Abstract
The role of the A2B adenosine receptor (AR) in prostate cell death and growth was studied. The A2B AR gene expression quantified by real-time quantitative RT-PCR and Western blot analysis was the highest among four AR subtypes (A1, A2A, A2B, and A3) in all three commonly used prostate cancer cell lines, PC-3, DU145, and LNCaP. We explored the function of the A2B AR using PC-3 cells as a model. The A2B AR was visualized in PC-3 cells by laser confocal microscopy. The nonselective A2B AR agonist NECA and the selective A2B AR agonist BAY60-6583, but not the A2A AR agonist CGS21680, concentration-dependently induced adenosine 3′,5′-cyclic monophosphate (cyclic AMP) accumulation. NECA diminished lactate dehydrogenase (LDH) release, TNF-α-induced increase of caspase-3 activity, and cycloheximide (CHX)-induced morphological changes typical of apoptosis in PC-3 cells, which were blocked by a selective A2B AR antagonist PSB603. NECA-induced proliferation of PC-3 cells was diminished by siRNA specific for the A2B AR. The selective A2B AR antagonist PSB603 was shown to inhibit cell growth in all three cell lines. Thus, A2B AR blockade inhibits growth of prostate cancer cells, suggesting selective A2B AR antagonists as potential novel therapeutics.
Keywords: Prostate cancer, Cancer, Adenosine receptor, A2B, G protein-coupled receptor (GPCR), Cell proliferation
Introduction
Prostate cancer is one of the most frequently diagnosed cancers in man [1], but the means of treatment of this disease is still limited. G protein-coupled receptors (GPCRs) are emerging targets for cancer [2, 3]. We recently reported that agonists of the G protein-coupled P2Y1 receptor could be potential novel drugs for prostate cancer [4].
It has been reported that the A2B adenosine receptor (AR) is one of those most abundantly expressed in the human prostate cancer cells [5]. However, the role of the A2B AR in prostate cancer has not previously been well explored, although it has been studied in several other types of tumors [6–9]. For example, Ryzhov et al. [8], using A2B AR knockout mice, demonstrated that host A2B AR promoted the growth of Lewis lung carcinoma. Ma et al. [6] demonstrated that A2B AR activation promoted colon carcinoma cell growth, which was significantly inhibited by a selective A2B AR antagonist, MRS1754. Stagg et al. [7] demonstrated both anti-CD73 antibody and a selective A2B antagonist PSB1115 slowed the growth of breast tumors. Cekic et al. [9] demonstrated that A2B AR antagonists, aminophylline (nonselective) and ATL801 (selective), blocked the growth of both breast tumors and bladder tumors.
In the present study, we found that the expression level of the A2B AR was the highest among the four AR subtypes in three commonly used prostate cancer cell lines. We subsequently studied the A2B AR signaling in regulating cell death and growth using PC-3 cells as a model. We further examined the role of the A2B AR in proliferation of the three most commonly used cell lines, PC-3 (androgen-independent), DU145 (androgen-independent), and LNCaP (androgen-dependent), using a selective A2B AR antagonist PSB603.
Materials and methods
Materials
Lactate Dehydrogenase (LDH) Assay Kit was purchased from Roche Applied Science (Indianapolis, IN, USA). XTT-based Toxicology Assay Kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). Caspase-3 Colorimetric Detection Kit was from Assay Designs (Ann Arbor, MI, USA). NECA (5′-N-ethylcarboxamidoadenosine), CGS21680 (2-[p-(2-carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine), TNF-α (tumor necrosis factor-alpha), cycloheximide (CHX), and doxorubicin (DOX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). MRS2365 ([[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxy-bicyclo[3.1.0]hex-1-yl]methyl]diphosphoric acid mono ester trisodium salt) and PSB603 (8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine) were purchased from Tocris Bioscience (R&D Systems, Inc., Minneapolis, MN, USA). Predesigned small interfering RNA (siRNA) for the A2B AR, negative control siRNA, and SYBR Green reagents were purchased from Applied Biosystems (Foster City, CA, USA). Annexin-FITC was from BD Biosciences (San Diego, CA, USA). LUF6210 (BAY60-6583) was synthesized at Leiden University, The Netherlands. All other reagents were from standard sources and were of analytical grade.
Cell culture
Human prostatic carcinoma cells, PC-3, DU145, and LNCaP (American Type Culture Collection (ATCC), Manassas, VA, USA), were cultured at 37 °C in a humidified incubator with 5 % CO2 in RPMI-1640 medium supplemented with 10 % FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 3 mM/l glutamine. Cells used in the present study were within 20 passages after purchasing from ATCC.
Detection of AR gene expression
Total mRNA in cells was isolated following the protocol of the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Reverse transcription was completed using Superscript III First Strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA, USA). The cDNA was then amplified by PCR with gene-specific primers for ARs and GAPDH on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol using SYBR Green PCR Master Mix. Amplification parameters were as follows: 1 cycle of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The primers were synthesized by Eurofins MWG Operon (Huntsville, AL, USA) and the sequences are as follows: A1 AR: forward 5′-CTA CCT AAT CCG CAA GCA GC-3′; reverse 5′-GTC ATC AGG CCT CTC TTC TGG-3′; A2A AR: forward 5′-AAC CTG CAG AAC GTC ACC A-3′; reverse 5′-GTC ACC AAG CCA TTG TAC CG-3′; A2B AR, forward 5′-GTG CCA CCA ACA ACT GCA CAG AAC-3′; reverse 5′-CTG ACC ATT CCC ACT CTT GAC ATC-3′; A3 AR, forward 5′-CAC CAC CTT CTA TTT CAT TGT CTC T-3′; reverse 5′-GGT ACT CTG AGG TCA GTT TCA TGT T-3′; and human GAPDH, forward 5′-ATT CCA TGG CAC CGT CAA GGCT-3′; reverse 5′-TCA GGT CCA CCA CTG ACA CGT T-3′. Quantitative analysis of data was performed using the ∆∆Ct method [10]. Values were normalized to GAPDH and were expressed as relative expression levels.
Measurement of cell growth
The assay method was previously described [4]. Briefly, cells were seeded in 96-well plates at a concentration of approximately 5,000 cells per well in 100 μl medium and cultured at 37 °C overnight. The A2B AR agonist or antagonist was incubated with cells at 37 °C for 24, 48, and 72 h. Absorbance was measured at 450 nm using XTT Toxicology Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer's protocol and converted to cell numbers based on a standard curve (cell numbers vs. absorbance values). The A2B siRNA (1 μM) was transfected at about 60–80 % cell confluency using Lipofectamine 2000 transfection reagent as instructed by the manufacturer (Invitrogen, Carlsbad, CA, USA).
Detection of the A2B AR using laser confocal microscopy
PC-3 cells were seeded on cover slips in six-well plates and grown for 48 h. Cells were then incubated with MitoTracker Red (Molecular Probes) for 45 min and washed with PBS, and fixed with cold fix solution (50 % methanol/50 % acetone) for 20 min at −20 °C. Cells were blocked with 10 % FBS for 1 h at room temperature, after washing with PBS. Anti-A2B AR antibody (Alomone Labs, Ltd., Israel) was added at 1:200 dilution and incubated with cells for 1 h. Cells were then washed three times with PBS before adding Alexa fluor-488 goat antirabbit IgG (Invitrogen, Carlsbad, CA, USA) and incubated for another 1 h. This was followed by washing three times with PBS and mounting with ProLong® Gold antifade mounting reagent with DAPI (Invitrogen, Carlsbad, CA, USA). Fluorescence images were obtained with a laser scanning Zeiss LSM-510 Meta Confocal Microscope (Carl Zeiss Inc., Jena, Germany).
Western blot analysis
Cells of about 80 % confluence were lysed using cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) and the cell lysates were stored at −80 °C. Protein concentration was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Cell lysates (30 μg protein/well) were analyzed under reducing conditions by SDS-PAGE and proteins were separated on 12 % Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose membrane by electroblotting. Membranes were blocked according to the manufacturer's instructions and probed with specific antibodies overnight at 4 °C. Subsequently, blots were probed with IRdye-conjugated secondary antibody for 1 h and then analyzed using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). In case of blots probed with control antigen of the respective antibody, both antigen and antibody were premixed and incubated for 1 h with constant agitation, and this ag–ab mixture was used as control for the respective AR antibody.
Cyclic AMP accumulation assay
Cells were planted in 96-well plates in 0.1 ml medium. After overnight incubation, the medium was removed and cells were washed three times with 0.1 ml DMEM, containing 50 mM HEPES, pH 7.4. Cells were then treated with the test agonists in the presence of rolipram (10 μM) and adenosine deaminase (3 U/ml) for 30 min. The antagonist was added 20 min before the addition of the agonist. The reaction was terminated by removing the supernatant, and cells were lysed upon the addition of 100 μl of 0.1 M ice-cold HCl. For determination of adenosine 3′,5′-cyclic monophosphate (cyclic AMP) production, the Sigma Direct cyclic AMP Enzyme Immunoassay kit was used following the instructions provided with the kit. The absorbance was measured with a microplate reader at 405 nm.
Assay of LDH release
Cells were first cultured in 24-well plates overnight with complete medium and then replaced with 500 μl fresh medium containing 1 % serum. The A2B AR agonist NECA was added 20 min before CHX (10 μg/ml) or DOX (10 μM) and the mixture was incubated at 37 °C for 24 h. If an antagonist was used, it was incubated with the cells 20 min prior to the addition of agonists. For the measurement, the culture medium was first centrifuged, and supernatant was carefully transferred to the corresponding wells of an optically clear 96-well flat bottom microplate in triplicate. LDH activity was measured using a LDH Cytotoxicity Detection kit (Roche Applied Science, Indianapolis, IN, USA) following the manufacturer's instructions. The absorbance of the samples was measured at 490 nm using SpectraMax5 Microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Measurement of caspase-3 activity
PC-3 cells were plated in six-well plates at a density of 2 × 105 cells per well and cultured to 80–90 % confluence. The cells were treated with TNF-α in the absence or presence of A2B AR agonist NECA for 8 h and were then lysed in RIPA lysis buffer (Thermo Fisher Scientific, Rockford, IL, USA) containing protease inhibitors, and the protein concentration was quantified. Caspase-3 activity was measured using the Caspase-3 Colorimetric Assay Kit (Enzo Life Sciences, Plymouth Meeting, PA, USA) following the manufacturer's instructions. Each sample contained 50–200 μg protein, and the absorbance in each well was measured at 405 nm using a microplate reader.
Detection of apoptosis of PC-3 cells using laser scanning confocal microscopy
The method used was as previously described by Blom et al. [11]. In brief, PC-3 cells were grown in two-chambered coverglass system (Nunc, Rochester, NY, USA) until 70–80 % confluence. The cells were washed with PBS and the medium was changed to RPMI medium with 1 % serum. Cells were incubated in the presence or absence of the A2B AR antagonist PSB-603 (1 μM) for 20 min followed by treatment with 1 μM of NECA for another 20 min. CHX (100 μM) was then added to the cell medium with reduced serum, and the cells were incubated for 6 h at 37 °C. After washing twice with PBS, cells were stained with Annexin-FITC (BD Biosciences, San Diego, CA, USA) for 15 min at room temperature. After staining, cells were washed once with PBS and microscopy proceeded. Cells were analyzed using a laser scanning confocal live cell imaging system (LSM 5 Live, Carl Zeiss, Germany).
Statistical analysis
EC50 values were calculated with Prism 5 (GraphPad, San Diego, CA, USA). Data were analyzed by analysis of variance (ANOVA) (followed by post hoc analysis) or via Student's t test to check the statistical difference among groups with P value less than 0.05 being considered significant. Results were expressed as mean ± SE.
Results
Gene expression levels of four subtypes of ARs in three prostate cancer cell lines
The gene expression levels of four AR subtypes, A1, A2A, A2B, and A3, in three prostate cancer cell lines, PC-3 (Fig. 1a), DU145 (Fig. 1b), and LNCaP (Fig. 1c), were compared using real-time quantitative RT-PCR analysis. The expression level of the A2B AR was the highest among four subtypes of ARs in all three cell lines (A2B > A2A > A1, A3). However, there is a quantitative difference in the A2A AR expression relative to that of the A2B AR in these three cell lines. The expression of the A2A AR was about 30-, five-, and onefold lower than A2B AR in PC-3, DU145, and LNCaP cells, respectively. Expression of A1 and A3 ARs was detectable but was shown to be much lower than that of the A2B AR.
Fig. 1.
a, b, and c Gene expression level of the A2B AR in comparison to A1, A2A, and A3 ARs in PC-3 (a), DU145 (b), and LNCaP (c) human prostate cancer cells. Total RNA was extracted and reverse-transcripted to cDNA and then amplified with gene-specific primers for ARs or GAPDH on a 7900HT Fast Real-Time PCR System. Results are expressed as mean ± SE from three separate experiments. Values of each experiment were normalized using GAPDH as an endogenous control and were expressed as relative expression levels. Quantitative analysis of data was performed using the ∆∆Ct method. The expression level of the A2B AR was expressed as 1. d Localization of the A2B AR in PC-3 cells by confocal laser scanning microscopy. Red MitoTracker Red (mitochondria), green Alexa Fluor-488 (A2B AR), blue DAPI (nucleus)
Detection of the A2B AR using laser confocal microscopy
The A2B ARs in PC-3 cells were visualized using laser confocal microscopy. Figure 1d shows that the staining by the anti-A2B AR antibody (green color) was localized mainly on the surface of the PC-3 cells.
Western blot analysis with all the four AR antibodies revealed that A2A and A2B antibodies were highly expressed followed by low expression of A3 and A1 ARs (Fig. 2). This result correlates with our findings from gene expression analysis.
Fig. 2.
Western blot analysis of the expression of all four subtypes of adenosine receptors A1, A2A, A2B, and A3 in all three prostate cancer cell lines used in the study. In each figure, left side blot was probed with the specified AR antibody alone and the right side panel was probed with a mixture of specific antigen + antibody and used as control. Lane 1 molecular weight marker, lane 2 cell lysates of DU145, lane 3 LNCaP, lane 4 PC3. Arrows indicate the approximate molecular weight of monomeric ARs
Cyclic AMP accumulation induced by agonists for A2B and A2A ARs
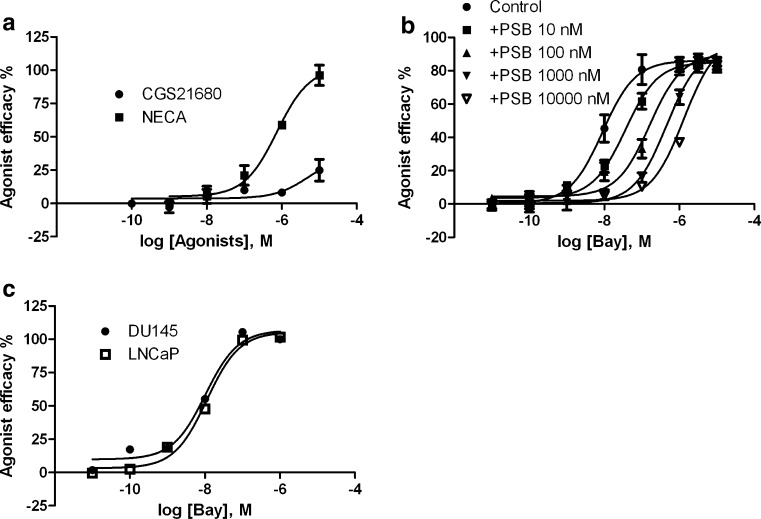
Both A2A and A2B ARs are Gs-coupled receptors and mediate activation of adenylyl cyclase and subsequent accumulation of cyclic AMP in cells. To confirm the functional role of the A2B AR in PC-3 cells, we first compared the ability of a nonselective A2B agonist NECA and an A2A-selective agonist CGS21680 to induce accumulation of cyclic AMP. Figure 3a shows that NECA, but not CGS21680, concentration-dependently induces accumulation of cyclic AMP, corresponding to an EC50 value of 469 ± 71 nM, suggesting a functional role of the A2B AR, which is consistent with its high expression in PC-3 cells as shown in Fig. 1. Figure 3b shows that the recently available A2B agonist BAY60-6583 also concentration-dependently induces cyclic AMP accumulation in PC-3 cells corresponding to an EC50 value of 7.2 ± 2.8 nM. Various concentrations of the selective A2B antagonist PSB603 shift the agonist curve to the right in a parallel manner corresponding to a KB value of 1.0 nM. Figure 3c shows that BAY60-6583 induces accumulation of cyclic AMP in both DU145 and LNCaP cells corresponding to respective EC50 values of 9.8 ± 1.6 and 12.2 ± 2.7 nM.
Fig. 3.
a Cyclic AMP accumulation in PC-3 cells stimulated by the nonselective A2B AR agonist NECA and the A2A AR agonist CGS21680. b Effect of the A2B AR-selective antagonist PSB603 on A2B AR agonist BAY60-6583-induced cyclic AMP accumulation in PC-3 cells. c BAY60-6583-induced cyclic AMP accumulation in DU145 and LNCaP cells. Results are expressed as mean ± SE from three experiments performed in duplicate. The EC50 values of NECA, BAY60-6583, and CGS21680 were listed in the text. The KB value of PSB603 was calculated to be 1.0 nM. PSB PSB603, BAY BAY60-6583
LDH release in PC-3 prostate cancer cells
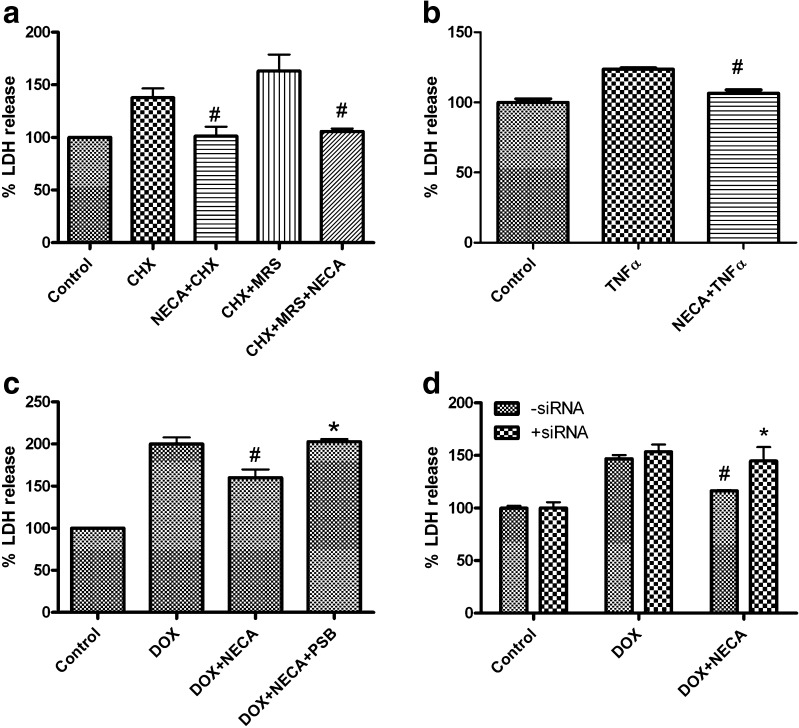
PC-3 cells, which almost exclusively express the functional A2B AR, were used as a model for further exploring the role of the A2B AR in prostate cancer cell death. Figure 4a shows that NECA (1 μM) decreases CHX-induced LDH release. It has been shown previously that the selective P2Y1 receptor agonist MRS2365 (1 μM) enhanced the effect of CHX to promote LDH release in PC-3 cells [4]. Here, we have shown that the MRS2365-induced enhancement of CHX-induced LDH release was also diminished by NECA (Fig. 4a). Figure 4b shows that NECA significantly diminishes LDH release induced by TNF-α (10 ng/ml). Figure 4c shows that DOX, a cytotoxic drug for prostate cancer shown to induce death of PC-3 cells [12], significantly increases LDH release, which is blocked by NECA. The selective antagonist of the A2B AR, PSB603, is shown to antagonize the effect of NECA (Fig. 4c). Figure 4d shows that siRNA specific for the A2B AR does not affect LDH release induced by DOX but reverses the effect of the A2B AR agonist NECA. The percentages of LDH release in the DOX + NECA group in the presence and absence of A2B AR siRNA were 147 ± 3.5 % and 116 ± 1.9 %, respectively, which were significantly different (P < 0.05). In control experiments, siRNA specific for an unrelated protein, GAPDH, did not reduce the effect of NECA.
Fig. 4.
Detection of cell death with an assay of LDH release in PC-3 cells. Agonists and/or CHX (10 μg/ml) (a) or TNF-α (10 ng/ml) (b) or doxorubicin (DOX, c) were added and incubated with the cells at 37 °C for 24 h. #Significantly different from corresponding groups in the absence of NECA (1 μM). MRS MRS2365 (1 μM), PSB PSB603 (1 μM). *Significantly different from DOX + NECA group (P < 0.05). d Cells were transfected with A2B AR siRNA (1 μM) at about 80 % confluency using Lipofectamine 2000 as instructed by the manufacturer. Cells were split to 24-well plates 24 h after transfection and incubated for an additional 24 h before the addition of DOX (10 μM) and/or NECA (1 μM). LDH release was measured 24 h after drug treatment. #Significantly different from the corresponding DOX group (P < 0.05). *Significantly different from the DOX + NECA control group in the absence of siRNA (P < 0.05)
Effect of NECA on TNFα-induced increase of caspase-3 activity
We next examined the role of A2B AR activation in the activity of the enzyme caspase-3, an early indicator of cell apoptosis. Figure 5 shows that TNF-α causes increase of caspase-3 activity in PC-3 cells, which is significantly diminished by NECA (1 μM). The percentage of caspase-3 activity in the presence of NECA (101 ± 1.7 %) is significantly different from that in its absence (133 ± 6.4 %) (P < 0.05). The A2B AR antagonist PSB603 did not produce any effect alone but blocked the effect of NECA.
Fig. 5.
Detection of apoptosis by measuring caspase-3 activity in PC-3 cells. Cells were plated in six-well plates at a density of 2 × 105 cells per well and cultured to approximately 80–90 % confluence for the experiments. The complete medium was replaced with fresh serum-free medium after washing with PBS for three times. Cells were then treated with the antagonist PSB603 (1 μM) for 20 min before the addition of the agonist NECA (1 μM) and incubated for another 20 min, followed by addition of TNF-α (10 ng/ml) for 8 h. Caspase-3 activity was measured using the Caspase-3 Colorimetric Assay Kit (Enzo Life Sciences, Plymouth Meeting, PA, USA). The protein concentration of each sample was quantified. Each sample contains 50–200 μg protein that was used in the assay. The absorbance in each well was measured at 405 nm with a microplate reader. Results are from three separate experiments performed in triplicate. #Significantly different from the TNF-α group (P < 0.05). *Significantly different from the TNF-α + NECA group (P < 0.05). PSB PSB603
Detection of morphological changes of apoptotic PC-3 cells with confocal laser scanning microscopy
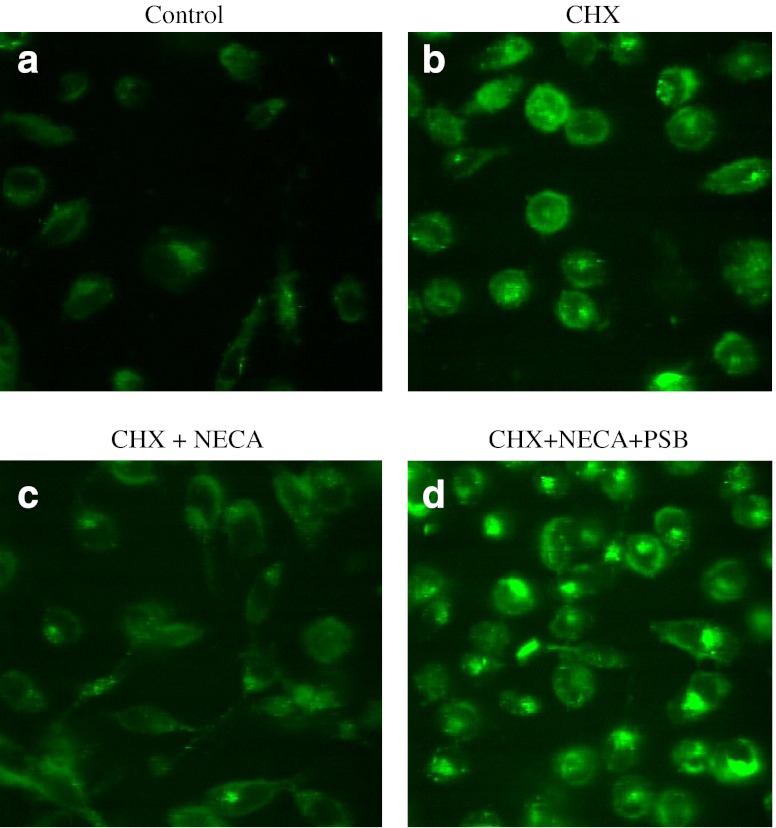
CHX has been shown in many studies to induce apoptosis with characteristics such as formation of blebs, chromatin condensation, and exposition of phosphatidyl serines at the plasma membrane. Therefore, we evaluated the protective effect of the A2B AR agonist NECA on CHX-induced apoptosis in PC-3 cells. In control cells (Fig. 6a), the nucleus was intact, and the morphology of PC-3 cells was not altered. As shown in Fig. 6b, cells treated with CHX exhibited formation of blebs and altered morphology, and the nucleus was fragmented. Annexin V binds to the extracellularly exposed phosphatidyl serine residues in cells that are undergoing apoptosis caused by CHX treatment. Pretreatment with NECA protected the cells against CHX-induced apoptosis, which was evident from Fig. 6c, in which the cells displayed decreased chromatin condensation and intact morphology. To confirm that the protective effect of NECA on CHX-induced apoptosis in PC-3 cells occurred through the A2B AR, a selective A2B antagonist PSB603 was used. Figure 6d shows that PC-3 cells pretreated with PSB603 plus NECA and CHX have characteristics similar to those of cells treated with CHX alone, which confirms that the protective effect of NECA was via the A2B AR.
Fig. 6.
Detection of morphological changes in CHX-induced apoptotic PC-3 cells using confocal microscopy. PC-3 cells grown to 70–80 % confluency were treated with 1 μM PSB603 (PSB) for 20 min before treatment with 1 μM NECA and/or 100 μM cycloheximide and were incubated for 6 h. Cells were then stained with FITC-Annexin-V and images were captured with confocal microscope (LSM 5 Live, Carl Zeiss, Germany). a Control. b CHX. c CHX + NECA. d CHX + NECA + PSB
Activation of the A2B AR-induced proliferation of prostate cancer cells
The activation the A2B AR has been shown to induce proliferation of colon carcinoma cells [6] and growth of breast tumors [7]. In the present study, the potential proliferative effect of A2B AR activation in PC-3 prostate cancer cells was examined using an XTT assay. Figure 7a shows that the nonselective A2B AR agonist NECA concentration-dependently promotes growth of PC-3 cells (measured 72 h after addition of NECA), corresponding to an EC50 value of 266 ± 78 nM. At 1 μM, NECA produced an effect close to maximum; thus, we selected this concentration in the following experiment. Figure 7b shows that NECA (1 μM) significantly enhanced growth of PC-3 cells, which was diminished by specific siRNA for the A2B AR (Fig. 7b). The selective A2A AR agonist CGS21680 (1 μM) did not promote cell growth (data not shown).
Fig. 7.
Measurement of A2B AR-promoted cell growth using an XTT assay. Cells were seeded in 96-well plates about 5,000 cells per well in a volume of 100 μl medium containing 10 % serum and cultured at 37 °C overnight. Cells were then treated with and incubated at 37 °C for 24, 48, or 72 h. a Concentration dependence of NECA-promoted PC-3 cell growth measured at 72 h after drug treatment. The EC50 value of NECA was calculated to be 266 ± 78 nM. b The effects of A2B AR siRNA (1 μM) on NECA (1 μM)-promoted growth of PC-3 cells. #Significantly different from control and siRNA groups (P < 0.05)
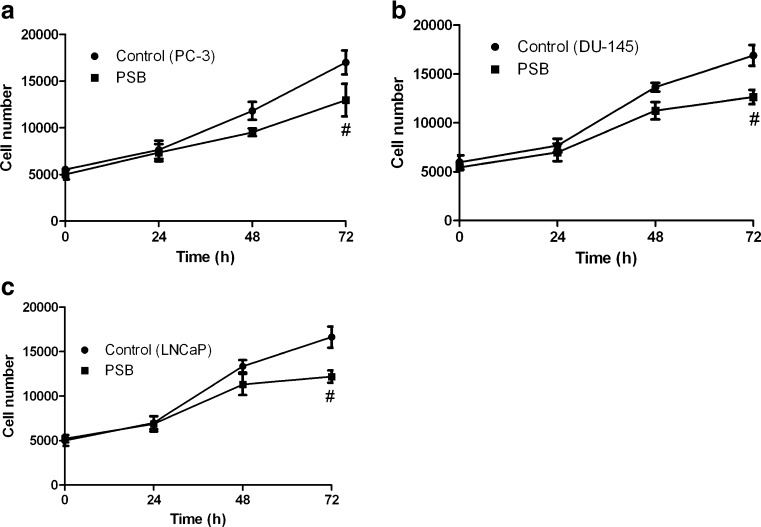
We further tested the role of A2B AR blockade in the proliferation of all three cell lines using the selective A2B AR antagonist PSB603. Figure 8 shows that PSB603 inhibits growth of all three types of commonly used prostate cancer cells.
Fig. 8.
Effect of the A2B AR antagonist PSB603 on the growth of PC-3 (a), DU145 (b), and LNCaP (c) cells. Cells were seeded in 96-well plates about 5,000 cells per well in a volume of 100 μl cell culture medium with 10 % serum and cultured at 37 °C overnight. Cell viability was measured using an XTT Assay Kit following the manufacturer's protocol. Absorbance values were transformed into cell numbers based on a standard curve measured with various known numbers of cells. PSB PSB603. Results are expressed as mean ± SE, from at least three separate experiments. #Significantly different from the control group (P < 0.05)
Discussion
In the present study, we explored the role of the A2B AR in the death and growth of prostate cancer cells. We found that A2B AR activation blocked cell death and promoted cell growth. Both the A2B AR antagonist PSB603 and siRNA specific for the A2B AR slowed cell growth, suggesting that selective A2B AR blockade could be useful for the treatment of prostate cancer.
The A2B AR has been identified as one of several GPCRs expressed at a considerably high level in some cancer tissues including prostate cancer [5]. However, the role of the A2B AR in prostate cancer has not been systematically investigated and reliably identified [13, 14]. This was, at least, in part, because many previous studies utilized adenosine and analogs that acted nonselectively on the ARs [13, 15] rather than selective A2B AR agonists or antagonists or siRNA specific for the A2B AR.
Regarding the expression profile of the four subtypes of ARs in prostate cancer cells, the results from the present study are in line with those from several previous reports [5, 15, 16]. Minelli et al. [15] suggested that the A2B AR was the highest expressed of the four ARs in PC-3 cells. Panjehpour et al. [16] demonstrated that in DU145 prostate cancer cells, the A2B AR was the highest expressed and the A3 AR was the lowest expressed (almost undetectable) among the four subtypes of ARs. The results from the present study are different from those of Aghaei et al. [14], who reported a different AR expression pattern with the A3 AR being highly expressed. Fishman et al. [17] reported that the A3 AR agonist IB-MECA at concentrations from 0.01 to 10 μM inhibited growth of PC-3 cells, but the expression profile of four ARs was not examined. The reason for this discrepancy is unclear. However, it should be noted that the A3 AR was not found to have a high expression level in primary prostate cancer tissue [5].
The XTT assay was used in the present study to measure cell growth and a stimulatory effect of NECA was detected. The direct interpretation for this is that A2B ARs mediated a proliferative effect. However, an antiapoptotic effect of NECA was also demonstrated in the same cells. Therefore, it is unclear if the effect of NECA is due to increased proliferation or decreased death or a combination of both, which could be addressed directly using a proliferation assay, such as the BrdU incorporation test.
Administration of the naturally occurring AR antagonists, i.e., caffeine and theophylline, has long been suspected to counteract the growth of cancer. Both caffeine and theophylline are weak and nonselective A2B antagonists, which might have a role in both cancer promotion and prevention depending on specific situations involved. In this study, a more potent and selective A2B AR antagonist, PSB603, has been clearly shown to inhibit the growth of prostate cancer cells. It remains to be seen how general is the antiproliferative effect of A2B AR antagonists in primary prostate cancer cells and other types of cancer cells.
The findings from the present study that the A2A AR is also relatively highly expressed in DU145 and LNCap cells, suggest that under some specific conditions, selective antagonists of the A2A AR may also have a therapeutic role in prostate cancer management. Consistent with this finding, a recent report from Kalhan et al. [18] also suggested that blockade of both A2A and A2B ARs may have a therapeutic role in endocrine tumors.
In addition to the high expression level of the A2B AR, P2Y1 receptors [4], which are activated by endogenous adenine nucleotides, and ectonucleotidases CD39 and CD73 [19], which together degrade adenine nucleotides to produce adenosine, are also abundantly expressed in prostate cancer cells. Stagg et al. [20] recently reported that CD73 deficiency suppressed prostate tumorigenesis in a mouse transgenic model of prostate cancer. In addition to their early findings that A2B AR antagonism blocked breast cell tumor growth [7], the authors show that the inhibitory effect of an anti-CD73 antibody on tumorigenesis depends on CD8+ T cells. Therefore, adenosine may act through its tolerogenic action leading to immune escape, in addition to a direct effect on tumor cell proliferation. On the other hand, CD39 might promote tumor cell growth by scavenging ATP rather than by generating adenosine. Indeed, ATP inhibits the growth of prostate tumor cells through the activation of P2X receptors [13]. Vascular CD39 directly promotes tumor cell growth [21]. It has been suggested that inhibition of either enzyme may find utility in cancer management. The present study demonstrated that A2B AR activation may have tumor-promoting effects, thus indicating that the tumorigenic effects of ectonucleotidases might be, at least in part, via the activation of the A2B AR, due to their ability to cleave ATP to produce adenosine.
In summary, the present study demonstrated that activation of the A2B AR promotes, and blockade of the A2B AR inhibits, the growth of prostate cancer cells. Thus, A2B AR blockade might be a novel therapy for prostate cancer patients.
Acknowledgments
This study was supported by the NIDDK Intramural Research Program, National Institutes of Health, Bethesda, MD, USA; the National Natural Science Foundation of China (no.: 30940072); Guangdong Province Science and Technology Program (2012B031800263); and Nanfang Hospital, Southern Medical University, Guangzhou, China. The authors thank Dr. Yafang Hu (Children's National Medical Center, Washington, DC, USA) for assistance in confocal microscopy experiments. We thank Prof. Ad IJzerman (Leiden University, The Netherlands) for providing LUF6210 (BAY60-6583).
Conflicts of interest
None.
Abbreviations
- BCA
Bicinchoninic acid
- Cyclic AMP
Adenosine 3′,5′-cyclic monophosphate
- CGS21680
(2-[p-(2-Carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine)
- CHX
Cycloheximide
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified eagle’s medium
- DOX
Doxorubicin
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IB-MECA
N6-(3-Iodobenzyl)adenosine-5′-N-methyluronamide
- MRS2365
(N)-Methanocarba-2′-deoxy-2-methylthio-adenosine-5′-diphosphate
- RPMI
Roswell Park Memorial Institute medium
- RT-PCR
Real time polymerase chain reaction
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- XTT
2,3-bis(2-Methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt
- NECA
5′-N-Ethylcarboxamidoadenosine
- PSB603
8-[4-[4-(4-Chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine
- TNF-α
Tumor necrosis factor-alpha
References
- 1.Damber JE, Aus G. Prostate Cancer. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 2.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 3.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 4.Wei Q, Costanzi S, Liu QZ, Gao ZG, Jacobson KA. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol. 2011;82:418–425. doi: 10.1016/j.bcp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 6.Ma DF, Kondo T, Nakazawa T, Niu DF, Mochizuki K, Kawasaki T, Yamane T, Katoh R. Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum Pathol. 2010;41:1550–1557. doi: 10.1016/j.humpath.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Blom WM, de Bont HJ, Meijerman I, Mulder GJ, Nagelkerke JF. Prevention of cycloheximide-induced apoptosis in hepatocytes by adenosine and by caspase inhibitors. Biochem Pharmacol. 1999;58:1891–1898. doi: 10.1016/S0006-2952(99)00268-3. [DOI] [PubMed] [Google Scholar]
- 12.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci USA. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132:536–546. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghaei M, Karami-Tehrani F, Panjehpour M, Salami S, Fallahian F. Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and -independent prostate cancer cell lines, LNcap-FGC-10, DU-145, and PC3. Prostate. 2011;72:361–375. doi: 10.1002/pros.21438. [DOI] [PubMed] [Google Scholar]
- 15.Minelli A, Bellezza I, Tucci A, Rambotti MG, Conte C, Culig Z. Differential involvement of reactive oxygen species and nucleoside transporters in cytotoxicity induced by two adenosine analogues in human prostate cancer cells. Prostate. 2009;69:538–547. doi: 10.1002/pros.20900. [DOI] [PubMed] [Google Scholar]
- 16.Panjehpour M, Movahedian A, Sadeghi H, Eghbali B, Yekdaneh A. Adenosine receptor expression in two different human cancer lines at molecular level. Iranian J Cancer Prevention. 2010;3:111–116. [Google Scholar]
- 17.Fishman P, Bar-Yehuda S, Ardon E, Rath-Wolfson L, Barrer F, Ochaion A, Madi L. Targeting the A3 adenosine receptor for cancer therapy: inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Res. 2003;23:2077–2083. [PubMed] [Google Scholar]
- 18.Kalhan A, Gharibi B, Vazquez M, Jasani B, Neal J, Kidd M, Modlin IM, Pfragner R, Rees DA, Ham J. Adenosine A2A and A2B receptor expression in neuroendocrine tumours: potential targets for therapy. Purinergic Signal. 2012;8:265–274. doi: 10.1007/s11302-011-9280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastie C, Saxton M, Akpan A, Cramer R, Masters JR, Naaby-Hansen S. Combined affinity labelling and mass spectrometry analysis of differential cell surface protein expression in normal and prostate cancer cells. Oncogene. 2005;24:5905–5913. doi: 10.1038/sj.onc.1208747. [DOI] [PubMed] [Google Scholar]
- 20.Stagg J, Beavis PA, Divisekera U, Liu MC, Moeller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]