Abstract
Objective
We hypothesized that radiation induced thoracic toxicity (RITT) of the lung, esophagus and pericardium share a similar mechanism, and aimed to examine whether genetic variation of transforming growth factor-beta1 (TGFβ1), tissue plasminogen activator (tPA) and angiotensin converting enzyme (ACE), are associated with RITT in patients with non-small cell lung cancer (NSCLC).
Material and methods
Patients with stage I-III NSCLC were enrolled and received radiotherapy (RT). Blood samples were obtained pre-RT and at 4–5 weeks during RT and plasma TGF-β1 was measured using an enzyme-linked immunosorbent assay. The DNA samples extracted from blood pre-RT were analyzed for the following frequent genetic variations: TGFβ1 509C/T, tPA −7351 C/T, and ACE I/D. RITT score was defined as the sum of radiation induced toxicity grades in esophagus, lung and pericardium.
Results
76 NSCLC patients receiving definitive RT were enrolled. Patients with TGFß1 509CC had higher mean grade of esophagitis (1.4±0.2 vs. 0.8±0.2, p=0.019) and RITT score (2.6±0.3 vs. 1.6±0.3, p=0.009) than T allele carriers. Although no significant relationship was observed between RITT and the tPA or ACE variants individually, patients with any high risk alleles (tPA CC or ACE D or TGFß1 509CC) had significantly higher grade of developing combined RITT (p<0.001). Patients with TGFß1 509CC had greater increase of plasma TGF ß1 levels at 4-5 weeks during-RT than T allele carriers (CC 1.2±0.2 vs. T 0.7±0.1, p=0.047).
Conclusion
This exploratory study demonstrated that sensitivity of radiation toxicity may be determined by genomic factors associated with TGFβ1 and genes involved in TGFβ1 pathway.
Keywords: single nucleotide polymorphism, toxicity, radiotherapy, non-small cell lung cancer
INTRODUCTION
Radiation is the mainstay local treatment for patients with inoperable stage I-III non-small cell lung cancer (NSCLC), and an adequate dose is essential for treatment success as increased radiation dose has been associated with reduction in risk of death(1). However, the majority of patients with stage III NSCLC cannot receive an adequate dose for tumor control without exceeding the “safe” dose limits of the adjacent critical structures such as lung, esophagus and heart. There are no reliable biologic markers to predict the risk of radiation-induced toxicity. Radiation doses during our standard practice are uniformly limited by the sensitive patient’s tolerance. Despite this conservative approach, some patients develop severe toxicity, while the majority do not, suggesting that genetic makeup may play a critical role in an individual’s response to radiotherapy and toxicity development(2).
Previous studies on radiation toxicity have been largely limited to specific organ toxicity; however, the biological mechanism of radiation toxicity in various organs at risk is likely to be similar. For example, inflammation is seen in esophagitis, pneumonitis and pericarditis. Genetic variations that lead to functional differences in proteins that participate in inflammatory processes may contribute to individual susceptibility to radiation induced thoracic toxicity (RITT) including esophagitis, pneumonitis, pericarditis and pericardial effusion in patients with NSCLC.
Transforming growth factor-beta1 (TGF-β1) plays a crucial role in radiation induced inflammation(3). Tissue plasminogen activator (tPA) has been shown to participate in TGFβ1 activation, the regulation of inflammation and the modulation of radiation-induced toxicity(4-5). In addition, angiotensin converting enzyme (ACE) and its inhibitors may mitigate radiation-induced lung injury(6, 7), possibly through the TGF-β1 pathway.
We hypothesized that the genetic variation of TGFβ1, tPA and ACE are associated with accumulated overall RITT involving inflammation of lung, esophagus and pericardium. We specifically selected the single nucleotide polymorphisms (SNPs), TGFβ1 509C/T, and tPA-7351 C/T, and the insertion (I)/deletion (D) polymorphism of ACE based on four criteria: 1) A minor allele frequency of at least 20% (~36% for rs1800469 TGFβ1 509C/T, 28% for rs2020918 tPA-7351 C/T and ~46% for rs4646994 I/D polymorphism of ACE; 2) Functional location such as in the promoter un-translated region or coding region of the gene; 3) Reported association between the variant and its protein level; 4) Reported association with radiation injury induced inflammation or fibrosis.
MATERIALS AND METHODS
Study Population
The study population included the patients enrolled in Institutional Review Board approved prospective imaging and biomarker studies between 2003 and 2009 at our institutes. Written informed consent was obtained from each patient. Eligible subjects included patients with stages I-III NSCLC undergoing radiation alone or combined radiation and chemotherapy. Exclusion criteria included a life expectancy of less than 6 months, biopsy-proven supraclavicular lymphadenopathy, malignant pleural or pericardial effusion, or noncontiguous involvement of the parietal pleura. All radiation was given using 3-dimension conformal techniques, as previously described(8, 9). Radiation dose was prescribed in majority of cases to a rate of 15-17% of lung normal tissue complication in our treatment studies, limited by 15% of normal tissue complication probability (NTCP) in imaging studies per standard care. Patients were seen weekly during-RT, and then 1, 3, 6, 12 and 24 months after RT and assessed for RITT. The minimum follow-up duration was 12 months for surviving patients. The RITT score was defined as the sum of radiation induced esophagus, lung and pericardium toxicity grades. Radiation induced lung toxicity included pneumonitis and fibrosis, radiation induced esophageal toxicity included dysphagia and odynophagia and radiation induced pericardium toxicity included pericardial effusion and pericarditis. All toxicities were graded prospectively per Common Terminology Criteria for Adverse Events v3.0 (CTCAE3.0 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctca ev3.pdf)
Sample Preparation and Plasma TGF-β1 Measurement
Blood samples were collected with Vacutainer® Plus collection tubes containing anticoagulant (K2EDTA) then placed in ice immediately after collection, and centrifuged within 3 hours of collection at 3000×g for 30 min at 4°C. Plasma TGFβ1 levels were measured at baseline (within 2 weeks before RT start), and 4-5 weeks during-RT. Plasma TGFβ1 levels were measured using a TGFβ1 specific Enzyme Linked Immunosorbant Assay (ELISA) (Human TGFβ1 DuoSet kit, R&D Systems Inc., Minneapolis, MN).
Genotypes
The buffy coat was collected and stored at −80°C. The genomic analyses were performed on the DNA isolated from the buffy coat drawn from NSCLC patients within 2 weeks before RT start.
Genomic DNA was extracted from the buffy coat samples with a DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) and sequencing primers for the SNPs of TGFβ1 509C/T, and tPA-7351 C/T were designed using Pyrosequencing Primer Design Version1.0 software. PCR reactions were carried out in a 30 μl volume with 1.5mmol/l Mg2+, 10 pmol of forward primer, and 10 pmol of reverse primer according to the following specifications: initial denaturation time of 5 min at 95°C followed by 45 cycles of 95°C for 30 s, 43°C for 30 s, 72°C for 30 s, and a final extension of 10 min at 72°C. Genotyping of TGFβ1 509C/T, and tPA-7351 C/T was carried out with Pyrosequencing Technology(10). The I/D polymorphisms of ACE were examined by electrophoresis on 1.8% agarose gels stained with ethidium bromide after PCR. Specific assay conditions are available upon request.
Statistical Analysis
The relationship between the various SNP’s and toxicity was assessed in numerous ways. In the primary analyses, genotype was treated as a binary covariate (CC vs T allele carrier for TGFβ1 and tPA, and I vs D allele carriers for ACE). The Cochrane-Armitage trend test was used to assess whether patients with one genotype tended to have higher (or lower) toxicity grades than patients with other genotypes. Separate analyses were performed in which genotype was treated as categorical and ordinal. Other analyses were based on RITT and on definition of sensitive patients as those with any of 3 sensitive genotypes. However, given the exploratory nature of this work, no adjustments for multiplicity were made. All tests were two-sided and a P < 0.05 was considered statistically significant. Data are presented as mean+standard error of mean. The software package SAS (V9.2, Cary, North Carolina) was used for analysis.
RESULTS
Patient and Genotypes
From 2003 to 2009, 76 patients with stages I-III NSCLC requiring radiation-based therapy were enrolled in the study. All NSCLC patients received radiotherapy (a median dose 66 Gy) and 56 of them (74%) underwent platinum-based concurrent chemotherapy. Among 56 received chemotherapy, 41 (73%) received pacitaxol + carboplatin; 8 (14%) received cisplatin and etoposide; the remaining received other chemo regimens. No significant differences in mean toxicity scores were found in lung (0.7±0.1 vs. 0.5±0.3 vs. 0.6±0.3, p=0.884), esophagus (1.0±0.2 vs. 1.3±0.6 vs. 1.6±0.3, p=0.435), pericardium (0.4±0.1 vs. 0.1±0.1 vs. 0.9±0.1, p=0.059) and combined RITT ( 2.1±0.3 vs. 1.9±0.7 vs. 3.0±0.8, p=0.381) among patients with pacitaxol + carboplatin, cisplatin + etoposide and other chemo regimens.
Patient-, disease-, and treatment-related characteristics according to the genetic variations are shown in Table 1. There were no significant differences between the genomic groups in age, gender, performance status, history of cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), tumor histology, disease stage, receipt of chemotherapy vs. radiotherapy only, and radiation dose received (p>0.05 for all); the only difference noted was in the distribution of tPA-7351 C/T genotype among the different tobacco use groups (p=0.02).
Table 1.
Patient characteristics and genetic variations
| Numbers of patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| TGFβ1 509C/T | tPA-7351 C/T | ACE I/D | |||||||
|
|
|||||||||
| Characteristics | CC (42) |
CT (34) |
TT (0) |
CC (32) |
CT (38) |
TT (6) |
II (33) |
ID (31) |
DD (12) |
| Age, years | p =0.818 | p =0.176 | p =0.626 | ||||||
| 43.4-68.0 (n=38) | 20 | 18 | 0 | 13 | 23 | 2 | 15 | 16 | 7 |
| 68.0-86.5 (n=38) | 22 | 16 | 0 | 19 | 15 | 4 | 19 | 14 | 5 |
| Gender | p =0.546 | p =0.919 | p =0.114 | ||||||
| Male (n=61) | 34 | 27 | 0 | 25 | 31 | 5 | 28 | 26 | 7 |
| Female (n=15) | 8 | 7 | 0 | 7 | 7 | 1 | 5 | 5 | 5 |
| KPS | p =0.072 | p =0.649 | p =0.650 | ||||||
| ≤80 (n=36) | 16 | 20 | 0 | 17 | 16 | 3 | 14 | 17 | 5 |
| >80 (n=40) | 26 | 14 | 0 | 15 | 22 | 3 | 19 | 14 | 7 |
| Weight loss | p =0.929 | p =0.960 | p =0.077 | ||||||
| <5 pounds (n=60) | 33 | 27 | 0 | 25 | 30 | 5 | 30 | 22 | 8 |
| ≥5 pounds (n=16) | 9 | 7 | 0 | 7 | 8 | 1 | 3 | 9 | 4 |
| Smoker | p =0.617 | p =0.022 | p =0.217 | ||||||
| Yes (n=63) | 34 | 29 | 0 | 23 | 36 | 4 | 30 | 26 | 7 |
| No (n=13) | 8 | 5 | 0 | 9 | 2 | 2 | 3 | 7 | 3 |
| COPD | p =0.836 | p =0.615 | p =0.066 | ||||||
| Yes(n=39) | 22 | 17 | 0 | 17 | 20 | 2 | 21 | 15 | 3 |
| No(n=37) | 20 | 17 | 0 | 19 | 15 | 3 | 12 | 16 | 9 |
| CVD | p =0.161 | p =0.121 | p =0.369 | ||||||
| Yes(n=32) | 21 | 11 | 0 | 13 | 16 | 3 | 16 | 13 | 3 |
| No (n=44) | 21 | 23 | 0 | 19 | 22 | 3 | 17 | 18 | 9 |
| Histology | p =0.565 | p =0.656 | p =0.646 | ||||||
|
Adenocarcinoma
(n = 12) |
7 | 5 | 4 | 7 | 1 | 6 | 4 | 2 | |
|
Squamous
carcinoma (n = 22) |
14 | 8 | 0 | 12 | 8 | 2 | 12 | 7 | 3 |
|
Not otherwise
specified (n=42) |
21 | 21 | 0 | 16 | 23 | 3 | 15 | 20 | 7 |
| Clinical stage | p =0.936 | p =0.489 | p =0.584 | ||||||
| I,II (n = 22) | 12 | 10 | 0 | 9 | 10 | 3 | 10 | 10 | 2 |
| III (n = 54) | 30 | 24 | 0 | 23 | 28 | 3 | 23 | 21 | 10 |
| Chemotherapy | p =0.282 | p =0.225 | p =0.437 | ||||||
| Yes (n = 56) | 33 | 23 | 0 | 21 | 31 | 4 | 24 | 23 | 12 |
| No (n = 20) | 9 | 11 | 0 | 12 | 7 | 2 | 11 | 7 | 2 |
| RT dose | p =0.645 | p =0.657 | p =0.150 | ||||||
| ≤66Gy (n=38) | 20 | 18 | 0 | 18 | 20 | 2 | 19 | 16 | 3 |
| >66Gy (n=38) | 22 | 16 | 0 | 16 | 18 | 4 | 14 | 15 | 9 |
Abbreviations: KPS=Karnofsky performance score; COPD=Chronic obstructive pulmonary disease; CVD=Cardiovascular Disease; RT=radiotherapy
Genotype and Thoracic Toxicity
The distribution of various grades of toxicity is shown in Figure1. Patients with the TGFß1 CC genotype had higher grade of esophagitis than T allele carrier patients (CC 1.4±0.2 vs. T allele carriers 0.8±0.2, p=0.019). There was no significant difference in mean toxicity grade of lung (CC 0.8±0.2 vs. T allele carriers 0.5±0.1, p=0.179), and pericardium (CC 0.4±0.1 vs. T allele carriers 0.3±0.1, p=0.42), although those with the CC genotype had higher grade toxicities than T allele carriers. TGFß1 CC patients had significantly greater mean scores for all RITT than T allele carriers (CC 2.6±0.3 vs. T allele carriers 1.6±0.3, p=0.009) (Figure 2).
Figure 1. The incidence of radiation induced thoracic toxicity.
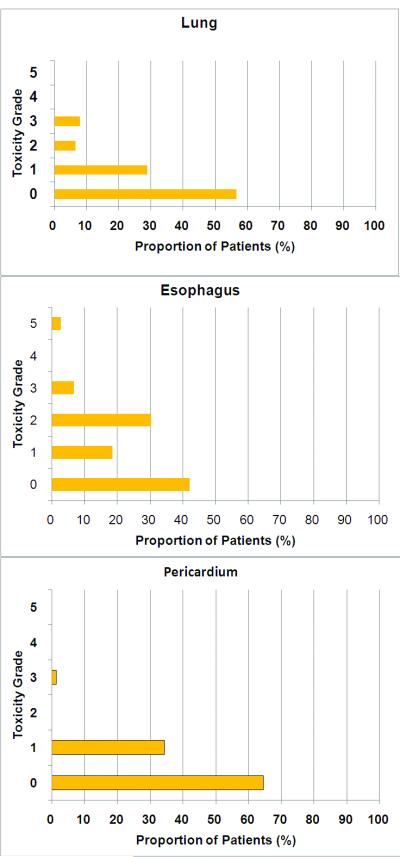
Toxicity grade was determined prospectively per CTCAE 3.0 standard.
Figure 2. Genotypes and radiation induced thoracic toxicity.
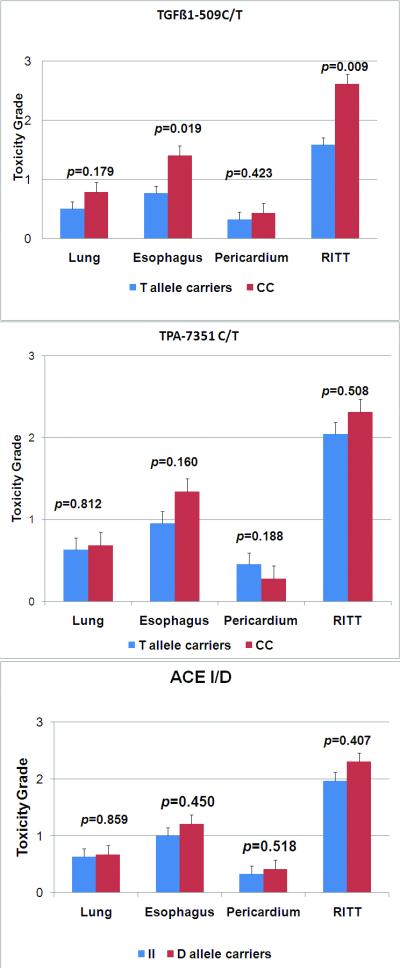
Toxicity grade was determined prospectively per CTCAE 3.0 standard. Transforming growth factor beta1=TGFβ1; ACE= angiotensin converting enzyme; tPA=tissue plasminogen activator; RITT=combined radiation induced thoracic toxicity.
No significant difference was observed in any individual RITT or mean grade of RITT either between tPA-7351 CC and T allele carriers, or between ACE I and D allele carriers.
Considering TGFβ1 CC, tPA CC, and ACE D allele carriers as sensitive genotypes, 9 patients without these sensitive genotypes had significantly lower mean toxicity scores of esophagus (0±0 vs. 1.3±0.1, p=0.002), and lung (0±0 vs. 0.8±0.1, p=0.021 and all RITT (0.3±0.2 vs. 2.4±0.2, p<0.001) than those with sensitive genotypes, although their pericardium toxicity was not significantly different (0.3±0.2 vs. 0.4±0.1, p=0.787). However, no significant differences in mean toxicity scores were found in lung, esophagus, pericardium and combined RITT among patients with 1, 2 or 3 sensitive genotypes (RITT score 2.4±0.3 vs. 2.3±0.3 vs. 2.6±0.7, p=0.912).
Genotype and plasma TGF ß1
In the entire group of patients, the mean pre-RT TGFß1 level was 10.7± 2.3 ng/ml, and the mean during-RT TGFß1 level was 6.0±0.7 ng/ml. No significant differences in TGFß1 levels were found at pre-RT and during-RT in patients with different genotypes of TGFß1 and tPA. Only patients with the ACE DD genotype had marginally higher pre-RT TGFß1 levels than patients with the II and ID genotypes (DD 23.6 ng/ml vs. II 9.0 ng/ml vs. ID 7.5 ng/ml, p = 0.05); there was no difference during-RT (p=0.346) or in the during-RT/pre-RT ratio (p = 0.433). However, patients with TGFß1 509CC had greater increase in plasma TGF ß1 level at 4-5 week during-RT than T allele carriers (TGFß1 ratio of during-RT/pre-RT were CC 1.2±0.2 vs. T allele carriers 0.7±0.1, p=0.047). (Table 2)
Table 2.
Genetic variations and plasma TGFβ1 level
| Plasma TGFβ1 level |
Genetic variations |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGFβ1 509C/T | tPA-7351 C/T | ACE I/D | ||||||||||
|
|
||||||||||||
| CC | CT | TT | p | CC | CT | TT | p | II | ID | DD | p | |
|
Pre-RT
(ng/ml) |
8.1± 1.2 |
13.8 ±4.9 |
- | 0.223 | 8.7± 1.4 |
12.6 ±4.5 |
10.3 ±5.4 |
0.734 | 9.0± 1.5 |
7.5± 1.1 |
23.6± 13.6 |
0.050 |
|
During-
RT (ng/ml) |
6.6± 1.0 |
5.3± 0.9 |
- | 0.333 | 5.5± 0.8 |
6.4± 1.0 |
7.0± 4.0 |
0.768 | 7.2± 1.2 |
5.2± 0.9 |
4.9± 1.2 |
0.346 |
| Ratio | 1.2± 0.2 |
0.7± 0.1 |
- | 0.047 | 1.3± 0.3 |
1.1± 0.2 |
0.8± 0.1 |
0.675 | 1.2± 0.2 |
1.0± 0.2 |
0.7± 0.3 |
0.433 |
DISCUSSION
The data from the current study indicate that genetic variation of TGFβ1 pathway genes may be associated combined RITT in lung, esophagus and pericardium. TGFβ1 −509 T allele carriers had significantly less severe radiation esophagitis (p=0.019) and mean grade of RITT (p=0.009) than TGFß1 CC patients. Patients with sensitive genotypes in this pathway had significantly higher grade of toxicity in lung, esophagus and RITT than those without sensitive genotypes (p<0.01)
The finding of the current study on TGFß1 pathway genotypes in all RITT has not been reported previously. From a biological standpoint, this finding is consistent with previous reports of TGFß1 in radiation normal tissue damage(11, 12) and that TGF-β1 levels may influence the pathologic process of radiation induced inflammation and fibrosis(3). Zhao et al(13-14) and Anscher et al(15) have previously reported that radiation-induced elevations of plasma TGF-β1 during RT is predictive of radiation induced lung toxicity (RILT). The combination of TGF-β1 and mean lung dose may help stratify the patients for their risk of RILT. A normal plasma level of TGFβ1 by the end of radiotherapy was more common in patients without RILT. Changes in plasma TGF-β1 levels during radiotherapy were found to be useful in identifying patients at low risk for complications after radiation up to 86.4 Gy(9). Fu et al reported that an elevated plasma TGFβ1 level at the end of RT is an independent risk factor for RILT(16). Novakova-Jiresova et al also observed a trend of plasma TGFβ1 concentration to decrease below the pre-treatment value during the RT treatment in patients who did not develop pulmonary complications after the RT treatment (17).
Overall the literature suggests a potential role of TGFβ1 in radiation lung damage and the value of using plasma TGFβ1 to predict RILT. However, measurement of plasma TGFβ1 is challenging for its reproducibility, as the plasma is easily contaminated with platelets or their degradation by-products, which can result from improper handling of blood samples. Testing with TGFß1 genotypes may accelerate the clinical application of TGFß1 in future clinical trial and practice. Genomic analysis is attractive for its resistance to variations in methodology of sample handing, as DNA is very stable. More importantly, risk assessment at baseline would provide an opportunity to individualize the entire course of treatment while assessment mid-treatment will only guide the remaining treatment.
TGFβ1 SNPs are associated with TGFβ1 plasma level. Shah et al reported the common functional SNPs of C-509T have been linked to a nearly two-fold difference in plasma levels of TGFβ1(18). In this study, we found that patients with TGFß1 509CC had higher elevation of plasma TGF ß1 level at 4-5 week during-RT than T allele carriers. This may help explain the correlation between TGF ß1 level elevation and higher risk of radiation induced lung toxicity which was reported in our previous publications(13-15). Therefore, it has been suggested that the molecular mechanism for the risk of radiation induced lung toxicities may be linked to the SNPs of C-509T in TGFβ1gene.
Yuan et al(2) also reported high risk of radiation pneumonitis in patients exhibiting CC in TGF-β1-509C/T. Wang et al reported that genotype of TGF-β1-509C/T polymorphism was associated with the incidence of radiation induced esophagus toxicity(19). Liu et al reported the pleiotropic effects of TGFβ1 on pericardial interstitial cells, implicating its effect for fibrosis and calcification in idiopathic constrictive pericarditis(20). The current study may serve as an independent validation of TGF-β1 in overall radiation injury in normal tissue. Patients expressing CC in TGFβ1-509C/T had significantly higher incidence of RITT and esophagitis and non-significant but increased toxicity score of lung and pericardium. If further validated in studies with a larger number of patients in a multicenter setting, TGFβ1-509C/T may be used as a convenient biomarker to individualize radiation therapy in lung cancer.
Considering TGFβ1, tPA, and ACE as parts of a single pathway, any functional variant of these proteins would change the sensitivity RITT of one individual. This was partly supported by the result of the current study in which patients without sensitive genetic variants in this pathway had significantly lower mean toxicity scores of esophagus (p=0.002), lung (p=0.021) and combined RITT (p<0.001) than that of patients with any sensitive variant. It seems that one functional SNP in the pathway was sufficient to predispose a patient at higher risk as there was no significant difference in toxicity grade between patients with 1, 2 or 3 sensitive variants.
However, we acknowledge that this study is limited by its sample size, which may explain why we did not detect a difference in any RITT either between tPA-7351 CC and T allele carriers, or between ACE II and D allele carriers. The insignificant result of RITT with genotypes of tPA and ACE may indicate that TGFβ1 plays a more important role than either tPA or ACE in the complicated signal transduction pathway for radiation induced normal tissue injury. Nevertheless, the association of the genotypes between TGF-β1 C-509T and grades of RITT support our hypothesis that radiation inflammation related toxicity of different organs may share a similar mechanism.
In summary, results from this hypothesis driven study indicate that functional TGF-β1 genotypes C-509T may play an important role in the development of RITT, and future radiation toxicity studies may be carried out in patient level based on biologic mechanism. The approach of combining all RITT may have a greater impact on individualized care, as it provides an opportunity to assess a patient as a whole rather than organ by organ. Further study with a larger number of patients (ideally in a multi-center setting) is needed to validate our findings of the genetic variations in accumulative RITT.
Figure 3. Radiation thoracic toxicity and sensitive genotypes in TGFβ1 pathway.
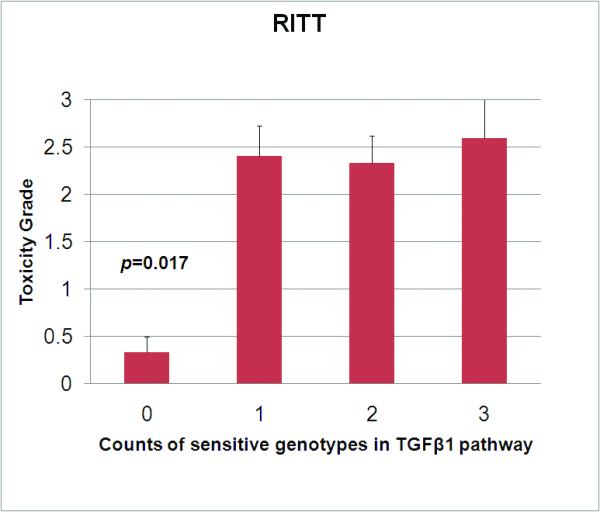
Toxicity grade was determined prospectively per CTCAE 3.0 standard. Presence of any one sensitive genotype (tissue plasminogen activator CC type, or angiotensin converting enzyme D type (DD or DI) or transforming growth factor beta1 (TGFß1) 509CC type) is associated with a significantly higher mean grade of combined radiation induced thoracic toxicity (RITT). Error bars show the standard error of mean. There seems no significant difference in the overall score of RITT in patients with 1, 2, or all 3 high risk variants.
ACKNOWLEDGEMENTS
This study was supported in part by: the Lance Armstrong Foundation through the American Association of Clinical Oncology-Career Developmental Award (ASCO-CDA) (FMK), R21CA127057 (FMK), R01 CA 142840-03 (FMK), Shandong Provincial Health Bureau (2007QW036), Foundation for Excellent Young Scientists of Shandong Province (BS2009YY012), NSFC30700196 and NSFC81172133 (SY); the Michigan Institute for Clinical and Health Research (UL1RR024986; KAS)
Footnotes
CONFLICTS OF INTEREST NOTIFICATION All authors have approved this manuscript and no actual or potential conflicts of interest exist.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGF{beta}1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–3378. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang HR, Cho SJ, Lee CG, et al. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 4.Stringer KA, Hybertson BM, Cho OJ, et al. Tissue plasminogen activator (tPA) inhibits interleukin-1 induced acute lung leak. Free Radic Biol Med. 1998;25:184–188. doi: 10.1016/s0891-5849(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 5.Hoogerwerf JJ, de Vos AF, Levi M, et al. Activation of coagulation and inhibition of fibrinolysis in the human lung on bronchial instillation of lipoteichoic acid and lipopolysaccharide. Crit Care Med. 2009;37:619–625. doi: 10.1097/CCM.0b013e31819584f9. [DOI] [PubMed] [Google Scholar]
- 6.Morrison CD, Papp AC, Hejmanowski AQ, et al. Increased D allele frequency of the angiotensin-converting enzyme gene in pulmonary fibrosis. Hum Pathol. 2001;32:521–528. doi: 10.1053/hupa.2001.24321. [DOI] [PubMed] [Google Scholar]
- 7.Molteni A, Wolfe LF, Ward WF, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 8.Chapet O, Kong FM, Quint LE, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63:170–178. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Ronaghi M. Pyrosequencing for SNP genotyping. Methods Mol Biol. 2003;212:189–195. doi: 10.1385/1-59259-327-5:189. [DOI] [PubMed] [Google Scholar]
- 11.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Sporn MB, Roberts AB. Transforming growth factor-beta. Multiple actions and potentialclinical applications. JAMA. 1989;262:938–941. doi: 10.1001/jama.262.7.938. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Wang L, Ji W, et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys. 2009;74:1385–1390. doi: 10.1016/j.ijrobp.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Sheldon K, Chen M, et al. The predictive role of plasma TGF-beta1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung Cancer. 2008;59:232–239. doi: 10.1016/j.lungcan.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Anscher MS, Kong FM, Marks LB, et al. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997;37:253–258. doi: 10.1016/s0360-3016(96)00529-9. [DOI] [PubMed] [Google Scholar]
- 16.Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001;50:899–908. doi: 10.1016/s0360-3016(01)01524-3. [DOI] [PubMed] [Google Scholar]
- 17.Novakova-Jiresova A, Van Gameren MM, Coppes RP, et al. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol. 2004;71:183–189. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Shah R, Hurley CK, Posch PE. A molecular mechanism for the differential regulation of TGF-beta1 expression due to the common SNP −509C-T (c. −1347C > T) Hum Genet. 2006;120:461–469. doi: 10.1007/s00439-006-0194-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Yang M, Bi N, et al. Association of TGF-beta1 and XPD polymorphisms with severe acute radiation-induced esophageal toxicity in locally advanced lung cancer patients treated with radiotherapy. Radiother Oncol. 2010;97:19–25. doi: 10.1016/j.radonc.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Bai C, Gong D, et al. Pleiotropic effects of transforming growth factor-beta1 on pericardial interstitial cells. Implications for fibrosis and calcification in idiopathic constrictive pericarditis. J Am Coll Cardiol. 2011;57:1634–1635. doi: 10.1016/j.jacc.2010.10.054. [DOI] [PubMed] [Google Scholar]


