Abstract
Background
The ability to spontaneously attend to the social overtures and activities of others is essential for the development of social cognition and communication. This ability is critically impaired in toddlers with autism spectrum disorders (ASD); however, it is not clear if prodromal symptoms in this area are already present in the first year of life of those affected by the disorder.
Methods
To examine whether 6-month-old infants later diagnosed with ASD exhibit atypical spontaneous social monitoring skills, visual responses of 67 infants at high-risk (HR) and 50 at low-risk (LR) for ASD were studied using an eye-tracking task. Based on their clinical presentation in the 3rd year, infants were divided into those with ASD, those exhibiting atypical development (HR-ATYP), and those developing typically (HR-TYP, LR-TYP).
Results
Compared to the control groups, 6-month-old infants later diagnosed with ASD attended less to the social scene, and, when they did look at the scene, they spent less time monitoring the actress in general and her face in particular. Limited attention to the actress and her activities was not accompanied by enhanced attention to objects.
Conclusions
Prodromal symptoms of ASD at six months include a diminished ability to attend spontaneously to people and their activities. A limited attentional bias towards people early in development is likely to have a detrimental impact on the specialization of social brain networks and the emergence of social interaction patterns. Further investigation into its underlying mechanisms and role in psychopathology of ASD in the first year is warranted.
Keywords: autism, infants at risk, eye-tracking, visual attention, social development, spontaneous monitoring
Identification of prodromal symptoms of autism spectrum disorder (ASD) in the first year of life is one of the key priorities in the field of autism research(1). Discovery of such behavioral or biological features would advance the understanding of which symptoms are primary and, consequently, facilitate the identification of underlying genetic and neurobiological mechanisms, improve early screening and diagnostic instruments, and inform about novel behavioral or biological treatment targets(2–6). Considering that ASD is usually not diagnosed until the early preschool age, work on identifying prodromal symptoms has historically taken the form of retrospective studies. In recent years, however, studies leveraging the increased risk in younger siblings of children with ASD due to genetic associations(7), have offered a powerful avenue for studying the emergence of ASD in statu nascendi.
Although prevalence estimates of ASD in general population range from 0.07% to 1.8%(8–12), conversion rates in prospective studies of high-risk (HR) infants average around 19% (13). In addition, up to 30% of HR siblings exhibit broader autism phenotype features that include social difficulties, rigidities, and language delays(5,14–17). At 12 months those later diagnosed with ASD exhibit delays and atypical characteristics in several domains including: eye contact, social smiling and vocalizations(18–22), initiation of joint attention and requesting(20), object exploration(23), response to name(24), and responses to distress of others(21,25). The delays and abnormal features are often pronounced as a large proportion of infants later diagnosed with ASD exhibit clinically relevant levels of symptoms by the age of 12 months(26).
Yet, identification of prodromal symptoms of ASD in the first months of life has proven more complex. Global behavioral measures that were once considered optimal candidates as early markers including eye contact, social smiling, and frequency of socially-directed vocalizations observed in a context of parent- or examiner-infant interactions have shown limited predictive value when measured at six months(15,18–20,27). Investigations into development of working memory(29) or vocalizations(22) have also yielded negative results at six months. Though, sensing vulnerability in their high-risk infants(28), parents might adopt additional strategies to engage their children (e.g., directive style or motion cues), which may be difficult to control for in parent-child interaction studies. Similarly, interpretation of negative findings in studies comparing only HR and LR infants is complicated by the fact that HR samples include infants with a broad range of outcomes. Despite these largely negative behavioral findings, a recent study suggests that atpyicalities in development of white matter might be detectable as early as at 6 months(30). Other positive findings include the presence of physical overgrowth (including head circumference, height and weight)(31), as well as atypical ERP responses to gaze shifts in 6–10 month olds later diagnosed with ASD(32).
Taken together, the extant, albeit still limited, evidence suggests that more readily measurable global behavioral symptoms associated with ASD begin to emerge between 6 and 12 months and further intensify in the 2nd year of life(5,18,19,33). It is plausible that differences in structural brain organization early in development may restrict the experience-dependent specialization of neural systems involved in social cognition, and, in this sense, precede the emergence of behavioral symptoms of ASD. However, the developmental mechanisms that link atypical neural development and its consequent impact on social cognition skills remain to be identified. An alternative possibility is that prodromal vulnerabilities in elementary aspects of attention and perception emerging in the first months of life may, by limiting access to critical social experiences, have a detrimental effect on specialization of neural networks involved in social cognition.
The present paper examines the spontaneous monitoring of complex dynamic social scenes in 6-month-old infants at high- and low-risk for ASD. Similar research efforts targeting toddlers with ASD have uncovered pronounced deficits in this domain(34–36). In one such study, spontaneous monitoring of an actress engaged in several kinds of activities (e.g., making a sandwich, speaking to the camera, shifting attention to various objects) were tested in 14- to 24-month-old clinic-referred toddlers with autism using eye tracking. Compared to developmentally delayed and typical controls, toddlers with autism showed particularly atypical visual responses when the actress tried to engage their attention using dyadic cues (i.e., eye contact and child-directed speech). In such a context, they showed diminished attention to the scene in general, and, when they looked at the scene, showed deficits in monitoring the actress’s face and mouth(34). In typical development, the ability to orient preferentially to direct eye contact and child-directed speech are present in a rudimentary form from the first few days of life(37,38,39). Given that learning about people is a highly experience-dependent process(40,41), the presence of abnormal attention to these essential social cues in early infancy is likely to detrimentally impact the development of social cognition and communication as well as developmentally-appropriate specialization of the neural networks involved in processing social stimuli. Thus, the ability to spontaneously regulate attention to social cues of others represents a highly promising area of inquiry for studying prodromal symptoms of ASD in high-risk infants.
In this study, we employed the same experimental procedure in 6-month-old infants at risk for ASD as that used in a study of 14–24 month old clinic-referred toddlers(34). We compared visual responses of infants later diagnosed with ASD to typically developing high-risk and low-risk infants as well as high-risk infants who had a history of clinically relevant delays and abnormalities in the 2nd year of life. We hypothesized that 6-month-old infants who later develop ASD would, in comparison to the other groups, show deficits in attending to complex social scenes in general and, in a manner similar to that observed in toddlers, would show difficulties attending to the speaker’s face and her mouth.
Methods and Materials
Participants
All infants (N=117) participated in a prospective study of infants at risk for ASD due to genetic liability. The sample consisted of 67 high-risk (HR) and 50 low-risk (LR) infants. To be considered ‘HR’ an infant had to have an older sibling with a diagnosis of ASD. The older sibling’s diagnostic status was ascertained based on a review of assessment records including ADOS-G(42) and/or ADI-R(43). Infants considered as LR had no history of ASD in 1st or 2nd degree relatives. All infants were enrolled in the study by the age of 6 months. Exclusionary criteria were gestational age below 34 weeks, any hearing or visual impairment, non-febrile seizure disorders, or known genetic syndrome. At six months, all infants underwent developmental assessment using the Mullen Scales of Early Learning(44) and completed the eye tracking procedure. Clinical best estimate (CBE) diagnosis was assigned by a team of expert clinicians based on the results of the Mullen Scales, Autism Diagnostic Observation Schedule-Generic(42), language assessments (either Communication and Symbolic Behavior Scales(45) or Reynell Developmental Language Scales III,(46) as well as medical and family history. The assessment instruments were administered by PhD-level psychologists and licensed speech and language pathologists. In 68% of cases CBE diagnosis was based on a 36-month assessment; the remaining 32% of high-risk infants were diagnosed based on a 24-month assessment. Based on these outcomes, the infants were divided into three groups: (1) infants with frank symptoms of ASD (n=15) (14 HR, 1 LR); (2) and (3) infants with no evidence for clinically significant symptoms in the 2nd or 3rd year (HR-TYP, n=19, LR-TYP, n=50); and (4) infants with clinically significant symptoms (e.g., language or other developmental delay or abnormal social-communication or repetitive behaviors) evident in the 2nd or 3rd year of life, but who did not meet criteria for ASD (HR-ATYP, n=33). Though developmental problems were transient in some HR-ATYP infants, for the purpose of this analysis they were included in this group in appreciation of their early atypical developmental course. Conversion rates to ASD amongst HR infants was 21%, well within the previously reported range (13). All parents signed an informed consent in adherence to the University Human Investigation Committee requirements.
Data from 38 out of 117 (32%) infants were not included in the analysis due to motion- or inattention-related eye-tracker calibration problems. There was no differential dropout by diagnosis in any of the four experimental conditions (see Procedure section): Sandwich (chi2(3)=1.63, p=.653); Dyadic Bid (chi2(3)=.97, p=.819); Joint Attention (chi2(3)=1.46, p=.693); and Moving Toys (chi2(3)=3.105, p=.376). Infants who were excluded due to calibration problems did not differ in chronological age (F(115)=1.42, p=.236), Mullen Scale visual reception (F(113)=2.63, p=.108), fine motor (F(1, 113)=2.86, p=.093), receptive language (F(113)=.09, p=.766) or expressive language (F(113)=.88, p=.349) age equivalent (AE) scores.
After the initial exclusions, a total of 84 infants were retained for analysis including 12 (80%) infants with ASD, 22 (67%) HR-ATYP, 15 (79%) HR-TYP, and 35 (70%) LR-TYP. Ninety-two percent of parents identified their child’s race as Caucasian and the distribution did not differ by group (chi2(3)=1.56, p=.667). The groups did not differ in terms of gender, chronological age, or AE scores on the Mullen visual reception, fine motor, receptive language, and expressive language scales at six months (Table 1). At 24 months the children did not differ in chronological age, though the clinically affected groups differed from non-affected toddlers in a predictable manner on measures of developmental and social-communicative functioning (Table 1).
Table 1.
Sample Characteristics at 6 and 24 Months
| 6 months
| |||||
|---|---|---|---|---|---|
| Characteristic | ASD (n=12) | HR-ATYP (n=22) | HR-TYP (n=15) | LR-TYP (n=35) | P value |
| Age (months) | 6.47 (.78) | 6.39 (.38) | 6.21 (.61) | 6.15 (.45) | .251 |
| Male Sex (%) | 64 | 83 | 55 | 55 | .218 |
| Mullen VR AE | 6.11 (.60) | 5.63 (1.10) | 6.00 (1.04) | 5.90 (0.79) | .515 |
| Mullen FM AE | 5.56 (1.51) | 5.32 (1.38) | 6.00 (1.41) | 6.12 (1.17) | .180 |
| Mullen RL AE | 5.11 (1.36) | 4.57 (1.50) | 5.14 (0.95) | 5.25 (1.26) | .339 |
| Mullen EL AE | 4.89 (.60) | 4.42 (1.16) | 4.79 (0.58) | 4.79 (0.80) | .431 |
|
| |||||
| 24 months | |||||
|
| |||||
| Age (months) | 24.3 (.78) | 24.6 (1.0) | 24.3 (.79) | 24.2 (.79) | .699 |
| ADOS-Toddler Total Score | 13.2 (6.9) | 7.1 (4.2) | 2.3 (1.8) | 3.2 (2.9) | .001a |
| Verbal DQ | 87 (35.8) | 101 (24.5) | 124 (11.8) | 124 (16.8) | .001b |
| Nonverbal DQ | 92 (20.7) | 102 (16.8) | 115 (11.2) | 117 (12.0) | .001c |
Abbreviations: AE=Age Equivalent (months), VR=Visual Reception, FM=Fine Motor, RL=Receptive Language, EL=Expressive Language
Verbal DQ: (RL AE + EL AE)/Age) * 100
Nonverbal DQ: (VR AE + FM AE)/Age) * 100
ASD > HR-ATYP > HR-TYP= LR-TYP
ASD = HR-ATYP < HR-TYP= LR-TYP
ASD = HR-ATYP < HR-TYP= LR-TYP
Stimuli
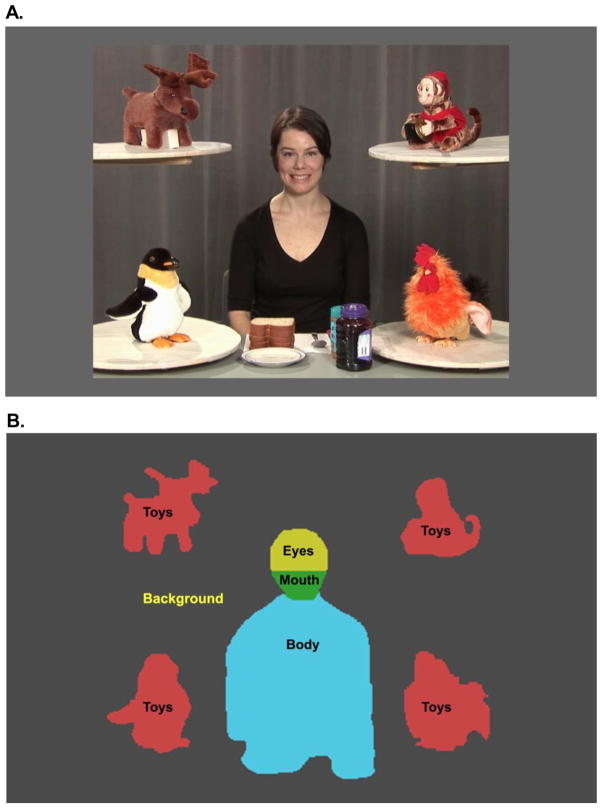
The stimuli were designed to capture, in a task-relevant fashion, the spontaneous regulation of visual attention in response to the ebbs and flows of social events. The stimulus consisted of a 3-minute video of an actress filmed in a setting containing four toys and a table with ingredients for making sandwiches (Figure 1a)(34). The video contained four types of activity (conditions) interspersed with one another, depicting a woman making a sandwich, occasionally looking at the camera and trying to engage the viewer using eye contact and child-directed speech, then going back to the sandwich, or looking at the toys positioned in the four corners of the screen, with toys sometimes remaining still and sometimes moving. The content of the actress’ speech was related to the events presented in the video and included greeting (“How are you, baby”), complimenting (“You are so cute!”), commenting (“Did you see it? It was so much fun!”), or referring to something the child saw just during calibration (“Did you see the tigers?”). There were no breaks in the video to re-engage or re-center the viewer’s attention, thus requiring the infants to adjust their gaze patterns depending on context, as they would in real life. In the Dyadic Bid condition the actress spoke to the camera using child-directed speech and looked straight into the lens of the camera (11 episodes, 69s), emulating a prototypical bid for dyadic engagement. In the Sandwich condition, the same actress looked down and quietly made a sandwich (2 episodes, 63s). Alternatively, the actress looked at toys located on shelves in the Joint Attention condition (4 episodes, 30s), and in the Moving Toys (4 episodes, 27s) (see34 for detailed description). The scene subtended 27 × 21 degrees of visual angle, the Face 3.9 × 5.6, Mouth 3.5 × 2.0, and each of the Toys 5.8 × 6.4.
Figure 1.
(A) Frame from video stimulus with (B) regions of interest (ROIs) used in analysis. The ROIs: Scene (Person + Toys + Background), Person (Face + Body), Toys, Eyes, and Mouth.
Apparatus
Gaze trajectories were recorded at a sampling rate of 60Hz using a SensoMotoric Instruments IView X™ RED eye tracking system. Eye tracking data were processed using custom software written in Matlab.(47) The software accommodated standard techniques for processing eye-tracking data including blink detection, data calibration, recalibration, and Region of Interest (ROI) analysis(48,49).
Procedure
Infants were seated in a car seat in a dark and soundproof room 75cm in front of a 24″ widescreen LCD monitor. Each session began with a cartoon video to help the child get settled. A five-point calibration procedure was then initiated with calibration points consisting of dynamic targets (e.g. a meowing, walking cartoon tiger). Subsequently, each participant was presented with the video described in the Stimulus section.
Analytic Strategy
Data reduction
The visual scene was divided into several regions of interest (ROIs) (Figure 1b). Dependent variables were based on the proportions of time spent examining each of the regions and included: (1) overall attention to the scene (%Scene), and (2) the proportion of attention directed towards the person (%Person) or toys (%Toys). We also examined the proportion of time spent looking at the face (%Face), as well as the eyes (%Eyes) and mouth (%Mouth). The proportion of the total looking time (%Scene) was standardized by the total duration of the video displayed; the remaining variables were standardized by the total looking time at the scene.
Statistical analysis
Primary hypotheses were tested using linear mixed effects models with group (4) as a between-group factor and condition (4) as a within-group factor. Considering that the task relied heavily on visual discrimination skills and attention to language, individual age equivalents on the Mullen Visual Reception and Receptive Language scales at 6 months were included into the model as covariates. All post-hoc contrasts are reported with a Tukey-Kramer correction for multiple comparisons. Effect sizes (Cohen’s d) are reported whenever applicable. Data analysis was implemented in SAS(50).
Results
Total Looking Time at the Scene
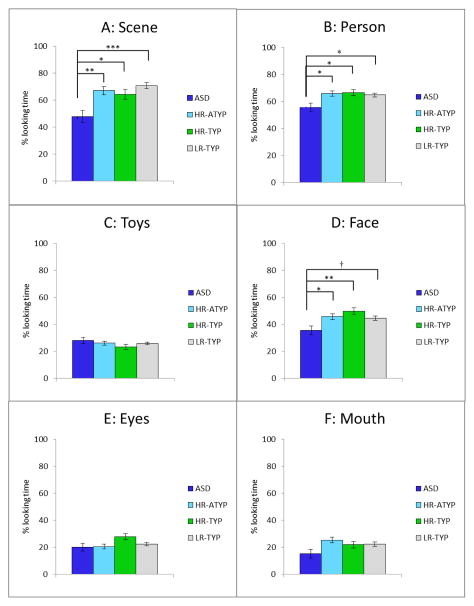
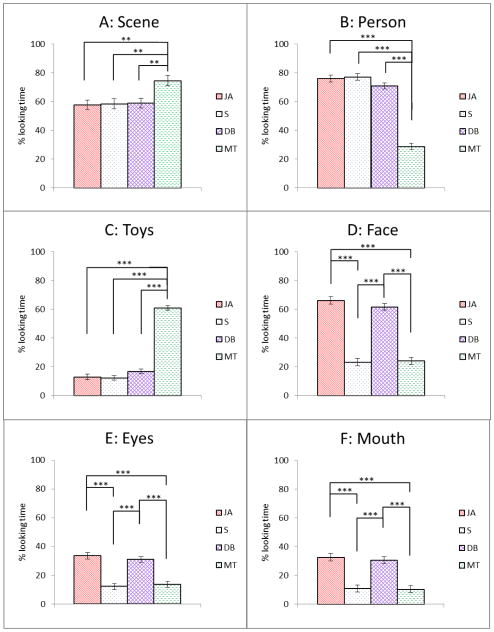
Each child contributed data to at least one condition and 85% contributed to 3 or 4 conditions such that the number of participants in each condition ranged from 6–10 in HR-ASD, 15–18 in HR-ATYP, 9–13 in HR-TYP, and 28–31 in LR-TYP. Loss of eye tracking data was attributed to saccades and blinks as well as inattention. A mixed effects model group x condition analysis performed on the average %Scene indicated a significant effect of group, F(2, 263)=7.25, p<.001 and condition, F(3, 263)=5.36, p=.002, but no group x condition interaction (p=.830). Post-hoc comparisons for group effects indicated that infants with ASD spent less time looking at the Scene than the three comparison groups (Figure 2a). All infants spent more time looking at the Scene in the condition involving moving toys (Figure 3a). The effect of VR (p=.007), but not RL (p=.339), was significant. The Cohen’s d effect sizes based on marginal means for planned comparisons between the ASD and the other groups were: d=.45 (HR-ATYP), d=.38 (HR-TYP), and d=.59 (LR-TYP).
Figure 2.
Looking time ratios for the four groups (marginal means, +/− 1 standard error) for the Scene, Person, Toys, Face, Eyes and Mouth Regions of Interest across all conditions. P values for planned contrasts are reported with Tukey-Kramer correction for multiple comparisons. † p < .065 * p < .05 ** p < .01 *** p < .001
Figure 3.
Looking time ratios for four conditions (marginal means, +/− 1 standard error) for each of the Regions of Interest. P values for post-hoc tests are reported with Tukey-Kramer correction for multiple comparisons. * p < .05 ** p < .01 *** p < .001
JA = Joint Attention; S = Sandwich; DB = Dyadic Bid; MT = Moving Toys
Attention to the Person and Toys
For subsequent analyses, we excluded all episodes (conditions) in which the child contributed less than 15% of valid eye tracking time, which amounted to 6% of all episodes (n=18).
Person
A group x condition analysis on %Person indicated a significant effect of group: F (3, 245)=3.41, p=.018, and condition: F(3, 245)=111.08, p<.001, and no interaction (p=.339). Infants with ASD spent a lesser proportion of time than the three remaining groups looking at the Person (Figure 2b) across all conditions. All infants spent the least proportion of time looking at the Person in the Moving Toys condition (Figure 3b). The effect of the VR (p=.007), but not RL (p=.321), was significant. The Cohen’s d effect sizes based on marginal means for planned comparisons between the ASD and the other groups were: d=.33 (HR-ATYP), d=.34 (HR-TYP), and d=.29 (LR-TYP).
Toys
A group x condition analysis on %Toys indicated no effect of group (p=.373) (Figure 2c), a significant effect of condition: F(3, 245)=192. 44, p<.0001, but no interaction (p=.903). As expected, all infants spent the highest proportion of time looking at the Toys in the Moving Toys condition (Figure 3c). Neither the VR (p=.386) nor the RL (p=.427) covariate contributed significantly to the model.
Attention to Facial Features
A groupxcondition analysis on %Face indicated a significant effect of group: F(3, 245)=3.92, p=.009, and condition (F(3, 245)=88.00, p<.001), but no interaction (p=.595). Infants with ASD spent a significantly lower proportion of time looking at the Face than the three remaining groups, though the contrast between ASD and LR-ATYP became marginally significant (p=.064) after correcting for multiple comparisons (Figure 2d). All infants spent more time looking at the actress’ face in the Joint Attention and Dyadic Bid conditions as compared to the Sandwich and Moving Toys conditions (Figure 3d). Effects of VR and RL covariates were both significant (p=.045 and p=.028, respectively). The Cohen’s d effect sizes based on marginal means for planned comparisons between the ASD and the other groups were: d=.32 (HR-ATYP), d=.47 (HR-TYP), and d=.33 (LR-TYP).
Subsequently, we examined the proportion of time spent looking at the eyes and mouth. A group x condition analysis on %Eyes showed no effect of group (p=.065) (Figure 2e), a significant effect of condition, F(3, 245)=27.14, p <.001 (Figure 3e), and no interaction effect (p=.412). Effects of VR (p=.785) and RL (p=.997) were not significant. All infants spent more time looking at the eyes in the two conditions that involved a social bid (Joint Attention and Dyadic Bid) as compared to the Sandwich and Moving Toys. An analogous analysis on % Mouth indicated only a significant effect of condition, F(3, 245)=24.71, p<.001) (Figure 3f), but no effect of group (p=.084) (Figure 2f), and no interaction (p=.785). The effect of the RL covariate was significant (p=.026), but the effect of the VR covariate was not (p=.072).
Discussion
The study examined spontaneous social monitoring in 6-month-old infants later diagnosed with ASD. The infants exhibited diminished attention in general to a social scene, and when they did attend, they spent less time monitoring the person and looked less at the person’s face. Interestingly, diminished attention to the person did not translate into enhanced attention to the highly perceptually and semantically attractive objects and suggest that at least in this experimental context, there is little evidence of prepotent salience of objects in those later diagnosed with ASD. Consistent with another report of 6-month-olds with ASD (27), no marked differences were found in the distribution of their attention to eyes or mouth. The attentional deficits were present not only in comparison to infants who are typically developing (both high- and low-risk) but also in comparison to high-risk infants who exhibited some ASD-related difficulties, suggesting an association of the noted attentional deficits with the full-blown syndrome rather than with intermediate phenotypes.
The positive findings regarding limited spontaneous attention to social scenes in infants later diagnosed with ASD reported in our study are contrary to negative reports based on face-to-face interactions with a parent or examiner(18–20,27). While the methodological difference between the current study and others is clear (eye tracking versus live interaction), we posit that the mechanism responsible for the discrepant results is alteration in adult behavior in response to infant characteristics during face-to-face engagement. Adults interacting with vulnerable infants may unknowingly employ subtle augmentative strategies to elicit attention and enhance responsiveness either with a more direct interaction style(28), or by using enhanced perceptual (moving one’s face within the infant’s line of vision) or affective (increasing intensity of facial expressions or prosodic features) cues. On the other hand, the video stimuli presented during eye tracking affords no such augmentation, thus the infants’ visual attention patterns under these conditions may represent a closer estimate of their spontaneous behavior in situations when scaffolding is absent, thus exposing the infants’ vulnerabilities. To directly test this hypothesis, a comparison of infants’ visual responses during the eye tracking task with those during a live face-to-face interaction, while accounting for the adult partner’s behavior, will be necessary.
The attentional patterns of 6-month-olds later diagnosed with ASD share some key similarities with the performance features noted in clinic-referred 14- to 24-month-old toddlers with autism viewing the same stimulus. The features include limited attention to the social scene, as well as the person (unpublished data) and her face(34). Furthermore, the deficits in spontaneous social monitoring in toddlers with autism were most pronounced in the condition when the actress tried to engage the child through eye contact and child-directed speech. In 6-month-olds, however, atypical attentional responses were not condition-specific, which may suggest that at this age, the deficits in social attention are still poorly differentiated and cut across a variety of contexts in which a person might appear in their visual field. Moreover, the infants did not display enhanced attention to the toys in any of the conditions, nor did they demonstrate the atypical distribution of attention to the mouth noted previously in toddlers (34,35).
Although cross-sectional, these findings suggest both continuity of social attention impairments in ASD as well as their evolution, from a limited attunement to people in general towards more specific impairments in attention towards their bids for social interaction and communication. These results are consistent with the interactive specialization hypothesis, which, in broad terms, suggests that in the postnatal period many brain regions are poorly specialized, but undergo fine-tuning to more specific classes of stimuli in an experience- dependent fashion(40,41,51). Examples of such specialization in the first year of life include the ‘other-species’(52) and ‘other race’(53) effects as well as the perceptual narrowing associated with the refinement of native language phoneme discrimination ability(54,55). In this vein, in typically developing infants, the early attunement to people facilitates access to a range of highly socially-relevant experiences and, consequently, the development of a more fine-tuned ability to spontaneously attend to people, their facial expressions and gestures. These skills are necessary for participation in complex dyadic routines (e.g., peek-a-boo game) and later in infancy, triadic interactions (e.g., joint attention). However, the limited salience of social stimuli observed here at 6 months, and possibly manifesting even earlier, may hinder the cortical specialization process that rapidly advances in non-affected infants, resulting in diverging attentional patterns between toddlers with ASD and control groups. Another intriguing, and not necessarily mutually exclusive, possibility is that interactive specialization is occurring in infants who go on to develop ASD, but that their specialization processes will emphasize other, non-social targets.
The stimuli employed in this experiment were complex and demanded rapid allocation of visual attention depending on the activity in which the person on the screen was engaged. Given the nature of the task (free-viewing) and type of stimulus (multimodal), performance captured here reflects a combination of top-down and bottom-up influences, which leave speculation regarding the underlying mechanisms relatively open. It is not clear, for instance, if the observed effects in the ASD group are due to deficits in the ability to detect and prioritize social stimuli for processing, the limited power of such stimuli to arouse positive emotions that enhance motivation and learning, or a more general deficit in regulation of attention to rapidly changing and complex stimuli, both social and nonsocial. Some aspects of social orienting such as preference for static faces appear to be spared at this age in those later diagnosed with ASD(57). Similarly, infants in our study were able to adjust their scanning strategies depending on the context (i.e., they looked more at the person’s face when she was talking than when she was making a sandwich). This may suggest that some aspects of processing of social information, both static and dynamic, might be intact at this age, which is very encouraging. However, it remains to be determined if their gaze patterns were driven by appreciation of the semantic significance of these areas or by low-level perceptual features (e.g., motion of the face or hands). To better understand the factors underlying the observed differences in infants later diagnosed with ASD as well as performance patterns in other high-risk infants, it will be necessary to parse the potential contributing factors experimentally. Furthermore, to understand how these deficits emerge in the first few months of life, it will be necessary to extend the investigation into the earlier postnatal months.
Conclusions
To our best knowledge, this is the first study to demonstrate behavioral prodromal features associated with ASD at six months, and as such, it complements positive findings regarding findings regarding atypical neural development occurring within the same developmental window(30,31). Limited attentional bias for people and their communicative cues may have important implications for the progressive specialization of the neural systems involved in social cognition, as well as for the emerging patterns of parent-child interactions(28), and thus, are potentially highly clinically relevant to the outcomes in high-risk infants. In this context, it will be essential to evaluate the role that variability in spontaneous social monitoring in infancy plays in the heterogeneity of syndrome expression later in toddlers(56). This study also highlights the possibility of identifying phenotypic features linked to visual attention for identifying infants at greatest risk for ASD in the first year of life.
Acknowledgments
The study was supported by the National Institute of Child Health and Development, PO1 HD003008, Project 1 (PI: KC), National Institutes of Mental Health R01 MH087554 (PI:KC), NIMH grants #1R03MH086732 (PI: S. Macari), R03 MH092618-01A1 (PI: F. Shic), Needleman Foundation, and the Associates of the Child Study Center. We would also like to thank Celine Saulnier, Amanda Steiner, Karen Bearss, Amy Carney, Elizabeth Simmons, Megan Lyons, Sarita Austin, and Rhea Paul for their contribution to the sample characterization as well as Jessica Bradshaw, Grace Chen, Marika Coffman, Alexandra Dowd, Eugenia Gisin, Mairin Meltvedt, Jessica Garzarek, Kerry O’Loughlin and Jessica Reed for assistance in data collection. We would like to thank Sophy Kim for her help in editing this manuscript. We wish to express our appreciation to the families and their children for their time and participation.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Interagency Autism Coordinating Committee. 2011 IACC Strategic Plan for Autism Spectrum Disorder Research. 2011 Jan; Retrieved from the Department of Health and Human Services Interagency Autism Coordinating Committee website at http://iacc.hhs.gov/strategic-plan/2011/index.shtml.
- 2.Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007 Mar;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 3.Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: Studies of infants at risk. Neural Networks. 2010;23(8–9):1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macari S, Campbell D, Gengoux G, Saulnier C, Klin A, Chawarska K. Predicting Developmental Status from 12 to 24 Months in Infants at Risk for Autism Spectrum Disorder: A Preliminary Report. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1521-0. Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14(2):81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AR, State MW. Recent Advances in the Genetics of Autism. Biological Psychiatry. 2007;61(4):429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Fombonne E. Epidemiology of Pervasive Developmental Disorders. Pediatr Res. 2009;65(6):591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 9.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area.[see comment] JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006 Jul;368(9531):210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Prevalence of Autism Spectrum Disorders — Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. CDC; Mar 30, 2012. [PubMed] [Google Scholar]
- 12.Kim YS, Leventhal BL, Koh Y, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. American Journal of Psychiatry. 2011:1–9. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 13.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011 Aug 15;2011:2010–2825. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007 Jul;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 15.Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, et al. Precursors to Social and Communication Difficulties in Infants At-Risk for Autism: Gaze Following and Attentional Engagement. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1450-y. Online First:1–11. [DOI] [PubMed] [Google Scholar]
- 16.Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007 Jan;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgiades S, Szatmari P, Boyle M, Hanna S, Duku E, Zwaigenbaum L, et al. Investigating phenotypic heterogeneity in children with autism spectrum disorder: a factor mixture modeling approach. Journal of Child Psychology and Psychiatry. 2012 doi: 10.1111/j.1469-7610.2012.02588.x. Online First. [DOI] [PubMed] [Google Scholar]
- 18.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005 Apr-May;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Ozonoff S, Iosif A, Baguio F, Cook I, Hill M, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):258–268. [PMC free article] [PubMed] [Google Scholar]
- 20.Rozga A, Hutman T, Young GS, Rogers S, Ozonoff S, Dapretto M, et al. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41(3):287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutman T, Rozga A, DeLaurentis AD, Barnwell JM, Sugar CA, Sigman M. Response to distress in infants at risk for autism: A prospective longitudinal study. Journal of Child Psychology and Psychiatry. 2010;51(9):1010–1020. doi: 10.1111/j.1469-7610.2010.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52(5):588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers S. A prospective study of response to name in infants at risk for autism.[see comment] Archives of Pediatrics & Adolescent Medicine. 2007 Apr;161(4):378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- 25.Hutman T, Rozga A, DeLaurentis A, Sigman M, Dapretto M. Infants’ pre- empathic behaviors are associated with language skills. Infant Behavior and Development. 2012;35(3):561–569. doi: 10.1016/j.infbeh.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The Autism Diagnostic Observation Schedule--Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(9):1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12(5):798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Parent– infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities. 2012;33(3):924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Noland JS, Reznick J, Stone WL, Walden T, Sheridan EH. Better working memory for non-social targets in infant siblings of children with Autism Spectrum Disorder. Developmental Science. 2010;13(1):244–251. doi: 10.1111/j.1467-7687.2009.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff JJ, GH, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, et al. Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang J. Early Generalized Overgrowth in Boys with Autism. Archives of General Psychiatry. 2011;68:1021–1031. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22(4):338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry. 2012;53(9):986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chawarska K, Macari S, Shic F. Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry. 2012;53(8):903–913. doi: 10.1111/j.1469-7610.2012.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shic F, Bradshaw J, Klin A, Scassellati B, Chawarska K. Limited Activity Monitoring in Toddlers with Autism Spectrum Disorder. Brain Research. 2010;1380:246–254. doi: 10.1016/j.brainres.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65(8):946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 37.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(14):9602–9605. [Google Scholar]
- 38.Kisilevsky BS, Hains SMJ, Brown CA, Cowperthwaite B, Stutzman SS, Swansburg ML, et al. Fetal sensitivity to properties of maternal speech and language. Infant Behavior and Development. 2009;32(1):59–71. doi: 10.1016/j.infbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Vouloumanos A, Hauser MD, Werker JF, Martin A. The Tuning of Human Neonates’ Preference for Speech. Child Development. 2010;81(2):517–527. doi: 10.1111/j.1467-8624.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 40.Greenough WT, Black JE, Wallace CS. Experience and brain development. In: Johnson M, Munakata Y, et al., editors. Brain development and cognition: A reader. 2. Malden, MA: Blackwell Publishers; 2002. pp. 186–216. [Google Scholar]
- 41.Johnson MH, Grossmann T, Kadosh KC. Mapping functional brain development: Building a social brain through interactive specialization. Developmental Psychology. 2009 Jan;45(1):151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- 42.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 2000. [Google Scholar]
- 43.Rutter M, Le Couter A, Lord C. ADI-R: Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 44.Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Serivce, Inc; 1995. AGS Edition. [Google Scholar]
- 45.Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales: Developmental Profile. 1. Baltimore, MD: Paul H. Brookes Publishing Co; 2002. [Google Scholar]
- 46.Edwards S, Fletcher P, Garman M, Hughes A, Letts C, Sinka I. The Reynell Developmental Language Scales III. London: NFER-Nelson: The University of Reading Edition; 1997. [Google Scholar]
- 47.MATLAB 7.8 (Release R2009a) [computer program]. Version. 2009. [Google Scholar]
- 48.Shic F. Computational Methods for Eye-Tracking Analysis: Applications to Autism. New Haven, CT: Computer Science, Yale University; 2008. [Google Scholar]
- 49.Duchowski AT. Eye Tracking Methodology: Theory and Practice. New York: Springer; 2003. [Google Scholar]
- 50.SAS Prioprietary Software 9.3 [computer program]. Version. Cary, NC, USA: 2002–2010. [Google Scholar]
- 51.Nelson CA, de Haan M, Thomas KM. Neuroscience of cognitive development: The role of experience and the developing brain. Hoboken, NJ: John Wiley & Sons Inc; 2006. [Google Scholar]
- 52.Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002 May;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- 53.Sangrigoli S, de Schonen Effect of visual experience on face processing: A developmental study of inversion and non-native effects. Developmental Science. 2004 Feb;7(1):74–87. doi: 10.1111/j.1467-7687.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheour M, Ceponiene R, Lehtokoski A, Luuk A, Allik J, Alho K, et al. Development of language-specific phoneme representations in the infant brain. Nature Neuroscience. 1998;1(5):351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- 55.Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindlom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992 Jan;255(5044):606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- 56.Campbell D, Shic F, Macari S, Chawarska K. Gaze patterns in response to dyadic bids at 2 years predict functioning at 3 years in Autism Spectrum Disorders: A Subtyping Analysis. doi: 10.1007/s10803-013-1885-9. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Bolton P, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]