind, one might be led to hypothesize that these different types of head movements may be subserved by separate circuits, analogous to the different subsystems involved in the control of eye movements. It is worth pointing out that many of the cells in the present sample clearly modulated their activity in association with both head-only movements and gaze shifts. If these neurons are, indeed, head cells, then the present data would seem to suggest that head-only movements and head movements associated with gaze shifts share some elements at the level of the SC.
Gaze coding versus separate eye and head control
In recent years, many studies have explored eye-head coordination in various brain regions with much disagreement about whether particular structures encode the eye and head components separately or as a gaze command (frontal eye fields: Bizzi and Schiller 1970; Chen 2006; Guitton and Mandl 1978a, b; Knight and Fuchs 2007; Tu and Keating 2000; caudal fastigial nucleus: Brettler and Fuchs 2002; Quinet and Goffart 2005; nucleus reticularis gigantocellularis: Freedman and Quessy 2004; Quessy and Freedman 2004; supplementary eye fields: Martinez-Trujillo et al. 2003b; nucleus raphe interpositus: Bergeron and Guitton 2002; Pare and Guitton 1990; Phillips et al. 1999; paramedian pontine reticular formation: Gandhi and Sparks 2007; Sparks et al. 2002; central mesencephalic reticular formation: Pathmanathan et al. 2006a,b).
This issue has also been discussed in the SC literature. Historically, the separate channel hypothesis was motivated largely by anatomical studies that demonstrated that projections to downstream oculomotor and neck muscle structures originate in different sublayers of the stratum griseum intermediale (Cowie and Robinson 1994; May and Porter 1992). However, electrophysiological studies have mainly supported the gaze hypothesis (Freedman and Sparks 1997a; Freedman et al. 1996; Klier et al. 2001; Martinez-Trujillo et al. 2003a; Munoz et al. 1991; Pare et al. 1994). In the monkey, Freedman and Sparks (1997a) found that the discharge of individual neurons was more strongly correlated with gaze than with either eye or head. Freedman et al. (1996) also reported that gaze shifts evoked by microstimulation of SC exhibited patterns of eye-head coordination that were highly similar to visually guided movements, even when initial eye position was varied. Because the head contribution to gaze normally varies depending on the initial position of the eyes in the orbits, their results are not easy to explain in the context of a separate eye and head cell hypothesis. If separate eye and head maps exist in SC, then one might expect stimulation to evoke the same combination of eye and head movements, regardless of initial eye position.
Superficially, the present results might be interpreted as a challenge to the gaze hypothesis. Some of the neurons in the present sample were active during coordinated eye-head gaze shifts and head-only movements, a result that may be interpreted as evidence that some neurons that are active for gaze shifts really encode head movements. The interpretation of this result, however, is not straightforward. For one thing, as mentioned in the preceding text, the function of the cells reported in this manuscript is unknown. Thus one should not jump to the conclusion that these neurons actively drive movement of the head as opposed to, for example, relaying a corollary discharge signal to cortex. Second, gaze-related burst neurons never significantly modulated their activity in association with head-only movements; cells that significantly modulated their activity for both gaze shifts and head movements generally were characterized by much weaker gaze-related bursts (i.e., Fig. 2B).
The interpretation of this latter result is also not straightforward. Given the different behavioral functions of head-only movements and head movements associated with gaze shifts, it is possible that some cells could participate in one type of head movement and not the other. Thus a neuron that discharges during gaze shifts but not head-only movements could, theoretically, be a “head movement associated with a gaze shift” cell.
With these considerations in mind, the present results are best interpreted as supporting the more general conclusion that SC is involved in the control of head movements in addition to its already-documented role in the control of gaze shifts. Identification of the precise nature of this role will require further study.
Acknowledgments
The authors are grateful to C. Bryant and K. Pearson for computer programming, B. Hughes and T. Wheeler for machining services, J. Buhrman and Dr. Charles Scudder for electronics assistance, and G. Duffy for general assistance.
GRANTS
This study was supported by National Eye Institute Grants EY-015485 to N. J. Gandhi and EY-015060 to M.M.G. Walton and the University of Pittsburgh Eye and Ear Foundation.
References
- Bergeron A, Guitton D. In multiple-step gaze shifts: omnipause (OPNs) and collicular fixation neurons encode gaze position error; OPNs gate saccades. J Neurophysiol. 2002;88:1726–1742. doi: 10.1152/jn.2002.88.4.1726. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Schiller PH. Single unit activity in the frontal eye fields of unanesthetized monkeys during eye and head movement. Exp Brain Res. 1970;10:150–158. doi: 10.1007/BF00234728. [DOI] [PubMed] [Google Scholar]
- Brettler SC, Fuchs AF. Role of caudal fastigial neurons during head-free gaze shifts in the monkey. Ann NY Acad Sci. 2002;978:505–506. doi: 10.1111/j.1749-6632.2002.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Bryant CL, Gandhi NJ. Real-time data acquisition and control system for the measurement of motor and neural data. J Neurosci Methods. 2005;142:193–200. doi: 10.1016/j.jneumeth.2004.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol. 2006;95:3528–3542. doi: 10.1152/jn.01320.2005. [DOI] [PubMed] [Google Scholar]
- Chen LL, Walton MM. Use of a central difference differential algorithm in combination with a flexible, sliding window for defining head movement onset and offset thresholds. Neural Control Move Abstr 9. 2004 [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron. 2006;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Olivier E. Priming of head premotor circuits during oculomotor preparation. J Neurophysiol. 2007;97:701–714. doi: 10.1152/jn.00670.2006. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol. 2002;88:2000–2018. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron. 2004;42:831–841. doi: 10.1016/s0896-6273(04)00267-3. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Olivier E, Pelisson D. Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J Physiol. 2004;556:675–681. doi: 10.1113/jphysiol.2004.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Smith MK, Robinson DL. Subcortical contributions to head movements in macaques. II. Connections of a medial pontomedullary head-movement region. J Neurophysiol. 1994;72:2665–2682. doi: 10.1152/jn.1994.72.6.2665. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Quessy S. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. II. Effects on metrics and kinematics of ongoing gaze shifts to visual targets. Exp Brain Res. 2004;156:357–376. doi: 10.1007/s00221-004-1840-2. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol. 1997a;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol. 1997b;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Experimental control of eye and head positions prior to head-unrestrained gaze shifts in monkey. Vision Res. 2001;41:3243–3254. doi: 10.1016/s0042-6989(01)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol. 2007;98:360–373. doi: 10.1152/jn.00252.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Walton MM. Superior colliculus activity associated with head movements. Soc Neurosci Abstr. 2006 211.212. [Google Scholar]
- Guillaume A, Pelisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and of the parameters of stimulation. Eur J Neurosci. 2001;14:1331–1344. doi: 10.1046/j.0953-816x.2001.01744.x. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pelisson D. Kinematics and eye-head coordination of gaze shifts evoked from different sites in the superior colliculus of the cat. J Physiol. 2006;577:779–794. doi: 10.1113/jphysiol.2006.113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor ” area in alert cat. II. Unit discharges associated with eye movements and neck muscle activity. Brain Res. 1978a;149:313–327. doi: 10.1016/0006-8993(78)90478-x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor ” area in alert cat. I. Eye movements and neck activity evoked by stimulation. Brain Res. 1978b;149:295–312. doi: 10.1016/0006-8993(78)90477-8. [DOI] [PubMed] [Google Scholar]
- Herrero L, Rodriguez F, Salas C, Torres B. Tail and eye movements evoked by electrical microstimulation of the optic tectum in goldfish. Exp Brain Res. 1998;120:291–305. doi: 10.1007/s002210050403. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Tectal control of spinal cord activity: neuroanatomical demonstration of pathways connecting the superior colliculus with the cervical spinal cord grey. Prog Brain Res. 1982;57:293–328. doi: 10.1016/s0079-6123(08)64135-7. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–632. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol. 2007;97:618–634. doi: 10.1152/jn.00256.2006. [DOI] [PubMed] [Google Scholar]
- Kutz DF, Dannenberg S, Werner W, Hoffmann KP. Population coding of arm-movement-related neurons in and below the superior colliculus of Macaca mulatta. Biol Cybern. 1997;76:331–337. doi: 10.1007/s004220050346. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA. 2002;99:13920–13925. doi: 10.1073/pnas.212500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Klier EM, Wang H, Crawford JD. Contribution of head movement to gaze command coding in monkey frontal cortex and superior colliculus. J Neurophysiol. 2003a;90:2770–2776. doi: 10.1152/jn.00330.2003. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Wang H, Crawford JD. Electrical stimulation of the supplementary eye fields in the head-free macaque evokes kinematically normal gaze shifts. J Neurophysiol. 2003b;89:2961–2974. doi: 10.1152/jn.01065.2002. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Visual Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D, Pelisson D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol. 1991;66:1642–1666. doi: 10.1152/jn.1991.66.5.1642. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol. 1993;70:559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Pare M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- Pare M, Guitton D. Gaze-related activity of brainstem omnipause neurons during combined eye-head gaze shifts in the alert cat. Exp Brain Res. 1990;83:210–214. doi: 10.1007/BF00232210. [DOI] [PubMed] [Google Scholar]
- Pare M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathmanathan JS, Cromer JA, Cullen KE, Waitzman DM. Temporal characteristics of neurons in the central mesencephalic reticular formation of head unrestrained monkeys. Exp Brain Res. 2006a;168:471–492. doi: 10.1007/s00221-005-0105-z. [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Presnell R, Cromer JA, Cullen KE, Waitzman DM. Spatial characteristics of neurons in the central mesencephalic reticular formation (cMRF) of head-unrestrained monkeys. Exp Brain Res. 2006b;168:455–470. doi: 10.1007/s00221-005-0104-0. [DOI] [PubMed] [Google Scholar]
- Peck CK. Neuronal activity related to head and eye movements in cat superior colliculus. J Physiol. 1990;421:79–104. doi: 10.1113/jphysiol.1990.sp017934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Beauchamp MS. Neural basis of visually guided head movements studied with fMRI. J Neurophysiol. 2003;89:2516–2527. doi: 10.1152/jn.00988.2002. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF. Action of the brain stem saccade generator during horizontal gaze shifts. I. Discharge patterns of omnidirectional pause neurons. J Neurophysiol. 1999;81:1284–1295. doi: 10.1152/jn.1999.81.3.1284. [DOI] [PubMed] [Google Scholar]
- Quessy S, Freedman EG. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. I. Characteristics of evoked head movements. Exp Brain Res. 2004;156:342–356. doi: 10.1007/s00221-003-1787-8. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head-unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol. 2005;93:2343–2349. doi: 10.1152/jn.00705.2004. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brain stem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp Brain Res. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004a;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol. 2004b;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Barton EJ, Gandhi NJ, Nelson J. Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts. Ann NY Acad Sci. 2002;956:85–98. doi: 10.1111/j.1749-6632.2002.tb02811.x. [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol. 1981;19:180–192. doi: 10.1159/000121641. [DOI] [PubMed] [Google Scholar]
- Straschill M, Schick F. Discharges of superior colliculus neurons during head and eye movements of the alert cat. Exp Brain Res. 1977;27:131–141. doi: 10.1007/BF00237694. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol. 2000;83:1283–1299. doi: 10.1152/jn.2000.83.3.1283. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Hoffmann KP, Miller LE. Correlation of primate superior colliculus and reticular formation discharge with proximal limb muscle activity. J Neurophysiol. 1999;81:1978–1982. doi: 10.1152/jn.1999.81.4.1978. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. Head and body movements evoked electrically from the caudal superior colliculus of rats: pulse frequency effects. Behav Brain Res. 1989;34:71–78. doi: 10.1016/s0166-4328(89)80091-9. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS. Two converging brainstem pathways mediating circling behavior. Brain Res. 1986;385:329–342. doi: 10.1016/0006-8993(86)91080-2. [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol. 2000;84:1103–1106. doi: 10.1152/jn.2000.84.2.1103. [DOI] [PubMed] [Google Scholar]
- Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol [A] 2002;188:89–108. doi: 10.1007/s00359-001-0275-5. [DOI] [PubMed] [Google Scholar]
- Werner W. Neurons in the primate superior colliculus are active before and during arm movements to visual targets. Eur J Neurosci. 1993;5:335–340. doi: 10.1111/j.1460-9568.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]


