Abstract
Adenosine is an important neuromodulator in the central nervous system involved in the regulation of wakefulness, sleep, learning and memory, fear and anxiety as well as motor functions. Extracellular adenosine is synthesized by the cell-surface ectoenzyme ecto-5′-nucleotidase (CD73) from 5′-adenosine monophosphate. While CD73 is widely expressed throughout the mammalian brain, its specific role for behaviour is poorly understood. We examined spatial working memory, emotional responses, motor coordination and motor learning as well as behavioural habituation in mice with a targeted deletion of CD73. CD73 knockout (CD73−/−) mice exhibit enhanced spatial working memory in the Y-maze and enhanced long-term behavioural habituation in the open field. Furthermore, impaired psychomotor coordination on the accelerating rotarod was found in CD73−/− mice. No changes in motor learning and/or anxiety-like behaviour were evident in CD73−/− mice. Our data provide evidence for a role of CD73 in the regulation of learning and memory and psychomotor coordination. Our results might be important for the evaluation of adenosine neuromodulators as possible treatments to ameliorate cognitive and motor deficits associated with neurodegenerative diseases.
Keywords: Ecto-5'-nucleotidase, Adenosine, CD73 knockout mice, Spatial working memory, Open-field habituation, Psychomotor coordination
Introduction
Adenosine is an important neuromodulator and homeostatic regulator in the central nervous system (CNS) involved in different physiological and pathological processes including wakefulness and sleep regulation [1, 2]. Adenosine is synthesized from adenosine triphosphate (ATP) that is dephosporylated to ADP and adenosine monophosphate (AMP) by the catalytic action of ecto-nucleoside triphosphate diphosphohydrolase (CD39). Adenosine can be directly released by neurons and glial cells or synthesized from extracellular AMP [3]. Extracellular adenosine is formed via the dephosphorylation of AMP by CD73 [1].
CD73 is a GPI-anchored cell-surface enzyme and belongs to the group of 5′-nucleotidases. While most 5′-nucleotidases are located intracellularly, ecto-5′-nucleotidase (also termed as low Km 5′-NT and CD73) faces the extracellular medium. Ectonucleotidases are widely distributed in the rodent CNS. CD73 catalytic activity has been detected in the hippocampus, inner/outer molecular layer of the dentate gyrus, molecular layer of the cerebellum and the cerebral cortex [4]. CD73 has been predominantly assigned to the surface of glial cells. However, neuronal localization of CD73 has also been reported [4].
Both, ATP and adenosine had been implicated in the modulation of learning and memory as well as synaptic plasticity [5, 6]. Extracellular ATP had been involved in long-term potentiation (LTP) in the hippocampus [7, 8]. Likewise, the activation of the brain’s adenosine A1 and A2a receptors (A1R and A2aR) by adenosine and its analogues inhibits LTP and LTD (long-term depression) [6, 9]. Furthermore, adenosine regulates the release of many neurotransmitters including glutamate, acetylcholine and dopamine [10–12].
It has been reported that ectonucleotidase activity in the brain is dynamically regulated during encoding and memory formation with phase-specific decreases and increases in hippocampal and cortical areas [13–16]. The lack of potent 5′nucleotidase inhibitors (but see [17]), however, prevented so far the study of the specific role of CD73 in these processes.
Recently, CD73 knockout (CD73KO) mice have been successfully generated. CD73KO mice still metabolise ATP to AMP [4, 18], but show a 90 % reduction of ATP to adenosine metabolism [3], and thus reduced endogenous adenosine as well as reduced adenosine receptor sensitivity [18–20]. Given the pivotal role of CD73 as a key regulator of purinergic signalling controlling the extracellular provision of adenosine, CD73KO mice provide a unique opportunity to examine the role of CD73 for wakefulness, sleep, memory and other behaviours. Here, we assessed possible changes in spatial working memory, behavioural habituation, psychomotor coordination and motor learning as well as anxiety-related behaviour in CD73−/− mice by using a broad-spectrum behavioural test battery.
Materials and methods
Animals
Eight CD73 knockout (CD73−/−) and 12 wild-type littermates (CD73+/+) at the age of 6 months were used. CD73−/− mice were generated by homologous recombination and activation of the Cre-loxP system. The targeted disruption of the CD73 gene has been described previously [18]. Two weeks prior to behavioural testing, the mice were housed individually in standard macrolone cages (type 2: 2 × 1 × 13 cm) with metal covers and sawdust bedding and free access to water and standard diet (Ssniff Spezialdiäten, Soest, Germany). Animals were maintained in a closed ventilated cage system (UniProtect Air Flow Cabinet, Ehret Company) under controlled temperature and humidity conditions. A reversed light/dark cycle with lights on between 7:00 a.m. and 7:00 p.m. was used. Experiments were conducted during the light phase. All experiments were performed in accordance with the national guidelines on animal welfare.
Open-field behaviour and behavioural habituation
Locomotor activity, anxiety-related responses and behavioural habituation were evaluated in the open-field test. The experimental protocol used was similar to the one previously described in [21]. The open-field apparatus was constructed of a 30-cm2 grey metal floor surrounded by walls of 40 cm height. The floor of the open field was virtually divided into nine equal-sized squares (10 × 10 cm). The whole apparatus was illuminated by ambient fluorescent ceiling lights and placed in a sound-attenuated chamber. The open-field test consisted of three trials of 10 min duration and a 24-h inter-trial delay. After each trial, the open field was cleaned with a solution of 50 % ethanol and thoroughly dried with paper towels. The animals’ behaviour was videotaped and analysed off-line with a semi-automated tracking device (EthoVision, Noldus, The Netherlands) by an observer blinded to the genotype of the mice. The behavioural parameters quantified during the 10-min-trials were: (1) locomotion: distance moved in cm; (2) centre time: time spent in the central part (10 × 10 cm) of the field; (3) corner time: time spent in the four corner squares (10 × 10 cm); (4) running speed: mean running speed (in centimetres per second) in the entire field and the central part (10 × 10 cm).
Behavioural habituation was determined by calculating a habituation index for each animal according to the procedure proposed by Bolivar [22]. The habituation index is a more sensitive measure of inter-trial open-field habituation that takes baseline differences into account. Hence, as a measure of activity decrease on the last trial relative to the first trial, the following habituation indices were calculated: (1) Habituation locomotion = Locomotion (trial 3) / (Locomotion (trial 3) + Locomotion (trial 1)) and (2) Habituation running speed = Running speed (trial 3) / (Running speed (trial 3) + Running speed (trial 1)). Habituation indices can range from 0.1 to 1.0 while a habituation index of 0.5 indicates no change in activity, and indices close to 0 reflect between-trial habituation.
Spontaneous spatial alternation in the Y-maze
Spontaneous alternation performance in the Y-maze was assessed according to the procedure by Zlomuzica and colleagues [21]. The Y-maze apparatus had an open roof and was constructed of black Plexiglas with three arms (7.5 cm wide × 18 cm long × 23.5 cm high) radiating from a triangle-shaped central platform. A coloured rectangular cue was located at the end of the wall of one arm, while the other two arms were identical and devoid of intra-arm cues. The Y-maze was placed in a sound-attenuated experimental chamber. Each animal received one trial of 5 min duration. A trial began with placing the animal on the central platform, allowing it to freely explore the three arms. The apparatus was cleaned before each trial with 50 % ethanol solution. An arm entry was scored when the mouse entered an arm with all four paws. The following parameters were calculated during 5-min trials: (1) total number of entries; (2) number of triplets: number of consecutive choices of each of the three arms, without re-entries during the last three choices and irrespective of the order of the chosen arms; (3) locomotion: total distance (in centimetres) travelled in the Y-maze; and (4) mean running speed in the Y-maze (in centimetres per second).
Psychomotor coordination and motor learning
Psychomotor coordination and motor learning were assessed with an accelerating rotarod (TSE systems; Bad Homburg, Germany). The rotating rod was made of black rubber, had an axis diameter of 3.5 cm and was elevated 10 cm off the floor. A total of six trials were performed on two subsequent days for each mouse (trials 1–3 on day1 and trials 4–6 on day 2). To control for possible effects of physical exhaustion, an inter-trial delay of 25–30 min was interposed between the three trials of a day. Each trial began with placing the mouse on the inactive drum, which thereafter was switched to an accelerating mode (increasing the speed to 40 rpm over a period of 5 min). The duration (in seconds) of active performance until the mouse fell off the drum was registered for each trial.
Anxiety-related behaviour in the light–dark box
The light–dark box was made of Plexiglas and consisted of a dark (24 cm width × 33 cm length × 30 cm height) as well as a white compartment with identical dimensions. An opening (10 cm width × 10 cm height) connected the dark with the white compartment. The white compartment was illuminated by a 60-W lamp providing a white light illumination of approximately 250 lx at the centre of the white compartment. The illumination density in the dark compartment was approximately 10 lx. Each mouse was individually placed in the light compartment. The following data were assessed: (1) latency to escape to the dark compartment (in seconds) and (2) total time spent (in seconds) in the light or dark compartments over 5 min. An entry into a compartment was scored after the mouse had entered the compartment with all four paws. After each trial, the apparatus was cleaned with a 50 % ethanol solution and dried with paper towels.
Statistical analysis
All data are expressed as mean ± SEM. Open field and rotarod data were analysed either by means of a one-way ANOVA or repeated measures ANOVA. Student’s T test for single groups was used to determine whether habituation indices were significantly different from a chance level value of 0.5. All other data were analysed by Student’s T test for dependent and independent groups. The P values given are two-tailed and were considered to be significant when P values lower than 0.05 were obtained.
Results
Open-field test
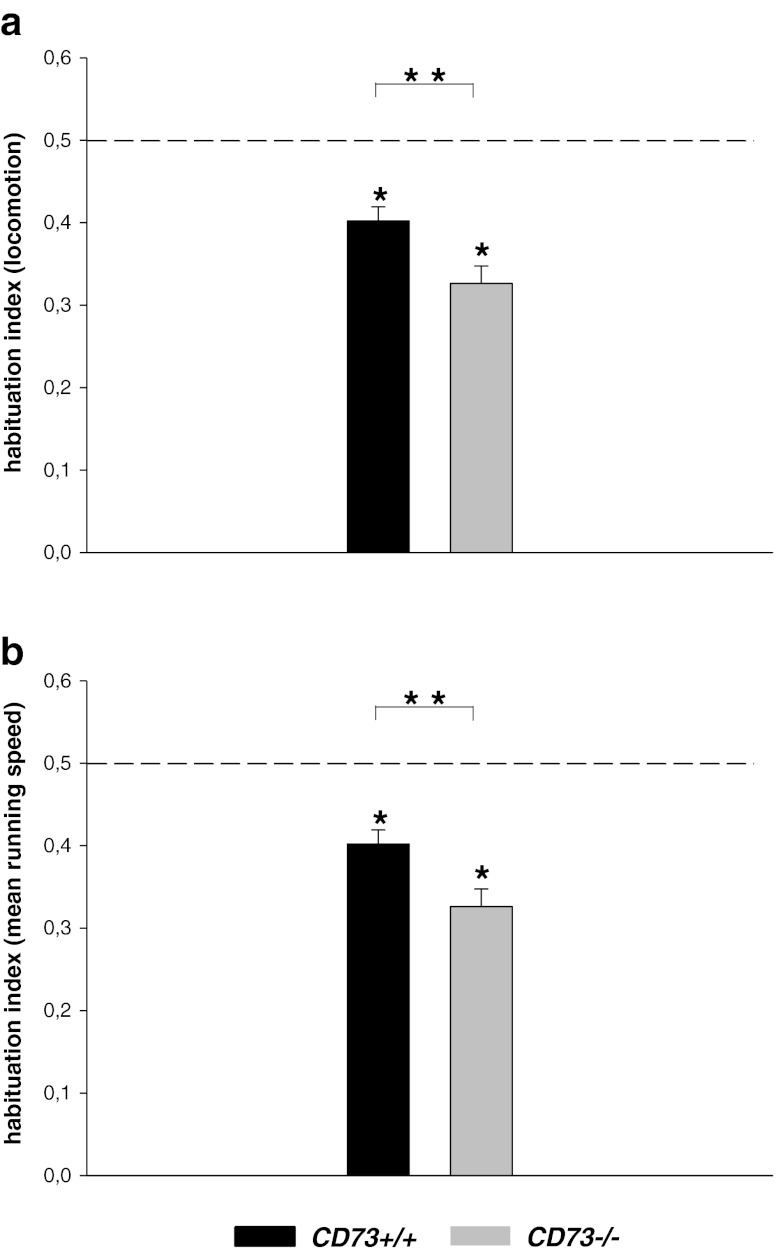
There were no significant differences for the open-field measures between CD73+/+ and CD73−/− mice (all P values > .05, see Table 1, Fig. 1) suggesting no changes in locomotor activity and/or anxiety-like responses in the open field in CD73−/− mice. CD73+/+ mice showed a habituation index for locomotion (0.40 ± 0.01 (mean ± sem)) and mean running speed (0.40 ± 0.01 (mean ± sem)) which was significantly smaller compared to a habituation index of 0.5 (Habituation locomotion: P = .001; Habituation running speed: P = .001; Student T test for single groups). These data suggest intact behavioural habituation to the open field in CD73+/+ mice. Likewise, the habituation index for locomotion (0.32 ± 0.02 (mean ± sem)) and mean running speed (0.32 ± 0.02 (mean ± sem)) in CD73−/− mice were significantly different from chance level (Habituation locomotion: P < .0001; Habituation running speed: P < .0001), suggesting no deficits in behavioural habituation to the open field. Furthermore, both the habituation index for locomotion (P = .012) and the habituation index for mean running speed (P = .012, T test for independent samples) were significantly smaller in CD73−/− mice compared to CD73+/+ mice, suggesting enhanced behavioural habituation in the open field in CD73−/− mice.
Table 1.
Locomotor activity and anxiety-like responses in the open field in CD73−/− mice. All data are expressed as mean (±sem)
| Open field parameters | Day | CD73+/+ | CD73−/− |
|---|---|---|---|
| Distance moved in cm | D1 | 1623. 03 (± 69.79) | 1851.94 (± 106.63) |
| D2 | 1144.49 (± 110.20) | 1126.73 (± 95.43) | |
| D3 | 1092.34 (± 63.43) | 916.16 (± 108.25) | |
| Mean running speed (cm/s) in the entire field | D1 | 5.41 (± 0.23) | 6.17 (± 0.35) |
| D2 | 3.81 (± 0.36) | 3.75 (± 0.31) | |
| D3 | 3.64 (± 0.21) | 3.05 (± 0.36) | |
| Time spent in the corners (s) | D1 | 157.6 (± 10.27) | 161.62 (± 8.11) |
| D2 | 147.13 (± 13.64) | 184.02 (± 15.06) | |
| D3 | 169.2 (± 11.88) | 197.87 (± 16.27) | |
| Time spent in the centre (s) | D1 | 43.26 (± 8.59) | 37.27 (± 6.02) |
| D2 | 41.43 (± 9.44) | 20.45 (± 4.63) | |
| D3 | 27.4 (± 8.33) | 13.6 (± 5.89) | |
| Mean running speed (cm/s) in the centre | D1 | 6.27 (± 0.55) | 7.52 (± 1.04) |
| D2 | 4.66 (± 1.03) | 6.04 (± 1.02) | |
| D3 | 7.61 (± 1.48) | 2.56 (± 0.41) |
Fig. 1.
Behavioural habituation in the open field in CD73KO mice. aBars represent mean (±sem) habituation index for locomotion. bBars represent mean (±sem) habituation index for mean running speed. Dashed line represents a habituation index of 0.5 (which means no behavioural habituation across the days, see “Materials and methods” section). The asterisk indicates p < 0.01, significantly different from a habituation index of 0.5; double asterisks indicate p < 0.05, Student T test for independent samples
Spatial working memory performance in the Y-maze
The CD73−/− mice showed an increased number of triplets compared to CD73+/+ mice in the spatial alternation task (T(18) = −2.20, P < .05; T test for independent samples; Fig. 2a). The increased number of triplets was presumably not due to increased exploratory locomotion in the Y-maze since there was no difference in the number of entries between CD73−/− and CD73+/+ mice (P = .20; T test for independent samples; Fig. 2b) and no genotype difference in locomotion (T(18) = −0.77, P = .44; T test for independent samples, Fig. 2c) or mean running speed (P > .05, Fig. 2d). These results suggest that spatial working memory performance is enhanced in CD73−/− mice.
Fig. 2.

Spontaneous spatial alternation performance in the Y-maze. aBars represent (± sem) number of triplets, bBars represent (± sem) number of entries, cBars represent mean (± sem) locomotion (distance moved in centimetres), dBars represent mean (±sem) running speed (in centimetres per second). The asterisk indicates p < 0.05; Student T test for independent samples
Psychomotor coordination and motor learning
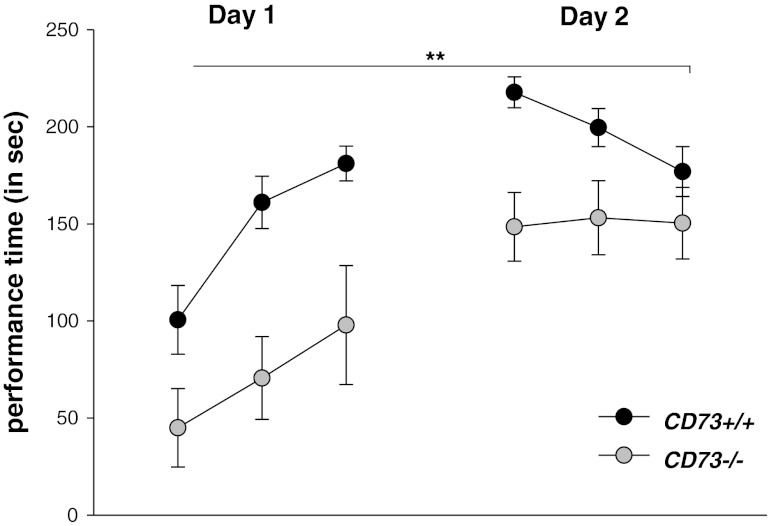
In both genotypes, the duration of active performance on the accelerating rotarod increased over the trials (Main effect of trials: F(5,90) = 24.51, P > .0001; repeated measures ANOVA) suggesting that, irrespective of genotype, the mice improved their coordination and balancing performance across the trials. No significant genotype × trial interaction for the active performance time on the rotarod was evident (F(5,90) = 1.98, P > .05). However, a significant genotype difference was found (main effect of genotype: F(1,18) = 13.08, P = .002, Fig. 3), indicating impaired motor coordination and balancing functions in CD73−/− mice.
Fig. 3.
Psychomotor coordination and motor learning. Circles represent mean (± sem) performance time (in seconds) on the accelerating rotarod on the indicated trials during day 1 and day 2 of acquisition. The double asterisks indicate p < 0.01, main effect of genotype, repeated measures ANOVA
Anxiety-related behaviour in the light–dark box
One CD73−/− mouse was excluded from data analysis due to insufficient exploratory behaviour in the light–dark box. Hence, the following analyses in the light–dark box were based on seven CD73−/− and 12 CD73+/+ mice. Both, CD73−/− and CD73+/+ mice, spent significantly more time in the dark compartment than in the white compartment (CD73−/− mice: T(6) = −12.18, P < .001; CD73+/+ mice: T(11) = −5.82, P < .001, Student’s T test for dependent measures; Table 2), suggesting that the white compartment induced anxiety-related responses in mice. There was no significant genotype difference for the time spent in the dark (T(17) = −0.67, P > .05; T test for independent samples) or white compartment (P > .05) between CD73−/− and CD73+/+ mice. Furthermore, the latency to escape to the white compartment was comparable in both genotypes (T(17) = 1.02, P = .31). These data suggest no significant changes in anxiety-related behaviour in the light–dark box in CD73−/− mice relative to CD73+/+ mice.
Table 2.
Anxiety-related behaviour in the light–dark box did not differ between CD73−/− and CD73+/+ mice
| Latency to enter the dark chamber | Time spent in the dark chamber | Time spent in the light chamber | |
|---|---|---|---|
| CD73−/− | 13.5 ± 3.5 | 228.7 ± 6.4 | 71.2 ± 6.4a |
| CD73+/+ | 26.0 ± 8.9 | 217.8 ± 11.6 | 82.1 ± 11.6a |
Mean (±sem) time spent (in seconds) in the two chambers and the latency to enter the dark chamber (in seconds) for CD73−/− (n = 7) and CD73+/+ (n = 12) mice
aTime spent in the light vs. the dark chamber, p < 0.05, one-tailed T test for paired data
Discussion
In this study, we - for the first time - provide evidence for specific behavioural alterations in mice with a targeted deletion of the CD73 gene. CD73−/− mice exhibited improved short-term spatial working memory performance in the Y-maze task and enhanced long-term open-field habituation relative to control mice. Conversely, a selective impairment in psychomotor coordination performance in the rotarod task was found in CD73−/− mice.
It has been shown that CD73−/− mice exhibit reduced adenosine formation and adenosine receptor activity [18–20]. The hippocampus exhibits high levels of G-protein coupled A1R and A2R [23]. Lesion and inactivation studies identified the hippocampus as an essential structure for the formation of spatial working memory [24]. The A1R has been implicated in putative cellular correlates of learning and memory formation such as LTP [25, 26] and LTD [6]. A2aR receptors are located at the mossy fibre-CA3 pyramidal cell and at the CA3-CA1 synapses which might also be relevant for synaptic plasticity [27, 28]. Pharmacological blockade of A1R ameliorates scopolamine-induced spatial memory deficits [29] and facilitates memory for inhibitory avoidance in rats [30]. A2R antagonism has been shown to block memory deficits induced by beta-amyloid peptides [31]. Similarly, A2aR KO mice showed enhanced working memory in the water maze and the radial maze tasks [32] and the genetic deletion of A2aR in the forebrain or striatum improved spatial working memory [33]. The selective enhancement of spatial working memory in A2aR deficient mice is in line with the improved spatial working memory performance of CD73−/− mice described in the present report. In rats, decreased ATP hydrolyzation was measured during the formation of a memory for an aversive event [13, 14]. A1R and A2R related agents [34] as well as inhibitors of nucleoside metabolism [35] have been proposed as possible procognitive treatments for neurodegenerative disorders including Alzheimer’s disease. While these findings suggest that the memory facilitation in CD73−/− mice might be correlated with decreased extracellular adenosine levels, opposed findings do also exist. For instance, increases in ATP levels and AMP hydrolysis were observed during the habituation to an open field in rats [15], which is in contrast to the present finding of enhanced behavioural habituation in the open field in CD73−/− mice. In order to know whether extracellular adenosine levels are indeed correlated with behavioural habituation in the open-field test, it would be necessary to combine the open-field test with in vivo microdialysis measurements of adenosine levels in the hippocampus and striatum of CD73−/− mice. However, it should also taken into account that, given the important role of ectonucleotidase during brain development and synapse maturation [36], possible compensational changes in the brains purinergic system of CD73 deficient mice might account for the discrepant results.
In addition to changes in memory performance, we also observed impaired rotarod performance in CD73−/− mice. High levels of A1R and A2aR have been detected in the cortico-striato-cerebellar pathway, which is important for the learning of psychomotor skills [37, 38]. A1R and A2aR can form heteromeric complexes with dopamine D2 and metabotropic glutamate mGlu5 receptors [39]. Ethanol-induced motor incoordination on the rotarod in rats can be attenuated via A1R-dependent mechanisms [40]. Likewise, striatal A1R and A2R activation induces rotational behaviour in response to dopaminergic stimulation [41]. Adenosine neuromodulation, particularly via A2aR in striatopallidal neurons, has been shown to reduce postsynaptic effects of dopamine depletion and ameliorate motor deficits in animal models of Parkinson’s disease (PD) [42, 43]. The activation of A2a/D2 and/or A2a/mGluR5 receptor heteromeres, which in turn affect D2 and mGluR5 receptor signalling, might account for the ameliorating effects of A2R antagonism on psychomotor symptoms in animal models of PD [39, 42, 43]. These and other studies support evidence for an important role of adenosine system in the control of striatal and cerebellar dependent psychomotor functions. CD73−/− mice display a >90 % reduction of adenosine synthesis, a reduced spike-dependent adenosine release and an inhibition of synaptic transmission by ATP in the cerebellum [3]. The latter finding is of special importance, since extracellular ATP acts as a fast transmitter in motor learning and psychomotor coordination [44].
The stimulation of presynaptic A1R decreases glutamate, acetylcholine, noradrenaline, 5-HT and dopamine release, while the stimulation of A2aR increases the release of glutamate and acetylcholine and decreases the release of GABA [45]. It is (in addition to the possible mechanisms suggested above) also possible that the behavioural effects of CD73 deficiency described here are due to modulation of neurotransmission in brain regions important for cognitive processes and motor function. As a perspective for follow-up studies, the contribution of specific adenosine receptors to the behavioural phenotypes of CD73−/− mice could be dissected using selective adenosine receptor related agents.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) trough grant no. DFG-DE 1149/6-1 to ED.
Abbreviations
- ADP
Adenosine diphosphate
- AMP
Adenosine monophosphate
- ATP
Adenosine triphosphate
- CNS
Central nervous system
- GPI
Glycosylphosphatidylinositol
- LTD
Long-term depression
- LTP
Long-term potentiation
References
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem. 2011;11:1047–57. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- 3.Klyuch BP, Dale N, Wall MJ. Deletion of ecto-5′-nucleotidase (CD73) reveals direct action potential-dependent adenosine release. J Neurosci. 2012;32:3842–3847. doi: 10.1523/JNEUROSCI.6052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–74. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 6.de Mendonça A, Almeida T, Bashir ZI, Ribeiro JA. Endogenous adenosine attenuates long-term depression and depotentiation in the CA1 region of the rat hippocampus. Neuropharmacology. 1997;36:161–7. doi: 10.1016/S0028-3908(96)00173-6. [DOI] [PubMed] [Google Scholar]
- 7.Wieraszko A, Ehrilch YH. On the role of extracellular ATP in the induction of long-term potentiation in the hippocampus. J Neurochem. 1994;63:1731–1738. doi: 10.1046/j.1471-4159.1994.63051731.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujii S. ATP- and adenosine-mediated signaling in the central nervous system: the role of extracellular ATP in hippocampal long-term potentiation. J Pharmacol Sci. 2004;94:103–106. doi: 10.1254/jphs.94.103. [DOI] [PubMed] [Google Scholar]
- 9.de Mendonça A, Ribeiro JA. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience. 1994;62:385–390. doi: 10.1016/0306-4522(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 10.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/S0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm BB. Purinoceptors in the nervous system. Pharmacol Toxicol. 1995;76:28–39. doi: 10.1111/j.1600-0773.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol. 1988;9:130–34. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 13.Bonan CD, Dias MM, Battastini AMO, Dias RD, Sarkis JJF. Inhibitory avoidance learning inhibits ectonucleotidase activities in hippocampal synaptosomes of adult rats. Neurochem Res. 1998;23:979–984. doi: 10.1023/A:1021084422228. [DOI] [PubMed] [Google Scholar]
- 14.Bonan CD, Roesler R, Pereira GS, Battastini AMO, Izquierdo I, Sarkis JJF. Learning-specific decrease in synaptosomal ATP diphosphohydrolase activity from hippocampus and entorhinal cortex of adult rats. Brain Res. 2000;854:253–256. doi: 10.1016/S0006-8993(99)02300-8. [DOI] [PubMed] [Google Scholar]
- 15.Pedrazza EL, Riboldi GP, Pereira GS, Izquierdo I, Bonan CD. Habituation to an open field alters ecto-nucleotidase activities in rat hippocampal synaptosomes. Neurosci Lett. 2007;413:21–4. doi: 10.1016/j.neulet.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Pereira GS, Mello e Souza T, Battastini AM, Izquierdo I, Sarkis JJ, Bonan CD. Effects of inhibitory avoidance training and/or isolated foot-shock on ectonucleotidase activities in synaptosomes of the anterior and posterior cingulate cortex and the medial precentral area of adult rats. Behav Brain Res. 2002;128:121–7. doi: 10.1016/S0166-4328(01)00312-6. [DOI] [PubMed] [Google Scholar]
- 17.Baqi Y, Lee SY, Iqbal J, Ripphausen P, Lehr A, Scheiff AB, Zimmermann H, Bajorath J, Müller CE. Development of potent and selective inhibitors of ecto-5′-nucleotidase based on an anthraquinone scaffold. J Med Chem. 2010;53:2076–86. doi: 10.1021/jm901851t. [DOI] [PubMed] [Google Scholar]
- 18.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flogel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sevigny J, Gear A, et al. Targeted disruption of CD73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 19.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zlomuzica A, Viggiano D, De Souza Silva MA, Ishizuka T, Gironi Carnevale UA, Ruocco LA, Watanabe T, Sadile AG, Huston JP, Dere E. The histamine H1-receptor mediates the motivational effects of novelty. Eur J Neurosci. 2008;27:1461–74. doi: 10.1111/j.1460-9568.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol Learn Mem. 2009;92:206–14. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochiishi T, Saitoh Y, Yukawa A, Saji M, Ren Y, Shirao T, Miyamoto H, Nakata H, Sekino Y. High level of adenosine A1 receptor-like immunoreactivity in the CA2/CA3a region of the adult rat hippocampus. Neurosci. 1999;93:955–967. doi: 10.1016/S0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- 24.Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–46. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- 25.Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujii S, Kato H, Ito K, Itoh S, Yamazaki Y, Sasaki H, Kuroda Y. Effects of A1 and A2 adenosine receptor antagonists on the induction and reversal of long-term potentiation in guinea pig hippocampal slices of CA1 neurons. Cell Mol Neurobiol. 2000;20:331–50. doi: 10.1023/A:1007014226224. [DOI] [PubMed] [Google Scholar]
- 27.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Fontinha BM, Delgado-García JM, Madroñal N, Ribeiro JA, Sebastião AM, Gruart A. Adenosine A(2A) receptor modulation of hippocampal CA3-CA1 synapse plasticity during associative learning in behaving mice. Neuropsychopharmacology. 2009;34:1865–74. doi: 10.1038/npp.2009.8. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki F, Shimada J, Shiozaka S, Ichikawa S, Ishii A, Nakamura J, Nonaka H, Kobayashi H, Fuse E. Adenosine A1 antagonists. 3. Structure activity relationships on amelioration against scopolamine- or N6-((R)-phenylisopropyl) adenosine-induced cognitive disturbance. J Med Chem. 1993;36:2508–2518. doi: 10.1021/jm00069a009. [DOI] [PubMed] [Google Scholar]
- 30.Pereira GS, Mello e Souza T, Vinadé ER, Choi H, Rodrigues C, Battastini AM, Izquierdo I, Sarkis JJ, Bonan CD. Blockade of adenosine A1 receptors in the posterior cingulate cortex facilitates memory in rats. Eur J Pharmacol. 2002;437:151–4. doi: 10.1016/S0014-2999(02)01307-9. [DOI] [PubMed] [Google Scholar]
- 31.Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–81. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, Zheng RY, Cai XH, Chen JF, He JC. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 33.Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, Chen JF. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–74. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–32. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- 35.Boison D. Modulators of nucleoside metabolism in the therapy of brain diseases. Curr Top Med Chem. 2011;11:1068–86. doi: 10.2174/156802611795347609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanojević I, Bjelobaba I, Nedeljković N, Drakulić D, Petrović S, Stojiljković M, Horvat A. Ontogenetic profile of ecto-5′-nucleotidase in rat brain synaptic plasma membranes. Int J Dev Neurosci. 2011;29:397–403. doi: 10.1016/j.ijdevneu.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Inhoff AW, Rafal R. Cerebellar structures and the programming of movement sequences. Behav Neurol. 1990;3:87–97. doi: 10.3233/BEN-1990-3203. [DOI] [PubMed] [Google Scholar]
- 38.Laforce R, Jr, Doyon J. Distinct contribution of the striatum and cerebellum to motor learning. Brain Cogn. 2001;45:189–211. doi: 10.1006/brcg.2000.1237. [DOI] [PubMed] [Google Scholar]
- 39.Ferré S, Ciruela F, Quiroz C, Luján R, Popoli P, Cunha RA, Agnati LF, Fuxe K, Woods AS, Lluis C, Franco R. Adenosine receptor heteromers and their integrative role in striatal function. Scientific World Journal. 2007;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dar MS. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Res Bull. 2001;55:513–20. doi: 10.1016/S0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 41.Popoli P, Pèzzola A, Scotti de Carolis A. Modulation of striatal adenosine A1 and A2 receptors induces rotational behaviour in response to dopaminergic stimulation in intact rats. Eur J Pharmacol. 1994;257:21–5. doi: 10.1016/0014-2999(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 42.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–54. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in Parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol. 2007;83:293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Brockhaus J, Dressel D, Herold S, Deitmer JW. Purinergic modulation of synaptic input to Purkinje neurons in rat cerebellar brain slices. Eur J Neurosci. 2004;19:2221–2230. doi: 10.1111/j.0953-816X.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- 45.Sperlágh B, Vizi ES. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and basal ganglia: pharmacological and clinical aspects. Curr Top Med Chem. 2011;11:1034–46. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]