Abstract
Women have coronary heart disease (CHD) later than men. This review describes studies of CHD risk factors or outcomes based on studies of premenopausal women followed through the menopause transition, and prospective cohort studies of younger or older women with CHD risk markers or disease outcomes in the context of their menopause history. Major early reports from both types of studies are included in order to put more recent work in context. Most attention has been paid to the Healthy Women Study (HWS), Study of Women’s Health across the Nation (SWAN), the Nurses’ Health Study (NHS), and the Rancho Bernardo Study (RBS) because they continue to produce recent publications designed to distinguish the effect of age from the effect of menopause. Understanding these differences has important implications for women’s cardiovascular health, but remains incomplete. Transition studies have relatively short (<10 years) follow up and exclude women with surgical menopause. Cohort studies suggest that women with oophorectomy are at greater risk for CHD than intact women, pointing to a greater risk from testosterone deficiency than from estradiol levels.
Introduction
How the type or timing of the menopause is related to atherosclerosis and coronary artery disease or, more generally, cardiovascular disease (CVD) remains unclear. This review considers two kinds of studies in women who are not patients, i.e., do not have CVD at baseline: 1) studies of repeated measures of change in CVD risk factors or CVD risk markers during the menopause transition; and 2) studies of CVD risk factors and outcomes in community-based cohorts that include information on the menopause. Until recently, transition studies were too small or too short for clinical outcomes, and most were cross-sectional. The cohort studies depend on age 50 as menopause, or self-reported year of last menstrual period or date of hysterectomy with or without oophorectomy; the latter rely on participants’ ability to remember remote dates and details. Results from both types of studies are complementary.
This review includes recent original and review papers with an emphasis on longitudinal work. This review does not include studies of pre- or post-menopausal hormone therapy, which pose other problems, including selection bias for who was prescribed hormones and the pharmacologic (not physiologic) doses used for treatment. Given word limitations, this review does not summarize other characteristics and consequences of the menopause. An excellent review of the menopause by Nelson was published in the Lancet in 2008[1].
The Menopause transition
The first large study in the United States to describe the menopause transition with a focus on CVD was the Healthy Women Study; this study measured changes in CVD risk factors in 541 healthy women ages 40–52 years at baseline when they reported menstrual bleeding within the past three months[2]. Women were then asked to report their menstrual bleeding and menopause status on monthly postcards. After approximately 2 ½ years, women who had a natural (nonsurgical) menopause, and who did not choose to use postmenopausal hormone therapy, had significant reductions in HDL cholesterol and increases in LDL cholesterol compared to women who were still premenopausal, changes expected to accelerate atherosclerosis. In this study, natural menopause did not affect weight, blood pressure, plasma glucose, or insulin levels, and risk factor changes did not reflect the number of calories consumed in the diet or expended in physical activity[2]. It was later shown that premenopausal measures of lipids predicted subclinical CVD based on coronary and aortic calcium [3, 4].
The Healthy Women Study was the model for the Study of Women Across the Nation (SWAN); an entire monograph on the SWAN results was published in 2011[5]; it included a highly recommended summary of many menopause studies and cardiovascular risk by Chae and Derby[6]. SWAN is the largest U.S. study to follow perimenopausal community-dwelling women through the menopause. At entry there were 3201 still-menstruating women age 42–52 years. Women completed annual interviews on their menses, and the frequency and severity of hot flushes and night sweats in the past two weeks; at the same time height and weight were measured, and blood for lipids and lipoproteins, serum estradiol, and FSH were obtained. A 2012 review reported 8-year lipid changes in the context of menopause status and symptoms[7]. In this SWAN paper, women reporting hot flushes 6 or more days a week had significantly higher LDL and HDL cholesterol and triglyceride levels. These associations largely persisted after adjusting for estradiol or FSH. Associations were strongest for lean women.
In a major SWAN analysis based on 10 years of follow up, only total and LDL cholesterol and apolipoprotein B changed within one year of the last menstrual period independent of age, ethnicity, or baseline weight; other common CVD risk factor changes were associated with age, not menopause [8]. SWAN was the first to present detailed changes in lipids anchored specifically to the timing of the final menstrual period.
In a SWAN ancillary study comparing subclinical atherosclerosis in premenopausal/early perimenopausal (n = 316) with late perimenopausal/postmenopausal (n = 224) women not using hormone therapy, aortic calcification, coronary artery calcium (CAC), and intimal medial thickness (IMT) were measured; results suggest the protective effects of HDL-cholesterol may be diminished during the menopause transition[9]. Analyses in this small subset of the SWAN study also showed a shift during the later menopause transition to higher HDL particle number and smaller HDL particle size, thought to be atherogenic changes[9].
In contrast to the lipid and lipoprotein changes, the ten-year follow up of the SWAN study showed that increases in blood pressure were related to chronological age and not related to the timing of the final menstrual period [8]. The risk of developing the metabolic syndrome (MetS) was unrelated to changes in estradiol or total testosterone, but increases in bio-available testosterone or SHBG were associated with increased risk of MetS[10]. In a nice overview, Chae and Derby[6] concluded that the 10-year SWAN study results did not support a strong influence of the menopause transition on glucose or insulin levels or on the risk of developing diabetes.
It has not been clear whether changes in sex hormones during or after the menopause precede or follow changes in body weight during the menopause transition. A SWAN paper based on 9 years of follow up of premenopausal women (baseline and up to three other visits 3, 6 and 9 years later) [11] that included 1528 women (mean age 46, who had not had a hysterectomy) reported that the association between waist girth and estradiol appeared to be bidirectional, suggesting that adiposity may lead to changes in estradiol levels and that estradiol levels may lead to changes in adiposity. In contrast current waist circumference predicted future SHBG, testosterone, and FSH, but not the reverse.
This SWAN paper provides the strongest evidence to date suggesting that waist circumference precedes significantly increased total testosterone and SHBG and low FSH, but not vice versa. Estradiol was completely different: estradiol and waist circumference were negatively associated in the early menopausal stages, and positively associated in later transitions stages. For each standard deviation higher current waist circumference future estradiol levels were lower by 0.15 SD in pre- and early-stage menopause transition, and higher by 0.38 SD in late peri- and post-menopause transitions. The authors conclude that the predominant temporal sequence is that weight gain and increasing waist girth lead to changes in sex steroids, rather than the reverse. Although they conclude that weight gain is primarily due to aging rather than to menopause-related estrogen-deficiency, the effect sizes in women were larger for adiposity’s effects on estradiol than the reverse, and were larger for waist circumference than for body weight, and were not explained by SHBG.
SWAN was not able to separate abdominal visceral from subcutaneous abdominal fat, or deal with the frequent changes in premenopausal estradiol levels (hour by hour and day by day), or the use of early estrogen immunoassays with less ability to detect low premenopausal estrogen levels than mass spectroscopy. Nevertheless, SWAN provides the strongest evidence to date suggesting that waist circumference predicts the magnitude of future estradiol levels to a much greater extent than the reverse.
Sleep disturbances
Perimenopausal women have more sleep disturbances than premenopausal women, but these disturbances are not always related to vasomotor symptoms. In SWAN, sleep disturbance was twice as common in women with vasomotor symptoms as in women without, and was associated with low estradiol levels [12]. These results differ from studies of symptomatic versus asymptomatic women as reviewed by Freedman [13], who found no difference in sleep disturbances in postmenopausal women with or without hot flashes and no evidence that hot flashes were related to estrogen deficiency or to objectively measured changes of body temperature. Freedman continues to make major contributions to the understanding of the etiology and treatment of hot flashes in relation to sleep, and to emphasize the separation between symptoms and objectively measured hot flashes.
Depression
Depression or depressed mood is a poorly understood but accepted risk factor for cardiovascular disease. The association of depressed mood with menopause was addressed using the 10th annual SWAN visit when 90% of participants had reached a natural menopause [14]. All women experienced a decrease in depressive and anxiety symptoms in the years after the menopause. There were relatively few women who had hysterectomy with or without oophorectomy, and they did not have more negative mood symptoms after surgery. (Different results were observed in African American women, but their numbers were small and differences may have been due to chance.) Because all SWAN women had to be in the menopause transition to participate, this could explain the inability to show an association of depressed mood with the menopause transition.
Cohort studies
One of the longest cohort studies of age at menopause and CVD is the Iowa Established Populations for the Epidemiological Study of the Elderly (Iowa EPESE), which included 1684 women from rural Iowa who were age ≥65 at baseline and were followed for up to 24 years[15]. Women whose reported menopause age was late (≥55 years) had an increased risk of all-cause and CVD mortality compared to women with an earlier menopause. The type of menopause (surgical 26% or natural 24%) was unrelated to these outcomes. Adjusting for confounding variables did not change the results. These results based on an exceptionally long follow-up challenge the conventional wisdom that women’s CVD risk is increased by the number of years of postmenopausal estrogen deficiency. One plausible explanation (suggested by the authors) is that women who survive past age 65 have positive health characteristics not shared by those who died before age 65. Women less than age 65 were not included in the IOWA EPESE cohort.
The Nurses’ Health Study (NHS), the largest U.S. cohort study of younger women, included 116,700 premenopausal US women age 30 to 55 years who had no known heart disease at baseline[16]. More than 95% were followed by mail between 1976 and 1982 (before the major promotion of postmenopausal estrogen in the U.S.) In this cohort, women who had a natural (nonsurgical) menopause and who had never taken postmenopausal estrogen had no increased risk of coronary heart disease compared to premenopausal women (adjusted rate ratio 1.12; 95% CI 0.8–1.8). Women who had a natural menopause and took estrogen also showed no difference in risk. In contrast, women who reported a bilateral oophorectomy had a 2-fold increased risk if they did not use post-menopausal estrogen.
These results were confirmed in a 2006 international meta-analysis of 18 published studies of age at menopause in relation to cardiovascular disease[17] (6 studies of menopause status, 9 of menopause age, and 3 of both age and status were reviewed with consideration of multiple covariates). The pooled relative risk for postmenopausal vs. premenopausal women was not significant (0.96, 95% CI 0.77–1.21) after adjusting for age and smoking, but was significant (2.62, 95% CI 2.05–3.35) in women who had had a bilateral oophorectomy. The authors concluded there was no convincing relationship between postmenopausal status in intact women and cardiovascular disease. Their analyses dramatically illustrate the difficulty of pooling data with so many different methods and definitions.
Perhaps remembered age at menopause is inaccurate by age 65. Against this bias is the observation that remembered age at menopause matched the separately reported time when estrogen was prescribed; further, the hazard ratio and percent dead by end of follow up were similar for those who did or did not know their age at menopause, suggesting survival bias did not explain the results.
A 2006 study by Kok et al.[18], based on data from the Framingham cohort study established in1948, included 695 women who had reached a natural menopause after at least two rounds of clinical evaluations that were conducted every two years for an average of 4.9 evaluations. The authors reported that a higher premenopausal serum total cholesterol level was statistically significantly associated with earlier menopause age (as were relative weight and blood pressure). Also, a decrease in relative weight was associated with a significant earlier age at menopause (each 1% higher premenopausal Framingham risk score was associated with a decrease of 1.8 years in age at menopause (95% CI −2.72 to 0.92), supporting the view that heart disease risk determines age at menopause and not vice versa. This is a novel explanation for inconsistent findings on rate of CVD in relation to age at menopause. Bittner’s thoughtful editorial on this methodologically challenging study [19] noted that it is critical to separate the influence of chronologic aging from that of menopause.
Atrial fibrillation
Atrial fibrillation (AF) increases with age and is common enough to eliminate the female CVD survival advantage, but is not related to the menopause, according to a unique recent Framingham Heart Study publication[20]; the authors examined the association of incident AF with menopausal age, studying women without prevalent AF at baseline. In a multivariate model adjusted for AF risk factors, incident AF did not differ between women with early menopausal age (<45 yrs) vs. late menopausal age (> 53 yrs); the Framingham Heart Study does not identify menopausal age as significantly increasing AF risk.
Sex hormones
The implications of increased CVD risk with long postmenopausal life without estrogen are large given the rapid growth and improved survival of the aging population. In the French Three-City prospective cohort study, high plasma levels of endogenous estradiol emerged as a new predictor of ischemic arterial disease in older postmenopausal women[21].
There is increasing interest in the importance of testosterone instead of estrogen as an explanation for the harmful effect of oophorectomy on cardiovascular risk. Testosterone is made in the stroma of the ovaries, and is maintained after all follicles (the main premenopausal source of estradiol) are lost. A study of individual data from more than 6000 postmenopausal women [22] from 13 prospective studies showed that all hormones were lower in older intact women. Androgens were lower in women who had had a bilateral oophorectomy than in naturally menopausal women. Smokers of 15+ cigarettes per day (known to have an earlier menopause) had higher levels of all hormones than nonsmokers, with larger levels for testosterone. Drinkers of 20+ g of alcohol per day also had higher levels of all hormones. Cardiovascular outcomes were not reported.
A recent SWAN analysis [23] showed that low SHBG and high free androgen index were strongly and consistently associated with elevated CV risk factors (higher insulin, glucose, hemostatic and inflammatory factors, and adverse lipids) independent of BMI. Low levels of estradiol were associated with elevated CV risk factors to much lesser degree.
These results are in contrast with hormone associations in older postmenopausal women in the Rancho Bernardo Study [24]. There were 639 postmenopausal women (age 50 to 91 years, median age 74) followed for a median of 12.3 years, who had 134 incident CHD events (45 nonfatal, 79 fatal, 10 coronary revascularizations). Age-adjusted CHD risks for total testosterone were similar for the four highest quintiles of total testosterone, and significantly higher for women in the lowest testosterone quintile (≤80 pg/ml) (Figure 1). Additional adjustment for estradiol, ovarian status, lifestyle, adiposity, or CHD risk factors had minimal influence on these results. Bioavailable testosterone showed a U-shaped association, such that age-adjusted CHD risk for the lowest and highest bioavailable T quintiles relative to the third were 1.79 (95% CI 1.03–3.16) and 1.96 (95% CI, 1.13–3.41), respectively. These results may be direct or indirect. In experimental studies, androgens suppress proinflammatory cytokine activity, inhibit apoptosis, and enhance vascular smooth muscle proliferation, important factors inhibiting atheroma formation and maintaining plaque integrity, as reviewed by Malkin and colleagues[25].
Figure 1.
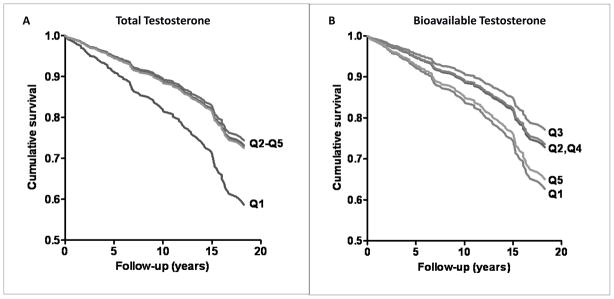
A. Total testosterone and 20-year incident CHD in women (adjusted for age, BMI, WHR, smoking, exercise, alcohol). B. Bioavailable testosterone and 20-year incident CHD in women (adjusted for age, BMI, WHR, smoking, exercise, alcohol) (Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab 2010;95:740–7, Copyright 2010, The Endocrine Society).
Conclusion
Transition studies have relatively short (<10 years) follow up and exclude women with surgical menopause. Cohort studies suggest that women with oophorectomy are at greater risk for CHD than intact women, pointing to a greater risk from testosterone deficiency than from estradiol levels.
This brief review is about women only. This writer strongly believes that a better understanding can be obtained by studying sex and gender differences in CHD. A recent publication [26] used U.S. and U.K. national heart disease mortality data for three birth cohorts (1916–25, 1926–35, and 1936–45). In all birth cohorts linear heart disease mortality rates peaked in men around age 45, and age-related increases were much slower thereafter. In women there was no linear accelerated increase in heart disease mortality rates at age 50, the usual age at menopause. In both sexes proportional increases fit the data better than absolute increases. The authors concluded that deceleration of the increase in male heart disease mortality in midlife explains sex differences better than postmenopausal estrogen deficiency in women.
Highlights.
Only total and LDL cholesterol and apolipoprotein B changed within one year of the last menstrual period.
Increasing importance of testosterone instead of estrogen to explain harmful effect of oophorectomy on cardiovascular risk
In adjusted analyses incident AF did not differ between early menopausal (<45 y) vs. late menopausal (>53 y) age.
Methodologically challenging but critical to separate out the influences of chronologic aging and menopause.
Acknowledgments
Dr. Barrett-Connor has received support for the Rancho Bernardo Study from NIH [National Institutes of Health/National Institute on Aging grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801].
Footnotes
This financial support does not represent a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Nelson HD. Menopause. Lancet. 2008;371(9614):760–70. doi: 10.1016/S0140-6736(08)60346-3. This review was first prepared by Nelson in 2005 as an Evidence Report/Technology Assessment for the Agency for Health Care and Quality. Her 2008 review includes 1996 to 2006 publications, a Cochrane review of menopause symptoms, including depression, mood, and quality of life, sleep disorder, urination disorder, vasomotor symptoms, somatic symptoms, vaginal disorder, and medications for these symptoms. She describes and references 12 international studies of the menopause transition, notes menopause status is more closely associated with vasomotor than psychological or physical symptoms, and argues against a universal menopausal experience. She reviews her Stages of Reproductive Aging Workshop (STRAW), which described seven stages of reproductive aging, from regular menstrual cycles, transition stages with irregular cycles and high FSH levels, and postmenopausal stages beginning with the final menstrual period, and lasting until the end of life. Nelson also discusses the TREMIN Research Program on Women’s Health with 12 different stages and substantially more individual variation, including skipping stages and moving back and forth between stages. [DOI] [PubMed] [Google Scholar]
- 2.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20-year follow-up in the healthy women study. Prev Med. 2007;45(4):302–8. doi: 10.1016/j.ypmed.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the healthy women study. Arterioscler Thromb Vasc Biol. 1999;19(9):2189–98. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 5.Santoro N. The Study of Women’s Health Across the Nation (SWAN) Obstet Gynecol Clin North Am. 2011;38(3):xvii–xix. doi: 10.1016/j.ogc.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 6**.Chae CU, Derby CA. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am. 2011;38(3):477–88. doi: 10.1016/j.ogc.2011.05.005. Chae and Derby have written an elegant review of the menopause transition which includes details and references for SWAN, the Healthy Women Study, The Melbourne Midlife Project, the Fels Longitudinal Study, and the Taiwan Chin-Shan Community Cardiovascular Cohort. Because these study designs and definitions are different, pooling of studies is not feasible. They note the limitations of each study, the difficulties in separating the effects of hormone deficiency from those of age, and highlight the first 7 years of the SWAN follow up showing total and LDL cholesterol and triglycerides peaked during late perimenopause and early postmenopause, and remained high. HDL also peaked but these changes appeared to be transient. Their analysis may be the first to present changes in lipids anchored specifically to the last menstrual period. [DOI] [PubMed] [Google Scholar]
- 7.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119(4):753–61. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause. 2011;18(4):376–84. doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14):1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Wildman RP, Tepper PG, Crawford S, et al. Do Changes in Sex Steroid Hormones Precede or Follow Increases in Body Weight during the Menopause Transition? Results from The Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012;97(9):E1695–704. doi: 10.1210/jc.2012-1614. This SWAN paper provides the strongest evidence to date suggesting that waist circumference precedes significantly increased total testosterone and SHBG and low FSH, but not vice versa. Estradiol was completely different: estradiol and waist circumference were negatively associated in the early menopausal stages, and positively associated in later transition stages. For each standard deviation higher current waist circumference future estradiol levels were lower by 0.15 SD in pre- and early- stage menopause transition, and higher by 0.38 SD in late peri- and post- menopause transition. The authors conclude that the predominant temporal sequence is that weight gain and increasing waist girth lead to changes in sex steroids, rather than the reverse. Although they conclude that weight gain is primarily due to aging rather than to menopause-related estrogen-deficiency, the effect sizes in women were larger for adiposity’s effects on estradiol than the reverse, and were larger for waist circumference than for body weight, and were not explained by SHBG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–90. [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005;118(Suppl 12B):124–30. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Gibson CJ, Joffe H, Bromberger JT, et al. Mood symptoms after natural menopause and hysterectomy with and without bilateral oophorectomy among women in midlife. Obstet Gynecol. 2012;119(5):935–41. doi: 10.1097/AOG.0b013e31824f9c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tom SE, Cooper R, Wallace RB, Guralnik JM. Type and timing of menopause and later life mortality among women in the Iowa Established Populations for the Epidemiological Study of the Elderly (EPESE) cohort. J Womens Health (Larchmt) 2012;21(1):10–6. doi: 10.1089/jwh.2011.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 17.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 18.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976–83. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 19.Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J Am Coll Cardiol. 2009;54(25):2374–5. doi: 10.1016/j.jacc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Magnani JW, Moser CB, Murabito JM, et al. Age of natural menopause and atrial fibrillation: the Framingham Heart Study. Am Heart J. 2012;163(4):729–34. doi: 10.1016/j.ahj.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarabin-Carre V, Canonico M, Brailly-Tabard S, et al. High level of plasma estradiol as a new predictor of ischemic arterial disease in older postmenopausal women: the three-city cohort study. J Am Heart Assoc. 1(3):e001388. doi: 10.1161/JAHA.112.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Key TJ, Appleby PN, Reeves GK, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105(5):709–22. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111(10):1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 24**.Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95(2):740–7. doi: 10.1210/jc.2009-1693. This is the first population-based study to show that low levels of total testosterone and high levels of bioavailable testosterone are independently related to higher risk of incident CHD events over a 20-year follow up in community-dwelling postmenopausal women. Women in the lowest 20% of total testosterone for this population (≤80 pg/ml) had 62% elevated risk of a first-ever CHD event, whereas women in the highest 20% of bioavailable testosterone (≥63 pg/ml) had 96% elevated risk, compared with women without extreme testosterone levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373–80. doi: 10.1677/joe.0.1780373. [DOI] [PubMed] [Google Scholar]
- 26.Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ. 2011;343:d5170. doi: 10.1136/bmj.d5170. [DOI] [PMC free article] [PubMed] [Google Scholar]


