Abstract
Depression is a common outcome for those having experienced early life stress (ELS). For those individuals, depression typically increases during adolescence and appears to endure into adulthood, suggesting alterations in the development of brain systems involved in depression. Developmentally, the nucleus accumbens (NAcc), a limbic structure associated with reward learning and motivation, typically undergoes dramatic functional change during adolescence; therefore, age-related changes in NAcc function may underlie increases in depression in adolescence following ELS. The current study examined the effects of ELS in 38 previously institutionalized children and adolescents in comparison to a group of 31 youth without a history of ELS. Consistent with previous research, the findings showed that depression was higher in adolescents than children with a history of ELS. Additionally, fMRI results showed atypical NAcc development, where the ELS group did not show a typical increase in NAcc reactivity during adolescence. Consequently, the ELS group showed NAcc hypoactivation during adolescence, and lower NAcc reactivity was correlated with higher depression scores. The results have important implications for understanding how ELS may influence increases in depression via neural development during the transition to adolescence and highlight the importance of identifying at-risk individuals in childhood, a potential critical period for depression-targeted intervention.
Keywords: Depression, ventral striatum, nucleus accumbens, early-life stress
1. Introduction
1.1
Early-life stress (ELS) can be defined as childhood exposure to events that negatively impact emotional or physical well-being to an extent that exceeds an individual's ability to cope (Gunnar and Quevedo, 2007). While biological responses to acute stress are considered to be an adaptive survival mechanism, high or chronic levels of stress may disturb normative brain development and negatively impact mental health (Anda et al., 2006; Lupien et al., 2009; Maniglio, 2009; Pirkola et al., 2005). Depression is a common outcome for those individuals with a history of ELS (Heim and Nemeroff, 2001; McEwen, 2000), with the magnitude of childhood adversity predicting the severity of lifetime depression (Kessler, 1997). Depression typically does not emerge until the adolescent period despite early environmental insults, and this relatively late onset of depression has been documented in both human and non-human animal models of ELS (Andersen and Teicher, 2008; Costello et al., 2002; Paus et al., 2008; Raineki, et al., 2012). Early-life stress effects have been found following both abuse (Anda et al., 2006; Maniglio, 2009; Raineki, et al., 2012) and maternal deprivation (Johnson, 2002; Loman and Gunnar, 2010), and the effects appear to endure into adulthood, suggesting that ELS alters the development of brain systems involved in depression.
1.2
In both human studies and non-human animal models, depression has been associated with altered neural activity in the ventral striatum (Epstein et al., 2006; Eshel and Roiser, 2010; Forbes et al., 2009; Monk et al., 2008.; Pizzagalli et al., 2009; Price and Drevets, 2010; Teicher et al., 2009), which includes the nucleus accumbens (NAcc). The NAcc is a dopamine receptor-rich limbic structure associated with reward learning and motivation in response to pleasurable stimuli (Ikemoto and Panksepp, 1999) such as the visual presentation of happy faces for humans (Monk et al., 2008). Developmentally, the NAcc response to pleasurable stimuli shows relatively late functional development, increasing significantly during the transition from childhood into adolescence, when it reaches a developmentally-normative peak in functional activity (Galvan et al., 2006; Ernst et al., 2005; van Leijenhorst et al., 2010; Geier et al., 2009; although see Bjork et al., 2004 for hypoactivity). We present findings to argue that deviation from this developmental trajectory coincides with the emergence of depressive behaviors in adolescence.
1.3
In adulthood, atypically low ventral striatum activity and depressive behaviors are commonly associated with stress exposure. For example, in a prospective examination of soldiers heading to combat, NAcc activity declined following stress exposure and was associated with clinical symptoms of depression (Admon et al., in press). Similarly, Nikolova et al. (2012) has shown that low ventral striatum activity predicts an association between recent stressful events and low positive affect in a group of emergent adults, suggesting that the association between stress and depression may be mediated by stress-related changes in the NAcc.
1.4
Similar effects of stress on striatal function have been observed during development. In animal models, electrophysiological and lesion studies have demonstrated a sensitivity of dopaminergic pathways (Powell et al., 2003; Hall et al., 1998; Jones et al., 1992), including the NAcc (Fulford and Marsden, 1998; Jones et al., 1992), and reduced responsiveness to reward (Lapiz et al., 2000) to early adverse experiences. Similar effects of ELS have been observed in humans. Dillon et al., (2009) found that participants with a history of early-life maltreatment displayed dampened behavioral responsiveness to reward and reduced activation in striatal structures. In a striking example of the enduring effects of ELS, Mehta et al., (2009) examined children who had experienced early maternal deprivation. As adolescents, these individuals showed hyporesponsivity in the ventral striatum in response to reward, and unlike in typical adolescents, ventral striatum activity was not modulated by reward value. Collectively, these findings suggest that ELS impacts the development of the ventral striatum, resulting in hyporeactivity, which adversely impacts reward and motivational processing.
1.5
In the current study we aim to further probe the association between early-life stress, depression, and alterations in ventral striatum development. We measured brain development with functional magnetic resonance imaging (fMRI). Although fMRI does not have enough resolution to identify the NAcc with confidence, our analyses focused on an anatomically defined region consistent with the location of the NAcc. For brevity's sake, we refer to this region as the NAcc throughout the manuscript. We examined children and adolescents with and without a history of ELS (institutional care in orphanages abroad) to examine age-related change in NAcc activity between childhood to adolescence. Additionally, we collected dimensional behavioral measures of depression to both chart its developmental course and to examine associations between NAcc activity and depression. Utilizing fMRI, we hypothesized that depression would ne higher in adolescents than in children following ELS, and this behavioral change would be paralleled by adolescent hypoactivity of the NAcc.
2. Experimental Procedures
2.1. Participants
Seventy-six individuals (42 ELS and 34 comparison, never-institutionalized) participated in an fMRI study whose characteristics are described in Table 1. Seven participants were removed due to excessive head motion (> 2.5 mm or 2.5 degrees of rotation). Therefore, our final sample of 69 participants, included 39 children between the ages of 5 and 10 years-old (24 ELS and 15 comparison) and 30 adolescents between the ages of 11 and 15 years-old (14 ELS and 16 comparison). We chose this age cut off based on previous research showing that depression is most pronounced among early adolescents (Brooks-Gunn and Petersen, 1991; Petersen et al., 1993), showing a sharp increase after age 10 (Kessler and Magee, 1993; Angold and Rutter, 1992). ELS youths were recruited via local international adoption agencies and family networks. The comparison group, comprised of non-adopted youths who had always lived with their families, was recruited via flyer advertisements within the surrounding community or from state birth records. Participants in the comparison group were only included if they were psychiatrically healthy and free of psychotropic medications. Based on parent-report of mental health from the Child Behavior Checklist questionnaire (CBCL; Achenbach & Rescorla, 2001), a standardized instrument to assess emotional or behavioral problems that includes DSM-Oriented Scales (Nakamura et al., 2009), and the Revised Child Anxiety and Depression Scales – Parent form (RCADS-P; Chorpita et al., 2000), none of the comparison participants scored within clinical range, and eight of the participants form the ELS group exhibited mental health characteristics within the clinical range (T scores>70 for internalizing or externalizing problems on the CBCL & depression or total anxiety on the RCADS-P). Four participants in the ELS group were taking psychotropic medications. The families of both the ELS youths ($85,001-$100,000) and the comparison group ($70,001-85,000) had a household income well above the median annual household income in the United States ($58,172; US Census Bureau, 2010) similar to the high socioeconomic status that has been observed in another sample of Midwest families who have adopted internationally (Hellerstedt et al., 2008). The protocol was approved by the Institutional Review Board at the University of California, Los Angeles. Parents of participants provided informed consent.
Table 1.
Characteristics for the Early-life stress (ELS) and comparison participants.
| ELS (n = 38; 24 children, 14 adolescents) | Comparison (n = 31; 15children, 16adolescents) | |
|---|---|---|
| Sex | 24 female; 14 male | 12 female; 19 male |
| Mean age in years (SD); range | 9.9 (2.6); 6-15 | 9.7 (3.1); 4-15 |
| Mean (SD) IQ; range | 101.1 (14.3); 72-126 | 111.2 (15.2); 76-143 |
| Country of origin | 29 Eastern Europe; 8 East Asia; 1 India | |
| Mean (SD) age in years when placed in institutional care ; range | .65 (1); 0-4 | |
| Mean (SD) age in years when adopted; range | 2 (2); 0.5-8 | |
| Mean (SD) time in years with adoptive family; range | 8 (3); 3-14 | |
| Mean (SD) parent reported quality of institutional caregiving (1=very poor caregiving, 10=very good caregiving); range | 6 (3); 1-10 | |
| Mean (SD) parent reported quantity of caregiving (1=too few caregivers, 10=many caregivers); range | 5 (3); 1-10 |
2.2. Procedures
2.2.1. MRI Task Paradigm
During the fMRI scan, participants completed two runs of an emotional faces task. The task consisted of a mixed design with one blocked variable (emotional valence: happy vs. fearful) and one event-related variable (emotion vs. neutral). During each run, participants viewed singly-presented faces that were either emotional or neutral. The order of runs was counterbalanced across participants, and the stimulus order within each run was randomized and fixed. To ensure that participants were paying attention, they were instructed to press a button with their index finger when they saw a neutral face. The faces were selected from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998). The faces were presented in color at a visual angle of approximately 15 degrees. The probability of a neutral face was 50% on any given trial. Stimuli were jittered (variable inter-trial interval ranging from 3000-9000 msec) and randomized based on a genetic algorithm (Wager and Nichols, 2003) in order to allow for unique estimates of the hemodynamic response for each trial type. Each run contained 48 trials (24 neutral faces, 24 fearful or happy faces). Each face remained on the screen for 500 msec.
2.2.2. Procedure
Children and adolescents came to the laboratory for two sessions. In the first session, behavioral measures were collected and participants were acclimated to the scanner environment with an MRI replica. The emotional faces task was administered in the MRI scanner on the second visit, which occurred on a separate day.
2.3. fMRI Data Acquisition
Scanning was performed on a Siemens Trio 3.0 Tesla MRI scanner. A standard radiofrequency head coil was employed. For each participant, an initial 2D spin echo image (TR=4000ms, TE=40ms, matrix size 256×256, 4mm thick, 0mm gap) in the oblique plane was acquired to allow configuration of slices obtained in the structural and functional scans. A whole-brain high-resolution, T1*weighted anatomical scan (MPRAGE; 192 × 192 in-plane resolution, 256 mm field of view [FOV] ; 192 mm × 1 mm sagittal slices) was acquired for each participant for registration and localization of functional data to Talairach space (Talairach and Tournoux, 1988). The emotional faces task was presented on a computer screen through MR-compatible goggles. The task was completed during two functional scans. T2*weighted echoplanar images were collected at an oblique angle of approximately 30 degrees (130 volumes/run, TR=2000, TE=30ms, flip angle =90 degrees, matrix size 64×64, FOV=192, 34 slices, 4mm voxel, skip 0mm, 24 observations per event type).
2.3.1. fMRI Data Analysis
Functional imaging data were preprocessed and analyzed using the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). All analyzed data were free of motion greater than 2.5 mm in any direction. TRs with motion greater than 2.5 mm in any direction were excluded (via censoring), and all participants had fewer than 15% of total TRs censored (mean % of censored TRs = 1.5%; mode=0%). Preprocessing of each individual's images included slice time correction to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, registration to the first volume of each run, spatial smoothing using a 6-mm Gaussian kernel (FWHM) to increase the signal to noise ratio, and transformation into the standard coordinate space of Talairach and Tournoux (Talairach and Tournoux, 1988) with parameters obtained from the transformation of each individual's high-resolution anatomical scan. Talairached transformed images had a resampled resolution of 3 mm3. Timeseries were normalized to percent signal change to allow for comparisons across runs and individuals. The functional runs were concatenated prior to creating two individual-level models for each participant to model activation.
2.3.1.1
In order to examine activation across the brain, each participant's individual-level model included regressors for each of the stimulus conditions (fearful faces, happy faces, neutral faces in the context of fearful faces, neutral faces in the context of happy faces) and accuracy. The regressors were created by convolving the stimulus timing files with the canonical hemodynamic response function. Six motion parameters were included as separate regressors. General linear modeling (GLM) was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled for each voxel's time course to control for correlated drift. Implicit baseline (BL) comprised unmodeled events (fixation) during the inter-trial intervals. At the group level, parameter estimates (beta weights) were extracted for each participant for the happy and fear conditions from anatomically-defined masks covering the bilateral NAcc as defined by AFNI's Talairach-Tournoux Atlas. Therefore, for the purposes of this study, each participant had an activation value for the happy (happy>BL) and the fear (fear>BL) condition. These values were subjected to analyses within SPSS that tested for ELS Group (Comparison, ELS) by Age Group (Children, Adolescents) interactions.
2.4. Behavioral Data Analysis
For each participant, d-prime was calculated based on hit rates and false alarm rates for each emotion block, producing two d-prime scores for each participant (neutral with happy, and neutral with fear). We also calculated the mean reaction time for correct hits to neutral in the context of happy or fear faces, producing two reaction time averages for each participant (neutral with happy, and neutral with fear). These values were subjected to analyses within SPSS that tested for ELS Group (Comparison, ELS) by Age Group (Children, Adolescents) effects.
2.5. Parent Report Questionnaires
Parents completed the Revised Child Anxiety and Depression Scales – Parent form (RCADS-P; Chorpita et al., 2000), which has been shown to be valid and reliable in developing samples (Chorpita et al., 2005). The RCADS-P is a parent report 47-item instrument that assesses symptoms of childhood anxiety disorders and depression continuously based on DSM-IV criteria. Each symptom on the scale is scored 1, “never”; 2, “sometimes”; 3, “often”; and 4, “always.” The questionnaire is reliable in terms of internal consistency (with Cronbach's alphas between 0.65 and 0.83 for the various subscales) and temporal stability (with 4-week test–retest correlations between 0.79 and 0.85), and displays reasonable parent–child agreement and good convergent and divergent validity. In the current study, two subscales were examined from the RCADS-P: a total anxiety score, which was computed by summing ratings on social phobia, panic disorder, separation anxiety, and generalized anxiety disorder items, and a total depression score. T-scores were calculated based on child gender and grade in school using the norms established by the RCADS-P. For the present study, we used the depression and total anxiety T-scores.
Parents also completed the Petersen Physical Development Scale (Petersen et al., 1988) for participants ages 10-years-old and older, which was used to classify pubertal status according to Tanner staging. This method is significantly less invasive than a physical exam and has been shown to have good reliability and validity (Petersen et al., 1998).
2.6. Child Report Questionnaires
For the purpose of confirming parent report, child and adolescent participants completed a child-friendly, 6-item scale mood assessment that was derived from the Positive and Negative Affect Schedule for Children (PANAS-C) (Laurent et al., 1999), which is designed to assess positive affect (PA) and negative affect (NA) in children. Laurent et al. (1999) reported acceptable alpha coefficients (.94 and .92 for negative affect, and .90 and .89 for positive) for the scale development and replication samples, respectively. Good convergent and discriminant validity were also reported, with the NA scale correlating positively with self-reports of depression, and the PA scale correlating negatively with depression. We chose to include 3 items on the PA scale (“Lately, how much have you been feeling:” Cheerful/Joyful/Delighted) and 3 items on the NA scale (“Lately, how much have you been feeling:” Frightened/Miserable/Sad) to abbreviate the testing session based on words that seemed most child-appropriate. Participants were asked to rate the degree to which they have felt during the past few weeks, on a scale of 1 (Very slightly or not at all) to 5 (Extremely). We also obtained acceptable alpha coefficients for the abbreviated version of the questionnaire (PA alpha=.77, NA alpha=.75). Responses were summed to create a PA score and an NA score for each participant.
3. Results
3.1. Depression scores between children and adolescence with a history of early-life stress
3.1.1
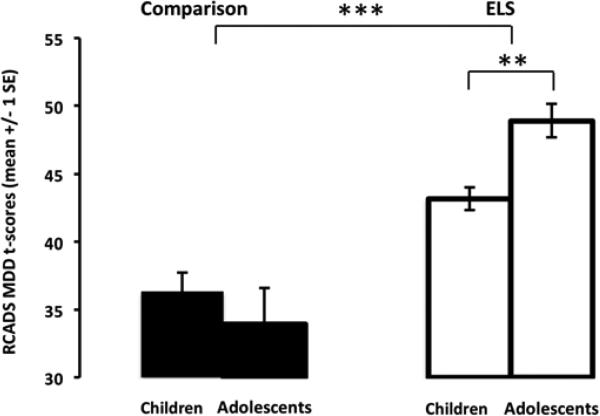
Data from the RCADS-P (Chorpita, 2000) was available for 67 participants. A 2×2 (ELSGroup×AgeGroup) analysis of variance (ANOVA) yielded a main effect of ELS group (F(1,63)=38.66, p<.001, partial η2=.38), such that the average RCADS-P MDD T-score was significantly higher for the ELS group (mean(SD);range=45.74(9.09);31.0-70.6) than for the comparison group (mean(SD); range=35.28(3.96); 30.4-46.5). This main effect was anticipated based on the selection of a healthy comparison group. There was a two-way interaction of ELSGroup×AgeGroup (F(1,63)=5.46, p<.025, partial η2=.08), and post hoc tests showed that for the ELS group only, depression scores were significantly different between age groups (F(1,36)=6.25, p<.025) (Figure 1)1. We also tested the relationship between pubertal status and RCADS depression scores by comparing participants who were pre-pubescent versus post-pubescent across those 10-15 years old. We performed a univariate ANOVA on RCADS-P MDD T-scores including the between-subject factor of pubertal status, controlling for age and group, and there were no effects of pubertal status on depression scores (p>.25).
Figure 1.
Depression is higher in adolescents than children following early life stress. The ELS group showed significantly greater depression scores (measured dimensionally) in adolescents than in children. In contrast, the comparison group did not show any age-related change in depression scores. ELS=early-life stress; MDD=major depressive disorder; **p < .025, ***p < .001
3.1.2. Anxiety scores between children and adolescence with a history of early-life stress
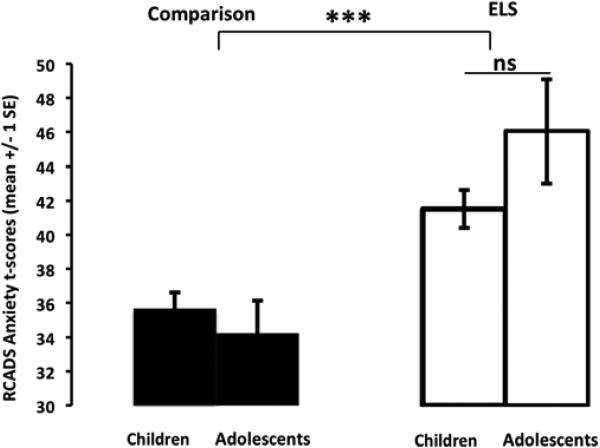
As anticipated based on the selection of a healthy comparison group, analysis of RCADS-P Total Anxiety T-score yielded a main effect of ELS group (F(1,64)=21.19, p<.001, partial η2=.25), such that anxiety scores were significantly higher for the ELS group(mean(SD); range=43.54(9.37); 28.9-72.9) than for the comparison group (mean(SD); range=35.03(5.41); 25.2-49.1). The ELSGroup×AgeGroup interaction that was observed in RCADS depression scores was not observed in RCADS anxiety scores (F(1,67)=2.47, ps>.05, partial η2=.04), showing that unlike the age-related differences in depression scores, anxiety scores remained constant over the two age groups (Figure 2).
Figure 2.
Total anxiety does not differ between children and adolescents following early life stress. The ELS group showed significantly greater anxiety scores than the comparison group, but did not show any age-related difference. ELS=early-life stress ;***p < .001
3.1.3
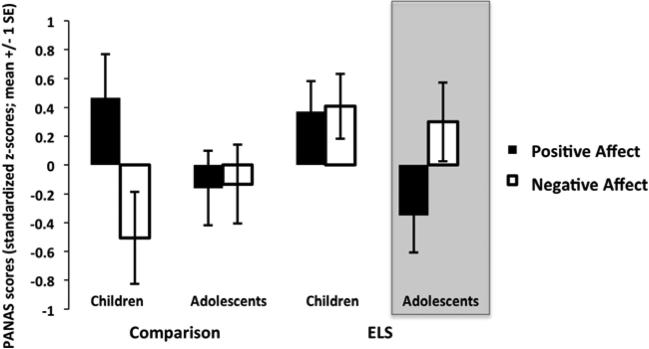
To confirm parent report, we included an abbreviated version of the PANAS-C to assess recent mood in all child and adolescent participants. Data were available for 69 participants. Two separate 2×2 (ELSGroup×AgeGroup) ANOVAs examining the dependent measures z-scores (standardized to the group mean) for positive affect (PA) and negative affect (NA) were performed. For positive affect, there was a main effect of age group (F(1,59) =6.77, p<.015, partial η2=.10), such that reported positive affect was high in all children and was significantly lower for adolescents. Post hoc t-tests showed that for the ELS group only, positive affect scores were significantly lower in adolescence than in childhood t(35)=2.13, p<.05. For negative affect, there was a main effect for ELS group (F(1,59)=5.96, p<.025, partial η2=.09), such that the ELS group reported significantly higher levels of negative affect than the comparison group. Taken together, as shown in Figure 3, adolescents with a history of ELS had the unique combination of low positive affect and high negative affect, based on visual inspection of the statistical main effects. These self-reported mood scores were consistent with parent reports of higher depression during adolescence following ELS.
Figure 3.
Self-reported mood. Based on visual inspection of the statistical main effects, adolescents with a history of ELS showed a unique combination of low positive affect and high negative affect. ELS = early life stress
3.2. fMRI Findings
3.2.1. Behavioral Task Performance
Participants were engaged in a simple task of pressing for neutral faces to ensure task engagement. Responses from one child were not included due to technical malfunction. Two separate 2×2×2 (Emotion×ELSGroup×AgeGroup) repeated measure ANOVAs were performed on the dependent measures of d-prime and reaction time during correct trials. For d-prime, there was a main effect of age group (F(1,64)=10.89, p<.002, partial η2 = .15), such that d-prime was higher in adolescents (mean(SD)=2.7(1.0)) than children (mean(SD)=1.9(1.1)). Additionally, there was an Emotion×AgeGroup interaction (F(1,64)=7.66, p<.007, partial η2 = .11). Post hoc t-tests showed that d-prime was higher for the neutral/happy face condition (mean(SD)=2.95(.93)) than d-prime for the neutral/fear condition (mean(SD)=2.43(1.29)) in adolescents (t(27)=2.69, p<.015), but there was no difference between the two conditions in children, t(39)=.66, ns. There were no main effects or interactions for reaction time (ps>.05).
3.2.2. NAcc Reactivity Following Early-life Stress
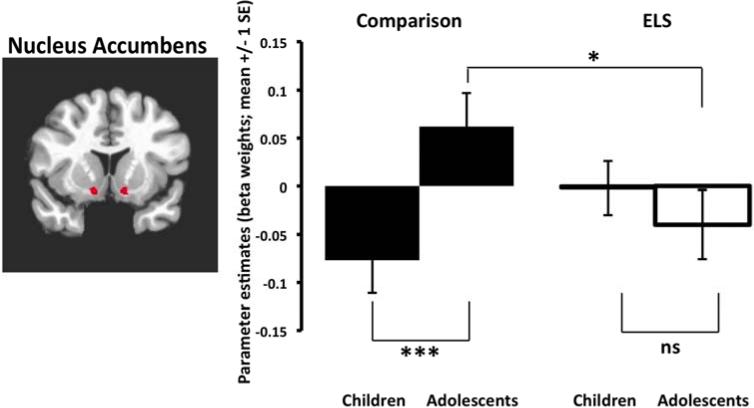
Beta weights from the anatomically-defined NAcc for happy and fear face conditions were extracted and submitted to a 2×2×2 ANOVA (Emotion×ELSGroup×AgeGroup). There was a significant ELSGroup×AgeGroup interaction (F(1,65)=7.03, p=.01, partial η2=.10). Post hoc independent samples t-test analyses reveal that, for the typically developing comparison group, mean NAcc activation significantly differed between child and adolescent groups (t(29)=2.33, p<.01), while no significant age group differences were seen in the ELS group (t(36)=.79, ps>.05) (Figure 4). Adolescents with a history of ELS exhibited significantly lower NAcc activation relative to the comparison adolescents (t(27)=2.26, p<.05) (Figure 4)2,3. We also tested the relationship between pubertal status and NAcc activation, comparing participants who were pre-pubescent versus post-pubescent across those 10-15 years old. We performed a univariate ANOVA on NAcc reactivity including the between-subject factor of pubertal status, controlling for age and group. There were no effects of pubertal status on NAcc activity (p>.91).
Figure 4.
NAcc Hypoactivation during adolescence following early-life stress. Left panel shows anatomically-defined NAcc regions selected for analysis. Right panel shows the normative developmental change in NAcc activity in children relative to adolescents in the comparison group. In contrast, the ELS group did not show any age-related change, demonstrating NAcc hypoactivity in adolescence. ELS = early life stress; *p<.05, ***p<.01
3.2.3. Association between NAcc activity and depression scores
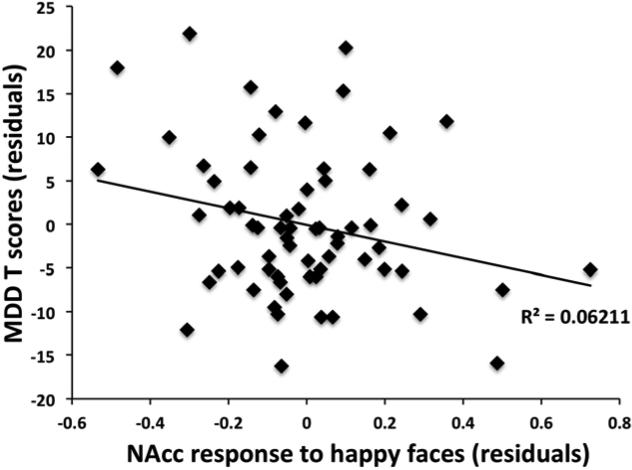
A linear regression was performed on the dependent measure of RCADS MDD T-scores and NAcc activation to happy and to fear entered as independent variables, controlling for age group and ELS group. As seen in Figure 5, this analysis showed that low NAcc activation while viewing happy faces predicted higher depression scores (beta= -.16, p<.05), and NAcc response to fearful face was not associated with depression (beta=.02, ns). When only the ELS group was examined, the association between NAcc response to happy faces and depression scores remained (beta = -.30, p<.05). Tests of mediation in the adolescents did not show a significant mediating effect of NAcc function on the association between ELS and depressive symptoms (beta weight of NAcc indirect effect = -.12, ns). Additionally, linear regression analyses on the dependent measures of PANAS positive and negative affect z-scores and the independent variables of NAcc activation to happy faces > BL and fear faces >BL did not reveal a significant association between the variables (p>.05).
Figure 5.
Negative association between depression scores and NAcc activation. Lower NAcc activation while viewing happy faces was inversely correlated with parent report of depression scores, controlling for age and ELS group. ELS = early life stress; MDD=major depressive disorder.
3.2.3.1. Whole-brain analysis
To confirm the association between depression scores and the anatomically defined NAcc, we also performed a voxel-wise whole-brain analysis regressing BOLD signal in response to happy faces with depression scores, covarying for age and ELS group. This analysis confirmed the inverse association between NAcc activity and depression scores, as shown in Table 2. Other regions that showed significant inverse associations with depression scores are listed in Table 2.
Table 2.
Results from whole-brain voxelwise regression of happy>BL with depression scores.+
| Anatomical Region | BA | x | y | z | voxel # | |
|---|---|---|---|---|---|---|
| Regions with inverse associations with depression | ||||||
| Superior Parietal Lobule | 7 | L | -31 | -61 | 56 | 65 |
| NAcc/Putamen/Amygdala | R | 23 | -61 | -10 | 51 | |
| Parahippocampal Gyrus | 28 | R | 20 | -13 | -10 | 28 |
Controlling for age and group, p<.001, corrected.
3.2.4 Institutional care related variables
We examined the associations between variables related to institutional care and RCADS-P depression T-scores as well as NAcc activation. Regression analyses controlling for current age showed that there were no associations between RCADS-P depression T-scores and age placed in institutional care (beta=.06, ns), age of adoption (beta=.18, ns), or time with family (beta=-.28, ns). Similarly, regression analyses controlling for age showed that there were no associations between NAcc response to happy faces > BL and age placed in institutional care (beta=.04, ns), age of adoption (beta=-.12, ns), or time with family (beta=.18, ns).
4. Discussion
4.1
The present study examined the association between early-life stress, depression, and underlying NAcc reactivity. Findings revealed that parent-reported depression was significantly greater in adolescents with a history of ELS than in children. Parent-report was corroborated by child and adolescent self-report measures of recent mood showing that adolescents, and not children, with a history of ELS experienced more negative affect in the absence of positive affect than any other group. Although ELS was associated with depression and anxiety, only depression scores showed age-related change, whereas anxiety was elevated in both age groups. These differential developmental patterns for anxiety and depression have been characterized previously (Angold and Rutter, 1992; Cole et al., 1998), with depression being infrequent in childhood and emerging in early adolescence. We examined NAcc reactivity as a potential correlate of the greater depression seen in adolescents.
4.2
Our findings support the hypothesis that ELS would be associated with hypoactivity of the NAcc during adolescence. Unlike the normative increase in NAcc activity during the transition from childhood to adolescence (Galvan et al., 2006; Ernst et al., 2005; van Leijenhorst et al., 2009; Geier et al., 2009) observed in the typically-raised comparison group, NAcc reactivity did not increase with age in the ELS group, and as a consequence, was hypoactive in the ELS adolescents. That is, there was no observable effect of ELS on NAcc reactivity during childhood, as both groups of children demonstrated relatively low levels of NAcc reactivity. It is possible that individual differences in NAcc activity that manifest in adolescence are the reason why individual differences in depression-related behaviors are typically observed during adolescence, and not in childhood. In support of this hypothesis, the effect of ELS on NAcc reactivity in the current study was observed in the adolescent group, the same group with highest depression symptoms. Importantly, lower NAcc activity was associated with higher levels of depression, consistent with the notion that hypoactivity in the NAcc and depressive behaviors are correlated. Our inability to find significant mediation was most likely predicated on the fact that group differences were only evident in the adolescents (not the children), and the adolescent sample alone may have been underpowered to test for indirect effects. Though we did not find an association between self-reported affect and NAcc reactivity, the limited response range of our child-appropriate positive affect measure most likely precluded our ability to find significant associations with neural activity. The current study did not observe associations between individual differences in adoption timing variables with depression behaviors or with NAcc reactivity. Taken together, these findings replicate earlier reports showing that depression associated with ELS is associated with NAcc hypoactivity. Moreover, the data suggest that depression typically emerges after childhood because it is after this point that group differences emerge in NAcc activity, with the ELS group failing to show the developmentally-typical rise in NAcc reactivity shown by the comparison group. Hypoactivity of the NAcc has been associated with the anhedonic aspects of depression (Wacker et al., 2009); therefore the NAcc hypoactivity observed in the current study may have contributed to the depressive behaviors in adolescents with ELS by increasing anhedonic states, a notion supported by the self-reported mood ratings in ELS adolescents (low positive affect, high negative affect). Our whole-brain analysis confirmed the inverse association between NAcc reactivity and depression. This analysis also showed associations between depression scores and superior parietal lobule and parahippocampal gyrus activity, two regions that have shown depression related phenotypes in previous studies (Fallucca et al., 2011; Milne et al., 2012).
4.3
ELS research in previously institutionalized individuals is unique in that this population is exposed to a temporally-discrete and severe stressor. Institutional care, which is sparse, unstable, and regimented (Smyke et al., 2007), is unfortunately a naturally occurring example of early-life stress in humans. Several research groups have observed that in institutional care, health care, nutrition, and safety needs are often met; however, necessary maternal input is lacking (Groark and McCall, 2011; Groark et al., 2011; Smyke et al., 2007; The St. Petersburg-USA Orphanage Research Team, 2008; Tirella et al., 2008; Vorria et al., 2003; Zych, 2006). Maternal deprivation is a potent stressor for the human infant (as reviewed in Tottenham, 2012), and not surprisingly, emotional health is at significant risk in previously institutionalized children (reviewed in Gunnar et al., 2000). Diagnostically, previously institutionalized children demonstrate an increased rate of internalizing and externalizing emotion regulation disorders such as anxiety, depression, and attention deficit hyperactivity disorder (Zeanah et al., 2009). Importantly, intervention studies suggest that many of the mental health effects of institutionalization are likely to be related to institutionalization itself rather than preexisting genetic or prenatal conditions of the child (Bos et al., 2011; Nelson et al., 2007; The St. Petersburg-USA Orphanage Research Team, 2008). Unlike most other studied populations with a history of ELS, previously institutionalized infants are removed from stress exposure through an adoption process into stable caregiving environments, thereby temporally isolating the duration of the stress exposure to the infant period. Despite this beneficial and dramatic “intervention” through international adoption, socio-emotional difficulties may remain or even exacerbate into adolescence (Colvert et al., 2008; Verhulst et al., 1990) suggesting that there may be long-term changes in neural systems associated with emotional processes following orphanage care.
4.4
There are limitations to the current study. First, due to the nature of international adoption, we do not have access to individual prenatal/developmental histories for previously-institutionalized youths. This is a common issue for investigators studying this population. However, randomized control intervention work suggests that institutionalization itself may be the most significant factor in children's developmental histories (Zeanah et al., 2009). However, an experimental benefit to studying this population is knowledge of a discrete period of deprivation. A second concern is the task used to assess NAcc activity. In the current study, we employed an emotional face processing paradigm, requiring only passive viewing of emotional faces interspersed with target neutral faces to maintain attention. Thus, a limitation of our design is that we did not employ a reward learning paradigm, which might be better suited for examination of the NAcc. However, in both healthy youths (Somerville et al., 2011) and those at risk for depression (Monk et al., 2008), emotional faces like happy have been effective in recruiting NAcc activity and effective in separating out diagnostic groups. Low ventral striatum reactivity in response to happy faces has been associated with clinical depression (Epstein et al., 2006). Similarly, low ventral striatum reactivity to happy faces has shown diagnostic specificity to depression, as another fMRI study showed that depression (not anxiety) was inversely correlated with NAcc response to happy faces (Keedwell et al., 2005). Importantly, our results replicate those of Mehta et al. (2009) who used a reward-learning task (i.e., a monetary incentive delay task) and showed NAcc hypoactivity in previously institutionalized adolescents. Lastly, while the present study did not find significant relationships between pubertal status and depression or NAcc activity, our sample was rather underpowered for these analyses. Due to the inherent relationship between age and puberty, future studies comparing larger samples of adolescents of the same age but different pubertal status would be useful for identifying specific effects of puberty and age on depression and NAcc functionality.
5. Conclusions
The results from the current study have important implications for understanding how early-life stress can influence the emergence of depression during adolescence. Consistent with previous research associating stress-induced dysfunction in the ventral striatum, in particular the NAcc, with depressive behaviors, we observed that NAcc function is hypoactive during adolescence following ELS. These findings highlight the importance of identifying individuals at risk for depression during childhood, as this may be a potential critical period for depression-targeted intervention.
Highlights.
Depressive behaviors higher in adolescents than children following early-life stress
Nucleus accumbens developmental trajectory following early life stress is aberrant
Thus, nucleus accumbens is hyporeactive in adolescence following early-life stress
Nucleus accumbens hyporeactivity is associated with depressive behaviors
Acknowledgments
This work was supported by NIMH R01MH091864 (NT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The two-way interaction of ELSGroup×Age for RCADS-P remained significant when age was examined in years with linear regression (p<.01). However, given the clinical findings that show a sharp increase in depression symptoms after age 10-years-old, we examined whether there was any statistical motivation in the current study to examine children versus adolescents. Examination of the regression lines showed that the predicted RCADS score for an ELS group member first fell outside of the 95% confidence interval of the comparison group at age 10 years old, indicating that the atypical trajectory for depression behaviors in the ELS group significantly deviated from the typical trajectory after this age. Therefore, subsequent analyses in this study proceeded to examine children versus adolescents.
To examine the possible impact of mental health status and psychotropic medication use on NAcc activation, we re-performed the univariate ANOVA on the beta weights extracted from NAcc covarying for the presence of a score in the clinical range (T score >70 for internalizing or externalizing problems on the CBCL or RCADS-P) and psychotropic medication use. There were no effects associated with mental health history or medication use (all p>.14), and the observed association between ELS and NAcc activity remained when mental health status and medication use was included as factors (p<.025).
Although the analyses throughout the study examined children versus adolescents, we also performed this analysis with age measured continuously in years. The ELSGroup×Age interaction for NAcc reactivity remained significant when age was examined continuously in years with linear regression (p<.01). However, piecewise regression analyses of mean NAcc activity indicated that the ELS group demonstrated two distinct rates of change of NAcc activation depending on age. For children under age 10-years-old, the rate of change was not significantly different from the comparison group's rate of change. However, for those 10-years-old and up, the groups differed in rates of change, with the comparison group showing increased NAcc activity with increasing age and the ELS group demonstrating no significant increase with age.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. University of Vermont, Department of Psychiatry; Burlington: 2001. [Google Scholar]
- Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex. doi: 10.1093/cercor/bhr369. in press. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256(3):174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neuroscience. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Angold A, Rutter M. The effects of age and pubertal status on depression in a large clinical sample. Development and Psychopathology. 1992;4:5–28. [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience and Biobehavioral Reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox N, Drury SS, McLaughlin K, Nelson C. Psychiatric outcomes in young children with a history of institutionalization. Harvard Review of Psychiatry. 2011;19(1):15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Petersen AC. Studying the emergence of depression and depressive symptoms during adolescence. Journal of Youth and Adolescence. 1991;20(2):115–119. doi: 10.1007/BF01537603. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM–IV anxiety and depression in children: A revised child anxiety and depression scale. Behaviour Research and Therapy. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt C, Gray J. Psychometric properties of the revised child anxiety and depression scale in a clinical sample. Behaviour Research and Therapy. 2000;43:309–322. doi: 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Cole D, Peeke LG, Martin JM, Truglio R, Seroczynski D. A longitudinal look at the relation between depression and anxiety in children and adolescents. Journal of Consulting and Clinical Psychology. 1998;66(3):451–60. doi: 10.1037//0022-006x.66.3.451. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Development and Psychopathology. 2008;20(2):547–67. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM. Development and natural history of mood disorders. Biological Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-ruth K, Pizzagalli DA. Childhood adversity is associated with left basal in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163(10):1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68(2):118–24. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Fallucca E, MacMaster FP, et al. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Arch Gen Psychiatry. 2011;68(5):527–33. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Effect of isolation- rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. Journal of Neurochemistry. 1998;70:384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T. a, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groark CJ, Call RBMC, Fish L. Characteristics of environments, caregivers , and children in three Central American orphanages. Infant Mental Health Journal. 2011;32(2):232–250. doi: 10.1002/imhj.20292. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Development and Psychopathology. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, et al. Isolation rearing in rats: Pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacology, Biochemistry and Behavior. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12(2):162–71. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens DA in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Adoption and the effect on children's development. Early Human Development. 2002;68(1):39–54. doi: 10.1016/s0378-3782(02)00017-8. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: Study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacology, Biochemistry and Behavior. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Keedwell P, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychological Medicine. 1993;23(3):679–690. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Mateo Y, Parker T, Marsden C. Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology (Berl.) 2000;152:312–320. doi: 10.1007/s002130000534. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Jr., Rudolph KD, Potter KI, Lambert S, et al. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews. 2010;34(6):867–76. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Karolinska Directed Emotional Faces. Psychology Section, Department of Clinical Neuroscience, Karolinska Institute; Stockholm: 1998. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maniglio R. The impact of child sexual abuse on health: A systematic review of reviews. Clinical Psychology Review. 2009;29(7):647–57. doi: 10.1016/j.cpr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- McEwen B. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- Mehta M, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(8):943–51. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Milne AM, MacQueen GM, et al. Abnormal hippocampal activation in patients with extensive history of major depression: an fMRI study. Journal of Psychiatry and Neuroscience. 2012;37(1):28–36. doi: 10.1503/jpn.110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at Risk for Major Depression. American Journal of Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Nakamura BJ, Ebesutani C, Bernstein A, Chorpita BF. A psychometric analysis of the Child Behavior Checklist DSM oriented scales. Journal of Psychopathology and Behavioral Assessment. 2009;31(3):178–189. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C, Zeanah CH, Fox N. a, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318(5858):1937–40. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological Psychiatry. 2012;72(2):157–63. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews. Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. American Psychologist. 1993;48(2):155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- Pirkola S, Isometsä E, Aro H, Kestilä L, Hämäläinen J, Veijola J, Kiviruusu O, et al. Childhood adversities as risk factors for adult mental disorders: Results from the Health 2000 study. Social Psychiatry and Psychiatric Epidemiology. 2005;40(10):769–77. doi: 10.1007/s00127-005-0950-x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with major depressive disorder. American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119:233–240. doi: 10.1016/s0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. Journal of Neuroscience. 2012;32(22):7758–65. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox N, Marshall PJ, Nelson C, Zeanah CH. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48(2):210–8. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–34. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The St. Petersburg-USA Orphanage Research Team The effects of early social-emotional and relationship experience on the development of young orphanage children. Monographs of the Society for Research in Child Development. 2008;73(3):1–262. 294–265. doi: 10.1111/j.1540-5834.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : an approach to cerebral imaging. Thieme; 1988. [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. Journal of Clinical Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirella LG, Chan W, Cermak S, Litvinova, Salas KC, Miller LC. Time use in Russian baby homes. Child: Care, Health and Development. 2008;34(1):77–86. doi: 10.1111/j.1365-2214.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Risk and developmental heterogeneity in previously institutionalized children. Journal of Adolescent Health. 2012;51(2 Suppl):S29–33. doi: 10.1016/j.jadohealth.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SRB, Crone E. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–9. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Althaus M, Versluis-DenBieman H. Problem behavior in international adoptees: I. An epidemiological study. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(1):94–103. doi: 10.1097/00004583-199001000-00015. [DOI] [PubMed] [Google Scholar]
- Vorria P, Papaligoura Z, Dunn J, van IJzendoorn MH, Steele H, Kontopoulou A, Sarafidou Y. Early experiences and attachment relationships of Greek infants raised in residential group care. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44(8):1208–20. doi: 10.1111/1469-7610.00202. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 2010;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson C. a, Fox N. a, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166(7):777–85. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zych M. Caregiver's perceptions of the ability of orphanages to meet child development needs: A Polish case study. Wilfrid Laurier University; 2006. [Google Scholar]