Abstract
Necrotizing enterocolitis (NEC) continues to be a devastating inflammatory disease of the newborn intestine. Despite advances in management, morbidity and mortality remain high. While it is clear that intestinal ischemia plays a large role in disease pathogenesis, attempts to link NEC to intestinal macrovascular derangement have been largely unsuccessful. More recently, there has been a concerted effort to characterize the pathologic changes of the intestinal microcirculation in response to intestinal injury, including NEC. This microcirculatory regulation is controlled by a balance of vasoconstrictor and vasodilator forces. Vasoconstriction is mediated primarily by endothelin-1 (ET-1) while vasodilation is mediated primarily by nitric oxide (NO). These chemical mediators have been implicated in many aspects of intestinal ischemic injury and NEC, with the balance shifting towards increased vasoconstriction associated with intestinal injury. With a proper understanding of these antagonistic forces, potential therapeutic avenues may result from improving this pathologic microcirculatory dysregulation.
Keywords: necrotizing enterocolitis, intestinal ischemia, intestinal microcirculation, endothelin-1, nitric oxide
Necrotizing enterocolitis (NEC) is an intestinal inflammatory condition of unknown etiology characterized by coagulation necrosis of the intestinal wall.1 It is predominantly a disease of prematurity and is one of the most common surgical emergencies in infants. Mortality has been estimated between 15% to 30%.2–7 Approximately 20%–40% of NEC patients require some form of surgical intervention.2,3,8–10 Three factors are felt to be necessary for disease initiation: intestinal mucosal injury, intestinal feeding and intestinal bacterial growth.11,12 Intestinal feeding produces bacterial proliferation, which can lead to bacterial invasion of the intestinal wall if the mucosa is damaged by some other mechanism. This leads to a cascade of inflammatory events with leukocyte adhesion and activation, complement activation, increased vascular permeability, cytokine release, localized vasoconstriction and localized ischemia/reperfusion (I/R) injury.13–15 It is clear that ischemia and the intestinal microcirculation play a role in these events, but it is unclear whether this role is as a primary initiator or simply a secondary response to intestinal injury.
Intestinal Embryology and Anatomy
To properly understand the physiology and pathology of intestinal injury, including NEC, a thorough understanding of intestinal anatomy is required. The foundation of intestinal anatomy is rooted in intestinal embryology. Beginning in the fourth week of gestation, the primitive gut begins to form as the head, tail and lateral folds incorporate the dorsal portion of the yolk sac.16–18 The three germ layers of the primitive gut differentiate into specific elements of the mature intestine. The endoderm forms the intestinal tract mucosa, liver and pancreas, while the splanchnopleuric mesoderm forms the connective tissue and muscular components, and ectodermal components contribute to the enteric nervous system.16–18
The primitive gut can be developmentally and anatomically divided into the foregut, midgut and hindgut. The foregut develops into the pharynx, esophagus, stomach, duodenum, pancreas, liver, biliary system and lower respiratory tract. The midgut forms the small intestine, cecum, appendix, ascending colon and proximal transverse colon. The hindgut forms the distal transverse colon, sigmoid colon, rectum and proximal portion of the anus.16–18 The intestinal vasculature, as well as the nervous system, develops in tandem, and the macrovascular elements follow a similar anatomic distribution, as described below.
Intestinal vasculogenesis begins as a response to rapid intestinal parenchymal growth.16 Mesodermal cells form blood islands incorporated into the mesodermal elements surrounding the wall of the yolk sac.17,18 These blood islands differentiate into hemangioblasts under the control of fibroblast growth factor-2 (FGF-2).18,19 Hemangioblasts can be divided into two separate groups. Peripheral hemangioblasts differentiate into angioblasts under the control of vascular endothelial growth factor (VEGF), which later form endothelial cells and primitive blood vessels.18,19 Once this primary vascular bed is established, additional vasculature is added via angiogenesis under the control of VEGF, platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF- β).20 Central hemangioblasts differentiate into hematopoietic stem cells, which further differentiate into their myeloid (monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes, dendrites) and lymphoid (T-cells, B-cells, NK-cells) lineages.18,19
Three major arterial branches from the dorsal aorta persist and mature to supply the mature derivatives of the primitive gut. The celiac artery supplies foregut derivatives, the superior mesenteric artery (SMA) supplies midgut derivatives and the inferior mesenteric artery (IMA) supplies hindgut derivatives.16–18 These major arterial trunks successively branch into smaller vessels until they eventually pierce the longitudinal and circular intestinal muscular layers to enter the submucosa.
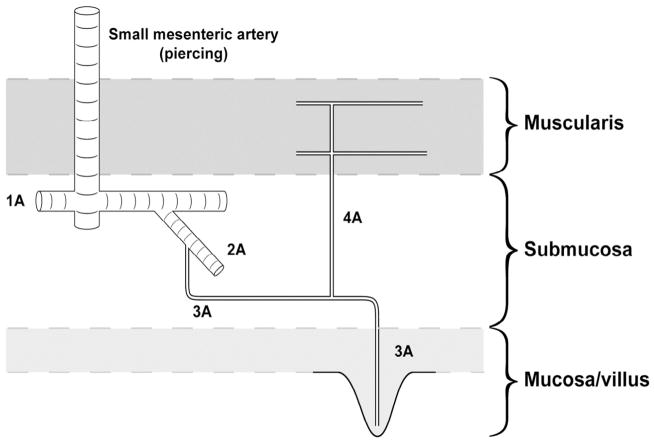
The intestinal microcirculation is visually summarized in Figure 1.21 The 1A arterioles branch into 2A arterioles. These 2A arterioles form vascular shunts with other 1A arterioles. These arterioles remain in the intestinal submucosa and represent a bridge between the intestinal micro- and macro-circulation. The 1A-2A plexuses are the primary sites of vascular resistance and thus are the primary regulators of intestinal blood flow.22–23 The 3A arterioles arise from the 2A arterioles and enter the intestinal mucosa. Each 3A arteriole enters a single villus to form a terminal capillary network. Prior to mucosal entry, 3A arterioles branch into 4A arterioles, which enter the intestinal muscular layers. The collecting venules from each villus drain into a mucosal sinus. They do not run in immediate proximity to their arterial counterparts until they reach the level of the submucosa.21
Figure 1. Schematic representation of the intestinal microcirculation.
Small mesenteric arteries pierce the muscularis layers and terminate in the submucosa where they give rise to 1A (1st order) arterioles. 2A (2nd order) arterioles arise from 1A arterioles. Although not shown here, these 2A arterioles merge with several 1A arterioles, thus generating an arteriolar plexus that serves to pressurize the terminal downstream microvasculature. 3A (3rd order) arterioles arise from 2A arterioles and proceed to the mucosa, giving off a 4A branch just before descent into the mucosa. The 4A vessels travel to the muscularis layers. Each 3A vessel becomes the single arteriole perfusing each villus. Reprinted with permission.21
Intestinal Circulatory Regulation
Regulation of intestinal blood flow can be divided into extrinsic and intrinsic elements.24 Extrinsic regulation refers to control from a site other than the intestine, often via the autonomic nervous system and cardiovascular reflexes. It typically functions to preserve systemic cardiovascular homeostasis, and can work at the expense of the local intestinal circulation.24 Intrinsic intestinal circulatory control refers to regulation produced by mediators that are formed and released locally in the intestine and its vasculature. Intrinsic regulation functions to preserve intestinal microcirculatory homeostasis locally to ensure delivery of oxygen and nutrients to the intestine.24 There is a balance between vasoconstrictive and vasodilatory influences in newborn intestine at the intrinsic level.21,25,26
Intestinal microcirculatory blood flow is largely based on resting vascular resistance. This is the impedance to flow across a regional circulation in the setting of steady-state hemodynamic conditions.25 Blood flow is inversely proportional to resistance, so increased resistance leads to decreased blood flow, with the corollary being true as well. Vascular resistance is inversely proportional to vessel radius to the 4th power. This implies that small vasoconstrictive or vasodilatory changes cause significantly larger changes in vascular resistance and blood flow. The newborn infant intestinal microcirculation is characterized by lower resting vascular resistance compared to that of older subjects. This leads to a higher rate of blood flow and an increased delivery of nutrients and oxygen.27,28
Necrotizing Enterocolitis and the Intestinal Macrocirculation
Investigators have explored potential relationships between the intestinal circulation and NEC-like intestinal injury for over forty years. Initial observations noted a correlation between perinatal asphyxia and subsequent gastrointestinal perforation.29 This was referred to as the diving reflex, as it was physiologically similar to the known cardiac output redistribution to the brain observed in diving mammals.30 It was speculated that extrinsic neurogenic blood flow redistribution from splanchnic organs to the brain resulted in intestinal ischemia. Initial studies in newborn piglets supported this hypothesis by demonstrating mucosal damage after acute asphyxia.31,32 This hypothesis fell out of favor as later studies noted that infants with NEC rarely suffered intrapartum asphyxia,33 that NEC rarely occurs in the 1st week of life,33 and that sustained adrenergic stimulation, a central facet of the diving reflex, does not cause sustained intestinal blood flow reduction, nor does it cause intestinal tissue hypoxia.34,35
Some data suggest that abnormal SMA blood flow parameters, with a high vascular resistance, are associated with intestinal dysmotility and early feeding intolerance36,37 and possibly later development of NEC.38 However, other attempts to link NEC to macrocirculatory intestinal blood flow derangements have yielded mixed results. Associations between NEC and exchange transfusions,39 umbilical artery catheters,40 or plasma hyperviscosity41 have not held up to investigational scrutiny. A number of postnatal factors that decrease mesenteric blood flow including caffeine administration,42,43 presence of a patent ductus arteriosus (PDA),44,45 indomethacin for treatment of PDA,46,47 hyperalimentation,48 and the use of continuous positive airway pressure49 have not been definitively linked to the development of NEC.
Because of these developments, much attention has turned towards the investigation of the intestinal microcirculation. There is emerging evidence that dysregulation at this level is associated with the development of NEC. In experimental rat models of NEC, intestinal microvascular blood flow to injured intestine is impaired50 and these animals demonstrate abnormal microvascular anatomy.51 Much of the focus on the intestinal microcirculation focuses on the balance between intestinal vasoconstriction controlled primarily by endothelin-1 (ET-1), and intestinal vasodilation controlled primarily by nitric oxide (NO).
Intestinal Microcirculatory Vasoconstriction: The Role of Endothelin-1
Endothelin-1 (ET-1) is a constitutively expressed vasoactive and mitogenic protein produced by endothelial cells52 that acts as the primary vasoconstrictor in neonatal intestinal vasculature.53 It exerts its biological effects by binding to endothelin receptor type A (ETA) and endothelin receptor type B (ETB).54–56 ET-1 is initially transcribed as prepro ET-1, a 212 amino acid protein. It is processed by nonspecific proteases to big ET-1, a 38 amino-acid peptide,57 and then to its final biologically active, 21 amino-acid form by endothelin converting enzyme.58 Under basal conditions, production of ET-1 is greater in younger compared to older subjects.53 In addition to its constitutive expression, increased production of ET-1 is locally stimulated by decreased blood flow, hypoxia and various inflammatory cytokines.59–61 ET-1 has both vasoconstrictive and angiogenic properties.53
Both ETA and ETB expression are higher in younger compared to older subjects.62 ETA is mainly expressed on vascular smooth muscle cells, where its activation induces sustained vasoconstriction.54 ETB is expressed on both vascular smooth muscle cells and endothelial cells. As with ETA, activation of vascular smooth muscle cell ETB results in vasoconstriction. On the other hand, stimulation of endothelial cell ETB leads to NO-mediated vasodilation.55,56 ETA receptor blockade has been shown to decrease intestinal vascular resistance, with a more profound effect in newborns compared to adults.63 ETB receptor stimulation has been shown to produce mild NO-dependent vasodilation in the newborn intestinal vasculature.26,53 In the neonatal intestinal vasculature, the effects of ETA (vasoconstriction) surpass those of ETB (predominantly vasodilation), thus the overall effect of ET-1 is vasoconstriction.
ET-1 has been extensively studied in many models of both animal and human intestinal injury, including NEC. Much of the pathogenic mechanistic detail has been explained in the setting of intestinal I/R injury. Intra-arterial ET-1 injection in rats leads to decreased intestinal perfusion and production of tissue damage,64 and leads to polymorphonuclear leukocyte (PMN) infiltration and oxidative stress as well as mucosal barrier dysfunction.65 These effects were attenuated by ETA blockade, but not by ETB blockade.66 ET-1 infusion in guinea pigs decreases submucosal terminal microvessel blood flow and increases microvascular permeability, effects that are also attenuated by ETA but not ETB blockade.67 Vasoconstriction occurs after intestinal I/R injury in 3-day old, but not 35 day-old swine, and is attenuated by ETA receptor blockade.63 In a rat model of I/R injury, pretreatment with ETA and ETB blockade decreased mucosal injury, decreased PMN infiltration and improved blood flow.68 In the same model, inhibition of endothelin converting enzyme, the final step in ET-1 post-translational protein modification, decreased intestinal mucosal injury.68
ET-1 expression is upregulated by a variety of inflammatory cytokines.65,66,68–73 In cultured endothelial cells, IL-1 increases expression of both ET-1 and ETA.61,74 IL-1β increases ET-1 expression and decreases ETB expression in cultured endothelial cells, and increases ETA expression in cultured smooth muscle cells. Infusion of IL-1β causes ileal vasoconstriction attenuated by ETA receptor blockade, which implies that that the vasoconstriction was ET-1- mediated.70
Ileal ET-1 mRNA expression was increased in a rat model of NEC, with compromised microvascular perfusion and decreased intestinal blood flow.75 In this model, topical ET-1 applied to the intestinal mucosa further increased intestinal permeability and microvascular dysfunction.75 Also in a rat model of NEC, microvascular blood flow was decreased to injured intestine, and was improved by ETA receptor blockade.76 In confirmed cases of human NEC there was increased ET-1 expression in NEC compared to non-NEC intestine, with ET-1 levels increasing with increasing intestinal injury. 71 Dissected subserosal arterioles showed relative vasoconstriction based on vessel diameter, flow rate and vascular resistance in arterioles proximal to NEC-injured intestine compared to vessels distal to the injury. ETA blockade led to vasodilation of the proximal arterioles but not the distal arterioles from NEC patients, and not the arterioles from non-NEC control patients. These data lend support to ET-1-mediated vasoconstriction via ETA in the setting of NEC.71
Intestinal Microcirculatory Vasodilation: The Role of Nitric Oxide
NO is the primary intestinal vasodilator in the newborn.27,28 It is produced by a family of NO synthases during conversion of L-arginine to L-citrulline.77 It exerts its biological effects in a paracrine fashion on smooth muscle cells by binding to and activating the heme moiety of soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP),78 which decreases intracellular calcium concentrations leading to vasodilation and decreased vascular resistance.79 The endothelial isoform, eNOS, is constitutively expressed, but can be increased by a variety of mechanical and chemical stimuli.25
SMA NO concentration is increased in younger compared to older subjects, and the increased vascular resistance caused by NO inhibition is more pronounced in younger compared to older subjects.25,27 Likewise, NO-mediated vasodilation is greater in newborn compared to older subjects.25,27 The most important physiologic stimulus to newborn eNOS activity is endothelial shear force. Increased blood flow causes increased eNOS-mediated NO production and vasodilation. Again, this response to shear force is greater in newborns compared to older subjects.26,80
Coordinated processes regulate the balance of ET-1 and NO. ET-1 stimulates eNOS activity and NO production via the ETB receptor to produce vasodilation.55,56 In cultured endothelial cells, ET-1 stimulates increased eNOS mRNA and protein expression.81 NO donors decrease ET-1 induction,82 while NO inhibitors increase both basal and stimulated ET-1 levels, and decrease shear-mediated down-regulation of ET-1 expression.59 NO has also been shown to decrease the binding affinity of ET-1 to ETA. 83
NO activity has also been studied in multiple animal and human models of intestinal injury, including NEC. In models of sustained low intestinal blood flow and intestinal I/R injury, there was increased vascular resistance in 3-day old piglets but no change in 35-day old piglets, due to decreased NO production.84,85 In intestinal I/R injury, there is a decrease of both basal NO and of stimulated NO from endothelial cell eNOS.86,87 In a rat model of NEC, blockade of NOS decreases intestinal microcirculatory blood flow.76 Also in a rat model of NEC, the well-known intestinal cytoprotective agent heparin-binding EGF-like growth factor (HB-EGF) improved microcirculatory blood flow50 by increasing both NO production and by increasing ETB expression.88 Rats with NEC demonstrated distorted, injured and broken arterioles, venules and capillaries. These anatomic microvascular changes were attenuated by treatment with HB-EGF via NO regulatory mechanisms.50
In human intestine resected for NEC, submucosal arterioles showed abnormal eNOS function compared to control specimens.89 The NEC arterioles constricted in response to pressure, failed to dilate or generate NO in response to acetylcholine, and failed to dilate in response to blood flow. However, these arterioles did dilate in response to exogenous NO, demonstrating functional smooth muscle. Asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of NOS, has also been implicated in the pathogenesis of NEC. In human infants with NEC, a decreased ratio of arginine to ADMA was associated with an increased incidence of NEC and higher NEC mortality.90
L-arginine, a major substrate for NO production, has also been studied in the setting of intestinal injury and NEC. In rat models of endotoxemia, decreased plasma arginine led to decreased small intestine blood flow.91 In a mouse model of NEC, L-arginine administration increased ileal NO and decreased intestinal injury.92 Likewise, in premature infants with NEC there was decreased plasma arginine levels both at NEC diagnosis and seven days prior to diagnosis.93,94 Also, a mutation in carbamoyl phosphate synthase, the rate-limiting enzyme in L-arginine production, has been linked to an increased risk of NEC in human preterm infants.95 Some data suggest that L-arginine administration increases serum NO production,96 and decreases intestinal injury in swine models of I/R97 and NEC98, mouse models of NEC99 and even in human NEC.100
Conclusion
Intestinal ischemia is clearly a hallmark of NEC, but whether it is a primary inciting factor or a secondary result of intestinal inflammation and mucosal injury is uncertain. The dynamic interplay between the vasoconstrictor forces dominated by ET-1, and the vasodilator forces dominated by NO, largely control regional microcirculatory blood flow and local intestinal ischemia in newborn intestine. Dysregulation of this delicate balance in favor of vasoconstriction has been noted in both animal models and in confirmed human NEC. As our understanding of these antagonistic interactions improve, modulation of these pathways represents a promising potential therapeutic strategy for this often devastating disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balance WA, Dahms BB, Shenker N, et al. Pathology of neonatal necrotizing enterocolitis: a ten year experience. J Pediatr. 1990;117:S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 2.Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;56:278–85. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 3.Luig M, Lui K, NSW ACT NICUS Group. Epidemiology of necrotizing enterocolitis. II. Risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41(4):174–9. doi: 10.1111/j.1440-1754.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 4.Guillet R, Stoll BJ, Cotton CM, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117(2):e137–42. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 5.Llanos AR, Moss ME, Pinzon MC, et al. Epidemiology of neonatal necrotizing enterocolitis: a population-based study. Paediatr Perinat Epidemiol. 2002;16(4):342–9. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Holman RC, Stoll BJ, Curns AT, et al. Necrotizing enterocolitis hospitalizations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin PW, Stoll BJ. Necrotizing enterocolitis. Lancet. 2006;368(9543):1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 8.Sankaran K, Puckett B, Lee DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39(4):366–72. doi: 10.1097/00005176-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Hudak ML, Tepas JJ, 3rd, et al. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol. 2006;26(6):342–7. doi: 10.1038/sj.jp.7211510. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R, Tepas JJ, 3rd, Mollitt DL, et al. Surgical management of bowel perforations and outcomes in very low-birth-weight infants (≤1,200 g) J Pediatr Surg. 2004;39(2):190–4. doi: 10.1016/j.jpedsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–54. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patole S. Prevention and treatment of necrotizing enterocolitis in preterm neonates. Early Hum Dev. 2007;83:635–42. doi: 10.1016/j.earlhumdev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MD, Clark JA, Saunders TA, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. Am J Physiol. 2006;290:G757–64. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 14.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–51. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh W, Caplan MS, Qu XW, et al. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark DA, Munshi UK. Development of the gastrointestinal circulation in the fetus and newborn. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3. Philadelphia, PA: WB Saunders; 2004. pp. 701–5. [Google Scholar]
- 17.Moore KL, Persaud TVN. The developing human: Clinically oriented embryology. 7. Philadelphia, PA: WB Saunders; 2003. [Google Scholar]
- 18.Sadler TW. Langman’s medical embryology. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 19.Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev Dyn. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Battegay EJ. Angiogenesis. Mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73:333–46. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 21.Nowicki PT. Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg. 2005;14:152–8. doi: 10.1053/j.sempedsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Bohlen HG. Determinants of resting and passive intestinal vascular pressures in rat and rabbit. Am J Physiol. 1987;253:G587–95. doi: 10.1152/ajpgi.1987.253.5.G587. [DOI] [PubMed] [Google Scholar]
- 23.Christensen KL, Mulvany MJ. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res. 1993;30:73–9. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- 24.Nowicki P. Intestinal ischemia and necrotizing enterocolitis. J Paediatr. 1990;117:S14–99. doi: 10.1016/s0022-3476(05)81125-4. [DOI] [PubMed] [Google Scholar]
- 25.Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology Clin Perinatol. 2002;29:23–39. doi: 10.1016/s0095-5108(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 26.Nankervis CA, Dunaway DJ, Nowicki PT. Determinants of terminal mesenteric artery resistance during the first postnatal month. Am J Physiol. 2001;280:G678–86. doi: 10.1152/ajpgi.2001.280.4.G678. [DOI] [PubMed] [Google Scholar]
- 27.Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol. 1995;268:G949–58. doi: 10.1152/ajpgi.1995.268.6.G949. [DOI] [PubMed] [Google Scholar]
- 28.Reber KM, Mager GM, Miller CE, et al. Relationship between flow rate and NO production in postnatal mesenteric arteries. Am J Physiol. 2000;280:G43–50. doi: 10.1152/ajpgi.2001.280.1.G43. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd JR. The etiology of gastrointestinal perforations in the newborn. J Pediatr Surg. 1969;4:77–84. doi: 10.1016/0022-3468(69)90186-9. [DOI] [PubMed] [Google Scholar]
- 30.Elsner R, Kenney DW, Burgess K. Diving bradycardia in the trained dolphin. Nature. 1966;212:407–8. doi: 10.1038/212407a0. [DOI] [PubMed] [Google Scholar]
- 31.Alward C, Hook J, Helmrath T, et al. Effects of asphyxia on cardiac output and organ blood flow in the newborn piglet. Pediatr Res. 1978;12:824–7. doi: 10.1203/00006450-197808000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Touloukian RJ, Posch JN, Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate: selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg. 1972;7:194–205. doi: 10.1016/0022-3468(72)90496-4. [DOI] [PubMed] [Google Scholar]
- 33.Stoll BJ, Kanto WP, Jr, Glass RI, et al. Epidemiology of necrotizing enterocolitis: a case control study. J Pediatr Surg. 1980;96:447–51. doi: 10.1016/s0022-3476(80)80696-2. [DOI] [PubMed] [Google Scholar]
- 34.Buckley NM, Jarenwattanon M, Gootman P, et al. Autoregulatory escape from vasoconstriction of intestinal circulation in developing swine. Am J Physiol. 1987;252:H118–24. doi: 10.1152/ajpheart.1987.252.1.H118. [DOI] [PubMed] [Google Scholar]
- 35.Nowicki PT, Miller CE, Hayes JR. Effects of sustained mesenteric nerve stimulation on intestinal oxygenation in developing swine. Am J Physiol. 1991;260:G333–9. doi: 10.1152/ajpgi.1991.260.2.G333. [DOI] [PubMed] [Google Scholar]
- 36.Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001;85:F42–5. doi: 10.1136/fn.85.1.F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robel-Tillig E, Knupfer M, Pulzer F, et al. Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radiol. 2004;34:958–62. doi: 10.1007/s00247-004-1285-6. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch EM, Sinha AK, Shanmugalingam ST, et al. Doppler flow velocity in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. 2006;118:1999–2003. doi: 10.1542/peds.2006-0272. [DOI] [PubMed] [Google Scholar]
- 39.Hardy JD, Savage TR, Shirodaria C. Intestinal perforation following exchange transfusion. Am J Dis Child. 1972;124:136–41. doi: 10.1001/archpedi.1972.02110130138023. [DOI] [PubMed] [Google Scholar]
- 40.Havranek T, Johanboeke P, Madramootoo C, et al. Umbilical artery catheters do not affect intestinal blood flow response to minimal enteral feedings. J Perinatol. 2007;27:375–9. doi: 10.1038/sj.jp.7211691. [DOI] [PubMed] [Google Scholar]
- 41.Leake RD, Thanopoulos B, Nieberg R. Hyperviscosity syndrome associated with necrotizing enterocolitis. Am J Dis Child. 1975;129:1192–4. doi: 10.1001/archpedi.1975.02120470042011. [DOI] [PubMed] [Google Scholar]
- 42.Hoecker C, Nelle M, Poeschl J, et al. Caffeine impairs cerebral and intestinal blood flow velocity in preterm infants. Pediatrics. 2002;109:784–7. doi: 10.1542/peds.109.5.784. [DOI] [PubMed] [Google Scholar]
- 43.Lane AJ, Coombs RC, Evans DH, et al. Effect of caffeine on neonatal splanchnic blood flow. Arch Dis Child Fetal Neonatal Ed. 1999;80:F128–9. doi: 10.1136/fn.80.2.f128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman-Ladd M, Cohen JB, Carver JD, et al. The hemodynamic effects of neonatal patent ductus arteriosus shunting on superior mesenteric artery blood flow. J Perinatol. 2005;25:459–62. doi: 10.1038/sj.jp.7211294. [DOI] [PubMed] [Google Scholar]
- 45.Shimada S, Kasai T, Hoshi A, et al. Cardiocirculatory effects of patent ductus arteriosus in extremely low-birth-weight infants with respiratory distress syndrome. Pediatr Int. 2003;45:255–62. doi: 10.1046/j.1442-200x.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 46.Christmann V, Liem KD, Semmekrot BA, et al. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatr. 2002;91:440–6. doi: 10.1080/080352502317371698. [DOI] [PubMed] [Google Scholar]
- 47.Pezzati M, Vangi V, Biagiotti R, et al. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr. 1999;135:733–8. doi: 10.1016/s0022-3476(99)70093-4. [DOI] [PubMed] [Google Scholar]
- 48.Havranek T, Thompson Z, Carver JD. Factors that influence mesenteric artery blood flow velocity in newborn preterm infants. J Perinatol. 2006;26:493–7. doi: 10.1038/sj.jp.7211551. [DOI] [PubMed] [Google Scholar]
- 49.Havranek T, Madramootoo C, Carver JD. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J Perinatol. 2007;27:704–8. doi: 10.1038/sj.jp.7211808. [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Radulescu A, Zorko N, et al. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterol. 2009;137:221–30. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Downard C, Grant S, Matheson P, et al. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg. 2011;46:1023–8. doi: 10.1016/j.jpedsurg.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1998;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 53.Nankervis CA, Nowicki PT. Role of endothelin-1 in regulation of the postnatal intestinal circulation. Am J Physiol. 2000;278:G367–75. doi: 10.1152/ajpgi.2000.278.3.G367. [DOI] [PubMed] [Google Scholar]
- 54.Davenport AP, O’Reilly G, Kuc RE. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br J Pharmocal. 1995;114:1110–6. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clozel M, Fischli W, Guilly C. Specific binding of endothelin on human vascular smooth muscle cells in culture. J Clin Invest. 1989;83:1758–61. doi: 10.1172/JCI114078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossi GP, Seccia TM, Nussdorfer GG. Reciprocal regulation of endothelin-1 and nitric oxide: relevance in the physiology and pathology of the cardiovascular system. Int Rev Cytol. 2001;209:241–72. doi: 10.1016/s0074-7696(01)09014-3. [DOI] [PubMed] [Google Scholar]
- 57.Bloch KD, Friedrich SP, Lee MN, et al. Structural organization and chromosomal assignment of the gene encoding endothelin. J Biol Chem. 1989;264:10851–7. [PubMed] [Google Scholar]
- 58.Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 59.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J PHysiol. 1993;264:H150–6. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 60.Kourembanas S, Marsden PA, McQuillan LP, et al. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054–7. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods M, Mitchel JA, Wood EG, et al. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin converting enzyme. Mol Pharmacol. 1999;55:902–9. [PubMed] [Google Scholar]
- 62.Su BY, Reber KM, Nankervis CA. Developmental expression of endothelin receptors in postnatal swine mesenteric artery. Pediatr Res. 2004;56:359–65. doi: 10.1203/01.PDR.0000134253.86014.B9. [DOI] [PubMed] [Google Scholar]
- 63.Nankervis CA, Schauer GM, Miller CE. Endothelin-mediated vasoconstriction in postischemic newborn intestine. AM J Physiol. 2000;279:G683–91. doi: 10.1152/ajpgi.2000.279.4.G683. [DOI] [PubMed] [Google Scholar]
- 64.Miura S, Fukumura D, Kurose I, et al. Roles of ET-1 in endotoxin-induced microcirculatory disturbance in rat small intestine. Am J Physiol. 1996;271:G461–9. doi: 10.1152/ajpgi.1996.271.3.G461. [DOI] [PubMed] [Google Scholar]
- 65.Oktar BK, Cocskun T, Bozkurt A, et al. Endothelin-1-induced PMN infiltration and mucosal dysfunction in the rat small intestine. Am J Physiol. 2000;297:G483–91. doi: 10.1152/ajpgi.2000.279.3.G483. [DOI] [PubMed] [Google Scholar]
- 66.Massberg S, Boros M, Leiderer R, et al. Endothelin (ET)-1 induced mucosal damage in the rat small intestine: role of ET(A) receptors. Shock. 1998;9:177–83. doi: 10.1097/00024382-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 67.King-VanVlack CE, Mewburn JD, Chapler CK, et al. Hemodynamic and proinflammatory actions of endothelin-1 in guinea pig small intestine submucosal microcirculation. Am J Physiol. 2003;284:G940–8. doi: 10.1152/ajpgi.00373.2001. [DOI] [PubMed] [Google Scholar]
- 68.Oktar BK, Gulpinar MA, Bozkurt A, et al. Endothelin receptor blockers reduce I/R-induced intestinal mucosal injury: role of blood flow. Am J Physiol. 2002;282:G647–55. doi: 10.1152/ajpgi.2002.282.4.G647. [DOI] [PubMed] [Google Scholar]
- 69.Anadol AZ, Bayram O, Dursun A, et al. Role of endogenous endothelin peptides in intestinal ischemia-reperfusion injury in rats. Prostaglandins Leukot Essent Fatty Acids. 1998;59:279–83. doi: 10.1016/s0952-3278(98)90142-9. [DOI] [PubMed] [Google Scholar]
- 70.Nowicki PT. IL-1beta alters hemodynamics in newborn intestine: role of endothelin. Am J PHysiol. 2006;291:324–9. doi: 10.1152/ajpgi.00042.2006. [DOI] [PubMed] [Google Scholar]
- 71.Nowicki PT, Dunaway DJ, Nankervis CA, et al. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr. 2005;46:805–10. doi: 10.1016/j.jpeds.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 72.Markel TA, Crisotomo PR, Wairiuko GE, et al. Cytokines in necrotizing enterocolitis. Shock. 2006;4:329–37. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 73.Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:51–4. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 74.Yoshizumi M, Kurihara H, Morita T, et al. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990;166:324–9. doi: 10.1016/0006-291x(90)91948-r. [DOI] [PubMed] [Google Scholar]
- 75.Ito Y, Doelle SM, Clark JA, et al. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res. 2007;61:180–4. doi: 10.1203/pdr.0b013e31802d77db. [DOI] [PubMed] [Google Scholar]
- 76.Downard C, Matheson P, Shepherd J, et al. Direct peritoneal resuscitation augments ileal blood flow in necrotizing enterocolitis via a novel mechanism. J Pediatr Surg. 2012;47:1128–34. doi: 10.1016/j.jpedsurg.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 77.Marletta MA. Nitric oxide synthase structure and metabolism. J Biol Chem. 1993;268:12231–4. [PubMed] [Google Scholar]
- 78.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochem Biophys Acta. 1999;1411:334–50. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 79.Russwurm M, Koesling D. Guanylyl cyclase: NO hits its target. Biochem Soc Symp. 2004;71:51–63. doi: 10.1042/bss0710051. [DOI] [PubMed] [Google Scholar]
- 80.Nowicki PT, Miller CE. Flow-induced dilation in newborn intestine. Pediatr Res. 1995;38:783–91. doi: 10.1203/00006450-199511000-00024. [DOI] [PubMed] [Google Scholar]
- 81.Marsen TA, Egink G, Suckau G, et al. Tyrosine-kinase-dependent regulation of the nitric oxide synthase gene by endothelin-1 in human endothelial cells. Pflugers Arch. 1999;438:538–44. doi: 10.1007/s004249900079. [DOI] [PubMed] [Google Scholar]
- 82.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–90. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goligorsky MS, Tsukahra H, Magazine H, et al. Termination of endothelin signaling: role of nitric oxide. J Cell Physiol. 1994;158:485–94. doi: 10.1002/jcp.1041580313. [DOI] [PubMed] [Google Scholar]
- 84.Nowicki PT. Effects of sustained flow reduction on postnatal intestinal circulation. Am J Physiol. 1998;275:G758–68. doi: 10.1152/ajpgi.1998.275.4.G758. [DOI] [PubMed] [Google Scholar]
- 85.Nowicki PT, Nankervis CA, Miller CE. Effects of ischemia and reperfusion on intrinsic vascular regulation in the postnatal intestinal circulation. Pediatr Res. 1993;33:400–4. doi: 10.1203/00006450-199304000-00017. [DOI] [PubMed] [Google Scholar]
- 86.Nowicki PT. Effects of sustained low-flow perfusion on the response to vasoconstrictor agents in postnatal intestine. Am J Pysiol. 1999;276:G1408–16. doi: 10.1152/ajpgi.1999.276.6.G1408. [DOI] [PubMed] [Google Scholar]
- 87.Nowicki PT. The effects of ischemia-reperfusion on endothelial cell function in postnatal intestine. Pediatr Res. 1996;39:267–74. doi: 10.1203/00006450-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Y, Brigstock D, Besner GE. Heparin-binding EGF-like growth factor is a potent dilator of terminal mesenteric arterioles. Microvasc Res. 2009;78:78–85. doi: 10.1016/j.mvr.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nowicki PT, Caniano DA, Hammond S, et al. Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr. 2007;150:40–5. doi: 10.1016/j.jpeds.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 90.Richir MC, Siroen MP, van Elburg RM, et al. Low plasma concentrations of arginine and asymmetric dimethylarginine in premature infants with necrotizing enterocolitis. Br J Nutr. 2007;97:906–11. doi: 10.1017/S0007114507669268. [DOI] [PubMed] [Google Scholar]
- 91.Prins HA, Houdijk AP, Wiezer MJ, et al. The effect of mild endotoxemia during low arginine plasma levels on organ blood flow in rats. Crit Care Med. 2000;28:1991–7. doi: 10.1097/00003246-200006000-00051. [DOI] [PubMed] [Google Scholar]
- 92.Cintra AE, Martins JL, Patricio FR, et al. Nitric oxide levels in the intestines of mice submitted to ischemia and reperfusion: L-arginine effects. Transplant Proc. 2008;40:830–5. doi: 10.1016/j.transproceed.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 93.Becker RM, Wu G, Galanko JA, et al. Reduced serum amino acid concentrations in infants with necrotizing enterocolits. J Pediatr. 2000;137:785–93. doi: 10.1067/mpd.2000.109145. [DOI] [PubMed] [Google Scholar]
- 94.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–8. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 95.Moonen RM, Paulussen AD, Souren NY, et al. Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res. 2007;62:188–90. doi: 10.1203/PDR.0b013e3180a0324e. [DOI] [PubMed] [Google Scholar]
- 96.Vukosavljevic N, Jaron D, Barbee KA, et al. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 97.Spanos CP, Papaconstantinou P, Spano P, et al. The effect of L-arginine and aprotinin on intestinal ischemia-reperfusion injury. J Gastrointest Surg. 2007;11:247–55. doi: 10.1007/s11605-007-0102-6. [DOI] [PubMed] [Google Scholar]
- 98.Di Lorenzo M, Bass J, Krantis A. Use of L-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg. 1995;30:235–40. doi: 10.1016/0022-3468(95)90567-7. [DOI] [PubMed] [Google Scholar]
- 9.Akisu M, Ozmen D, Baka M, et al. Protective effect of dietary supplementation with L-arginine and L-carnitine on hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. Biol Neonate. 2002;81:260–5. doi: 10.1159/000056757. [DOI] [PubMed] [Google Scholar]
- 100.Amin HJ, Zamora SA, McMillan DD, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–31. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]