Abstract
Extracellular ATP and its hydrolysis product, adenosine, acting through specific receptors collectively named purinergic receptors, regulate female fertility by influencing the endometrial fluid microenvironment. There are four major groups of ecto-nucleotidases that control the levels of extracellular ATP and adenosine and thus their availability at purinergic receptors: ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), ecto-nucleotide pyrophosphatase/phospho-diesterases (E-NPPs), ecto-5′-nucleotidase (5′NT), and alkaline phosphatases (APs). The aim of the present work is to characterize the expression and distribution of ecto-nucleotidases in human endometrium along the menstrual cycle and after menopause, to evaluate their potential utility as fertility markers. We examined proliferative, secretory and atrophic endometria from women without endometrial pathology undergoing hysterectomy. We show that the ecto-nucleotidases are mainly present at endometrial epithelia, both luminal and glandular, and that their expression fluctuates along the cycle and also changes after menopause. An important result was identifying NPP3 as a new biological marker of tubal metaplasia. Our results emphasize the relevance of the study of purinergic signaling in human fertility.
Keywords: Ecto-nucleotidases, Endometrium, Purinergic signaling, Fertility, CD39, CD73, NPP
Introduction
During the menstrual cycle, in response to autocrine, paracrine and endocrine factors, the human endometrium undergoes morphological and functional changes essential for uterine receptivity, affecting glands, stroma and luminal epithelium. The first phase is characterized by a proliferative endometrium and is governed by estrogens, while after ovulation, the secretory phase, influenced by progesterone, prepares the endometrium for embryo implantation [1].
Extracellular nucleotides, such as ATP, and nucleosides, such as adenosine, are autocrine and paracrine molecules that play important roles in reproduction [2]. In the uterus, extracellular ATP is needed for the initiation and maintenance of myometrial contractions [3]; it contributes to the regulation of the uterine fluid microenvironment by regulating endometrial Cl− secretion [4], Na+ absorption [5] and cervical mucus production [6]. P2X and P2Y nucleotide receptors have been identified in the female reproductive tract [7–9], with changes in the expression along the cycle [10] and during implantation [11] and pregnancy [8]. Furthermore, extracellular ATP treatment of sperm improves its fertilizing capability [12–14], thus potentially improving the outcome of assisted reproduction techniques.
Extracellular adenosine, the dephosphorylated product generated from the hydrolysis of ATP, coordinates early post-implantation events [15], and also exerts control of myometrium contractions [16]. Importantly, adenosine is a key molecule for sperm capacitation, the series of changes that sperm undergo in the female reproductive tract to acquire fertilizing ability [17–19].
For the reasons stated above, the study of the mechanisms controlling the levels of extracellular ATP and adenosine in the female reproductive system, the endometrium in particular, is necessary. The concentrations of extracellular ATP and adenosine are controlled by specific nucleotide-hydrolyzing enzymes expressed at the cell surface called ecto-nucleotidases [20]. Different families of enzymes are responsible for these activities and, alone or acting sequentially, they generate adenosine from adenine nucleotides (i.e., ATP, ADP or AMP): (1) the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family includes four plasma membrane-bound members: NTPDase1 (CD39), NTPDase2, NTPDase3 and NTPDase8 [21–23]; these enzymes are differentially expressed and hydrolyze nucleoside triphosphates and diphosphates to their monophosphate derivatives; (2) the ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) family has three members (NPP1-3) capable of hydrolyzing nucleoside triphosphates to monophosphates and PPi, such as ATP to AMP and PPi [24]; (3) the 5′-nucleotidase family has only one member attached to the outer plasma membrane, the ecto-5′-nucleotidase (CD73), a glycosyl phosphatidylinositol-linked membrane-bound glycoprotein that efficiently hydrolyzes AMP to adenosine [25]; (4) the alkaline phosphatase (AP) family includes ubiquitous enzymes, such as the placental AP (PLAP) and the tissue nonspecific AP (TNAP), with broad substrate specificity, including adenine nucleotides and pyrophosphate, releasing inorganic phosphate [26]. The generated adenosine can be further inactivated by other enzymes such as adenosine deaminase (ADA), which can be expressed as a soluble ectoenzyme, or as membrane-associated enzyme often forming larger complexes with CD26/dipeptidyl peptidase IV, converting adenosine to inosine. Moreover, ATP can be re-synthesized via backward ecto-phosphotransfer reactions catalyzed by enzymes such as adenylate kinase and nucleoside diphosphate kinase [23].
In spite of their obvious importance, very little is known of the ecto-nucleotidases expression in endometrium. A few studies have been conducted in mice; NTPDase1 and 2 were identified in myometrium [27], and ecto-5′-nucleotidase in endometrium, where the expression and activity fluctuate along the estrous cycle and with pregnancy, pointing to hormonal regulation of extracellular adenosine levels in this organ [28, 29]. However, to our knowledge, there are no available data concerning the expression of ecto-nucleotidases in human endometrium and their possible changes along the cycle.
The study of protein expression in remodeling cyclic human endometrium, and its comparison to postmenopausal endometrium, is crucial for understanding the physiology of reproduction. In the present work, we characterize for the first time the expression of human endometrial nucleotide-converting ectoenzymes, in both cyclic and postmenopausal endometria.
Methods
Samples
The ethical principles of this study adhere to the Declaration of Helsinki, and all the procedures were approved by the ethics committee for clinical investigation of Bellvitge Hospital. Endometrial samples were obtained from hysterectomy specimens without endometrial malignancy at the Service of Gynecology of Bellvitge Hospital. Fresh samples were cut, embedded in O.C.T freezing media (Tissue-Tek®; Sakura Finetk, Zoeterwoude, the Netherlands), snap-frozen in a Shandon Histobath™ 2 (Neslab Instruments Inc., USA) at the Service of Pathology and stored at −80 °C until used. Alternatively endometrial samples were obtained from the Tumor Bank of Bellvitge Biomedical Research Institut (IDIBELL).
Eight proliferative, 12 secretory and 32 atrophic endometria were used in this study. Endometrial dating was done at the Service of Pathology.
Demographic description of the samples and the factors that indicated the need for hysterectomy are summarized in Table 1.
Table 1.
Patient demographics
| Type of endometrium | Number of cases | Age (years) Average (range) | Indication of hysterectomy (number of cases) |
|---|---|---|---|
| Proliferative | 8 | 44.3 (39–49) | Leiomyomas (3) |
| Prolapse (2) | |||
| Cervical neoplasia (2) | |||
| Ovarian neoplasia (1) | |||
| Secretory | 12 | 43.9 (32–54) | Leiomyomas (8) |
| Cervical neoplasia (3) | |||
| Ovarian neoplasia (1) | |||
| Atrophic | 32 | 62.7 (47–80) | Leiomyomas (2) |
| Prolapse (24) | |||
| Cervical neoplasia (2) | |||
| Ovarian neoplasia (4) |
Reagents
Primary antibodies used in this study are listed in Table 2. Secondary antibodies used were: horseradish peroxidase-conjugated goat anti-mouse (EnVision™ + system; DAKO, Carpinteria, CA, USA), Alexa Fluor 488- or 555-goat anti-mouse or anti-rabbit, and Alexa Fluor 488-donkey anti-goat (Life Technologies, Paisley, UK). To-Pro®-3 (Life Technologies) was used as a nuclear marker.
Table 2.
List of primary antibodies used for immunolabeling experiments
| Antibody specificity | Name/clone | Source | Supplier | Dilution |
|---|---|---|---|---|
| Ecto-5′-nucleotidase (CD73) | 4G4 | Mouse | Abcam (ab81720) | 1/50 |
| NTPDase1 (CD39) | BU-61 | Mouse | Ancell (188–020) | 1/500 |
| NTPDase3 | B3S10 | Mouse | http://ectonucleotidases-ab.com/ | 1/500 |
| CD26 | 202–36 | Mouse | Abcam (ab3154) | 1/100 |
| NPP1 | Anti-NPP1 | Goat | Abcam (ab40003) | 1/250 |
| NPP3 | NP4D6 | Mouse | Abcam (ab90754) | 1/100 |
| Human placental alkaline phosphatase (PLAP) | 8B6 | Mouse | Sigma (A2951) | 1/1000 |
| Alkaline phosphatase, tissue non-specific (TNAP) | [3H414(TRA-2-49)] | Mouse | Abcam (ab17973) | 1/50 |
| CD31 | Anti-CD31 | Rabbit | Abcam (ab28364) | 1/50 |
| Alpha smooth muscle actin (α-SMA) | Anti-αSMA | Rabbit | Abcam (ab5694) | 1/200 |
Immunolabeling experiments
Sections (10 μm) were obtained with the Cryostat Leica CM1950 (Leica, Wetzlar, Germany), put onto poly-l-lysine covered glass slides, and fixed in 10 % phosphate-buffered formalin mixed with cold acetone (Merck, Darmstadt, Germany) for 2.5 min.
For immunolabeling experiments samples were rinsed with PBS and pre-incubated for 1 h at room temperature (RT) with PBS containing either 20 % normal goat serum or 20 % horse serum (Gibco, Paisley, UK) and 0.2 % gelatin (Merck). Slices were then incubated overnight at 4 °C with the primary antibodies at the dilutions indicated in Table 2. All dilutions were made in PBS. After three washes in PBS, tissue sections were incubated with the suitable secondary antibodies for 1 h at RT. Secondary antibodies alone were routinely included as controls for the experiments. Nuclei were counterstained with haematoxylin or, alternatively, in fluorescence assays, To-Pro®-3 was used to visualize the nuclei.
Samples were observed and photographed under light Leica DMD 108 microscope or under Leica TCS-SL spectral confocal microscope (Leica).
Immunohistochemical staining was independently evaluated by at least two observers. Staining distribution was recorded. Label intensity was scored as negative (−), weak (+), intermediate (++) or strongly positive (+++).
In situ AMPase and ATPase activity experiments
For the histochemical localization of AMPase and ATPase activity, the Wachstein/Meisel lead phosphate method [29, 30] was performed. Briefly, fixed tissue sections were pre-incubated for 1 h at RT in 50 mM Tris-maleate buffer, pH 7.4 containing 2 mM CaCl2 and 0.25 M sucrose. Enzymatic reaction was performed for 1 h at 37 °C in the same buffer supplemented with 5 mM MnCl2, 2 mM Pb(NO3)2, 3 % dextran T250 and 2.5 mM levamisole, as an inhibitor of the AP activity, and in the presence of either 1 mM AMP or 200 μM ATP as a substrate. For CD73 inhibition experiments, 1 mM α,β-methylene-ADP (α,β-meADP) was added to both pre-incubation and enzymatic reaction buffers. The substrate was omitted in the control experiments. The reaction was revealed by incubation with 1 % (NH4)2S v/v for exactly 1 min, and nuclei were counterstained with haematoxylin. Samples were then dehydrated, mounted with DPX mounting medium, and observed and photographed under light Leica DMD 108 microscope.
In situ alkaline phosphatase activity experiments
The histochemical localization of AP was addressed by using the Gossrau method [31] with some modifications. Briefly, fixed slices were washed twice in Tris 0.1 M HCl buffer, pH 7.4 containing 5 mM MgCl2, and then pre-incubated with the same buffer at pH 9.4 for 15 min at RT. Enzymatic reaction was started by adding 200 μl of the revealing reagent BCIP (Sigma-Aldrich, St. Louis, MO, USA) for 7 min at RT, and stopped with Tris 0.1 M HCl buffer, pH 7.4. For AP inhibition experiments, 5 mM levamisole was added to both pre-incubation and enzymatic reaction buffers. In control experiments the revealing reagent BCIP was omitted. Nuclei were counterstained with methyl green dye for 10 min, dipped briefly in alcohol, mounted in aqueous mounting medium (Fluoromount; Sigma-Aldrich) and observed under light Leica DMD 108 microscope.
Results
Table 3 compiles the results of all the immunolabelings performed.
Table 3.
Summary of the main findings on ecto-enzyme expression in cyclic (proliferative and secretory) and atrophic endometria
| NTPDase1 (CD39) | NTPDase3 | NPP1 | NPP3 | PLAP | TNAP | CD26 | 5′-NT (CD73) | |
|---|---|---|---|---|---|---|---|---|
| Proliferative | ||||||||
| Surface epithelium | − | − | +++ | − | +++ | +++ | − | − |
| Glandular epithelium | ||||||||
| Functional layer | − | + | + | + | ++ | +++ | − | ++ |
| Basal layer | − | ++ | + | ++ | + | ++ | − | +++ |
| Endometrial stromal cells | ++ | − | − | − | − | − | − | ++ |
| Spiral arteries | ++ | +++ | − | − | − | ++ | + | − |
| Secretory | ||||||||
| Surface epithelium | − | +++ | +++ | +++ | − | − | − | − |
| Glandular epithelium | ||||||||
| Functional layer | − | ++ | − | ++ | + | +++ | +++ | ++ |
| Basal layer | − | +++ | − | +++ | − | ++ | ++ | +++ |
| Endometrial stromal cells | +++ | − | − | − | − | + (*) | − | +++ |
| Spiral arteries | ++ | +++ | − | − | − | ++ | + | − |
| Atrophic | ||||||||
| Surface epithelium | − | − | +++ | − | +++ | +++ | − | − |
| Glandular epithelium | − | ++ | ++ | − | + | +++ | + | +++ |
| Endometrial stromal cells | ++ | − | + | − | − | − | − | ++ |
− no immunostaining, + weak positive staining, ++ strong staining, +++ strongest staining
Asterisk in the TNAP column of secretory endometrium indicates that the label is only present in a narrow area of the stroma subjacent to luminal epithelium
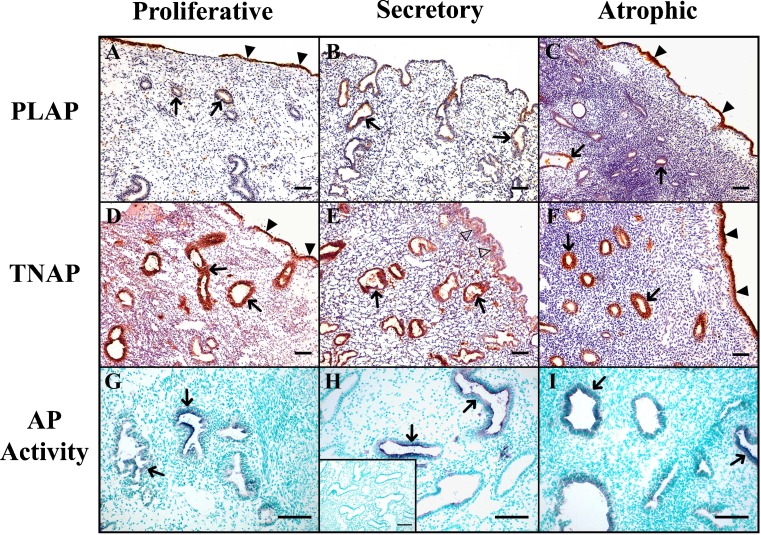
Our results show that PLAP and TNAP were expressed in both luminal and glandular epithelia of endometrium (Fig. 1), but were absent in the luminal epithelium of secretory endometria. Moreover, in this type of endometrium, TNAP was present in the stroma subjacent to the luminal epithelium. Besides this change in the enzyme distribution, there were no other significant variations along the cycle or when compared with the atrophic endometrium. Immunolabeling was stronger for TNAP than for PLAP in all the mentioned structures, especially in glands, where the PLAP staining was very weak. In situ activity experiments demonstrated AP activity in the locations of the immunodetected proteins, and this activity was completely inhibited with the AP inhibitor levamisole, confirming the specificity of the activity. As expected, AP was also detected in endothelial cells.
Fig. 1.
Immunolocalization of PLAP (a, b, c), TNAP (d, e, f) and AP in situ histochemistry (g, h, i), in proliferative (a, d, g), secretory (b, e, h) and atrophic (c, f, i) endometria. PLAP and TNAP were immunodetected in the glands (arrows) of all types of endometrium, and in the luminal epithelium of proliferative an atrophic endometria (filled arrowheads). TNAP was also immunodetected at the stroma subjacent to the luminal epithelium in secretory endometrium (e, empty arrowheads). Microphotographs g, h, and i show blue deposits corresponding to AP in situ activity and nuclei are stained in green. Inset in h corresponds to the activity experiment in the presence of the inhibitor levamisole, and shows complete AP inhibition. Scale bars = 100 μm
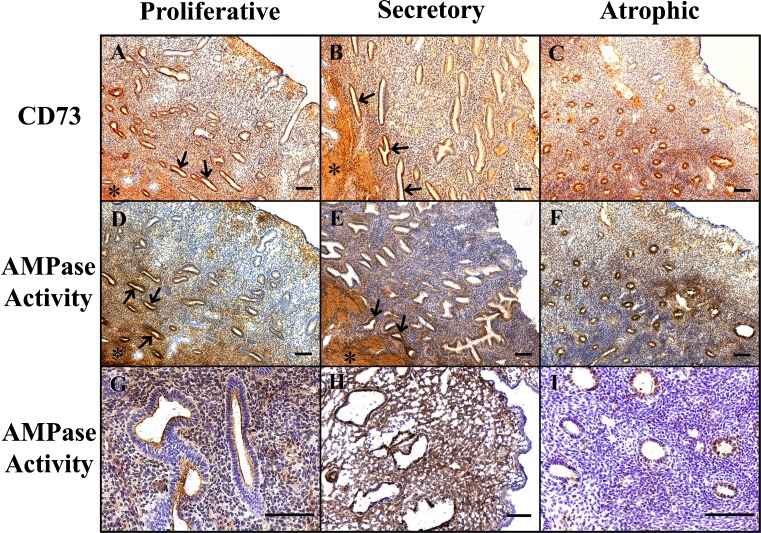
Ecto-5′-nucleotidase (CD73) was expressed and active in glandular epithelium and in stroma in both cyclic and atrophic endometria (Fig. 2). Labeling in the basal layer glands was much more intense than in the functional layer. Luminal epithelium was not stained. In situ activity experiments demonstrated AMPase activity in the structures where the enzyme was immunodetected, and this activity was completely inhibited by the specific ecto-5′-nucleotidase inhibitor α,β-meADP. Although the enzyme is present throughout the cycle, an increase in the expression and activity in the stroma was consistently observed in the secretory phase.
Fig. 2.
Immunolocalization of ecto-5′-nucleotidase (5′-NT)/CD73 (a, b, c), and AMPase in situ histochemistry (d–i), in proliferative (a, d, g), secretory (b, e, h) and atrophic (c, f, i) endometria. 5′-NT was immunodetected in the glands and the stroma of all types of endometrium. Dark brown deposits in microphotographs d–i correspond to the AMPase in situ activity. g Detail of glands of a proliferative endometrium showing AMPase activity at the luminal side of the glandular epithelium and at the stroma. h Magnification of a secretory endometrium showing intense stromal AMPase activity. i An activity experiment performed on atrophic endometrium in the presence of the inhibitor α,β-meADP, and shows complete inhibition of AMPase activity. Note that 5′-NT expression and AMPase activity are stronger in the glands of basal layer (arrows) and that myometrium is also intensely labeled (asterisks). Scale bars = 100 μm
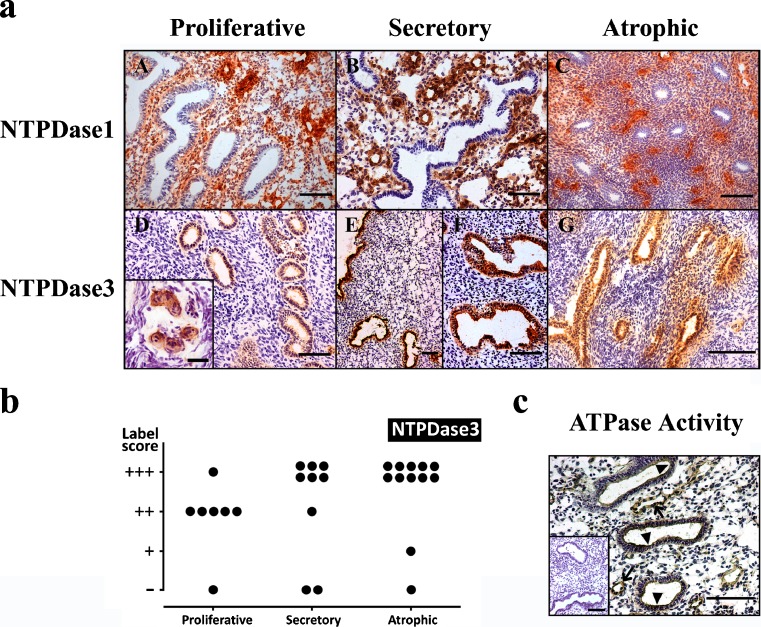
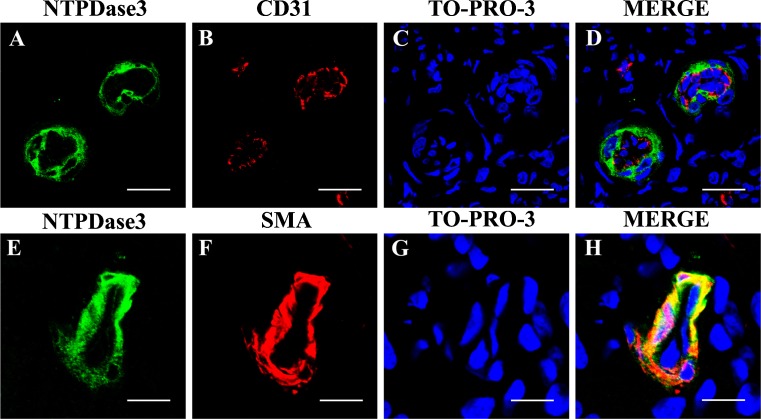
As expected, NTPDase1 (CD39) was expressed in the endothelial cells of the stromal blood vessels (Fig. 3a). Sparse cells at the stroma were also positive for NTPDase1 staining, probably being macrophages and other immune system cells. Labeling was never seen in association with either glands or luminal surface. On the contrary, NTPDase3 was expressed by glandular and luminal epithelia, in both cyclic and atrophic endometria. An increase in the expression in both epithelia was observed in the secretory phase. These changes in the expression in glands are represented in Fig. 3b. Interestingly, NTPDase3 was also detected in the endometrial spiral arteries. This expression is associated with the muscle layer but not with the endothelium as confirmed by double immunostainings performed with anti-SMA and anti-CD31 antibodies, respectively (Fig. 4). NTPDase3 was not detected in the myometrial arteries or in other blood vessels. The in situ ATPase activity was detected in the above reported structures, coinciding with NTPDase1 and NTPDase3 expression (Fig. 3c). NTPDase2 was not detected in endometrial epithelia, neither luminal nor glandular (data not shown).
Fig. 3.
a Immunolocalization of NTPDase1 (a, b, c) and NTPDase3 (d, e, f, g) in proliferative (a, d), secretory (b, e, f) and atrophic (c, g) endometria. NTPDase1 was immunodetected in the stromal blood vessels of all types of endometrium. NTPDase3 was immunodetected in the luminal and glandular epithelia of all types of endometria. Note that label is stronger in the secretory (e, f) than in the proliferative endometrium (d) and that in atrophic endometrium label is also very high. NTPDase3 was also immunodetected in spiral arteries (inset in d). Scale bars = 100 μm except for the inset, where it is 25 μm. b Label intensity score of NTPDase3 in the glandular epithelium of proliferative, secretory and atrophic endometria. Maximal score is found in secretory and atrophic endometria. c ATPase in situ histochemistry in glands (arrowheads) and in stroma, especially in blood vessels (arrows). The inset corresponds to an activity experiment performed in the absence of substrate. Scale bar = 100 μm
Fig. 4.
Confocal fluorescence images of endometrial spiral arteries labeled with antibodies against NTPDase3 (a, e) and CD31 (b) or SMA (f). Nuclei were labeled with To-Pro®-3 (c, g). Merge images showed colocalization between NTPDase3 and SMA (h) but not between NTPDase3 and CD31 (d). Scale bars 20 μm (a–d) and 10 μm (e–h)
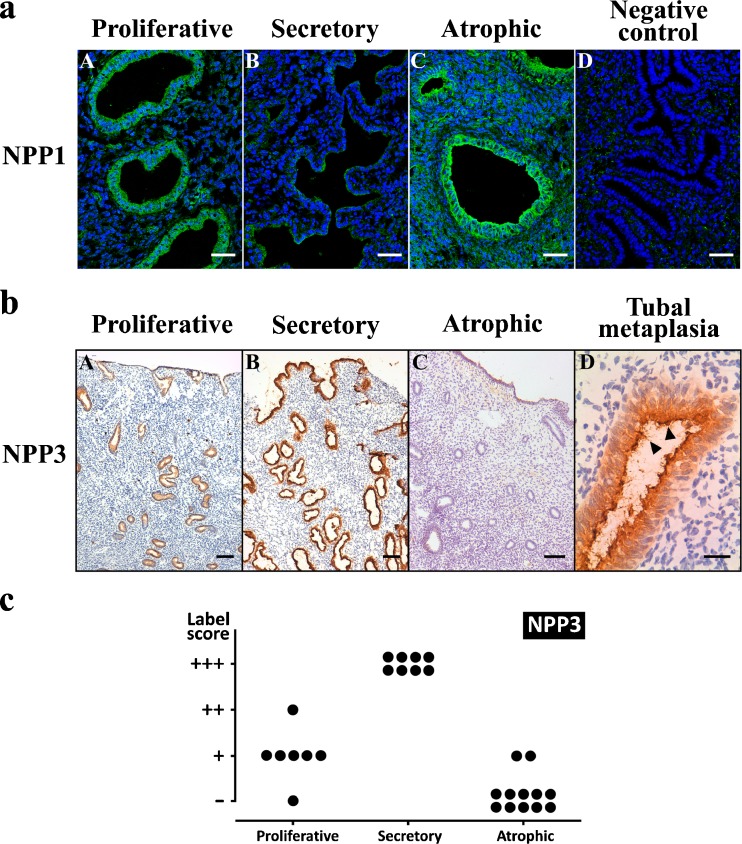
NPP1 was expressed in luminal epithelia of all types of endometrium and in glands of proliferative and especially atrophic endometrium, and was absent in secretory endometrium (Fig. 5a). NPP3 was expressed in glands only in cyclic endometria but with marked changes in the amount of expression along the cycle, being maximal in the secretory phase (Fig. 5b). These changes in glandular NPP3 expression are represented in Fig. 5c. NPP3 was also expressed in luminal epithelium exclusively in secretory endometria. Importantly, no labeling was seen in atrophic endometria except in cases of tubal metaplasia where strong labeling was seen in association with metaplastic glands (Fig. 5b). NPP2 was not detected in endometrial epithelia, neither luminal nor glandular (data not shown).
Fig. 5.
a Confocal fluorescence images of proliferative (a), secretory (b) and atrophic (c) endometria labeled with anti-NPP1. d A negative control of the experiment in which the primary antibody was omitted. NPP1 was immunodetected in the glandular epithelia of proliferative (a) and atrophic (c) endometria. Scale bars = 40 μm. b Immunolocalization of NPP3 in proliferative (a), secretory (b) and atrophic (c) endometria, and in a case of tubal metaplasia (d) in an atrophic endometrium. NPP3 was immunodetected in the luminal and glandular epithelia of cyclic endometria (a, b) but is maximal at the secretory phase (b). Note that the label is absent in the atrophic endometrium (c), and that it is present in the tubal metaplastic gland (d). Arrowheads point to the cilia in the tubal metaplastic epithelium. Scale bars = 100 μm (a–c) and 25 μm (d). c Label intensity score of NPP3 in the glandular epithelium of proliferative, secretory and atrophic endometria. Maximal score is found in secretory endometria
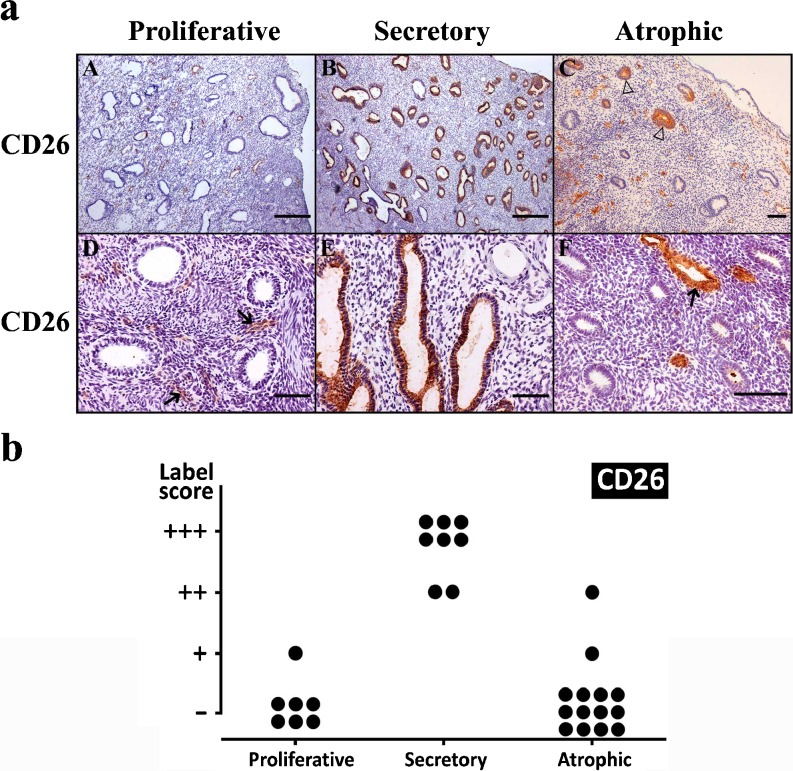
CD26 was detected in endometrial glands, with maximal expression in the secretory phase, coinciding with previous findings [32]. We add here new information by studying also the postmenopausic endometria, showing that CD26 is only weakly expressed in these endometria (Fig. 6). It is noticeable that in atrophic endometria, CD26 expression is not homogeneous amongst all the glands and that only a few glands were positive for this labeling. Immunostaining was also detected in the endothelial cells.
Fig. 6.
a Immunolocalization of CD26 in proliferative (a, d), secretory (b, e) and atrophic (c, f) endometria. CD26 was immunodetected in the blood vessels (arrows) and in glandular epithelia of secretory endometrium (b, e). Note that only a few isolated glands are labeled in atrophic endometrium (c, empty arrowheads). Scale bars = 500 μm (a, b) and 100 μm (c–f). b Label intensity score of CD26 in the glandular epithelium of proliferative, secretory and atrophic endometria. Maximal score is found in secretory endometria
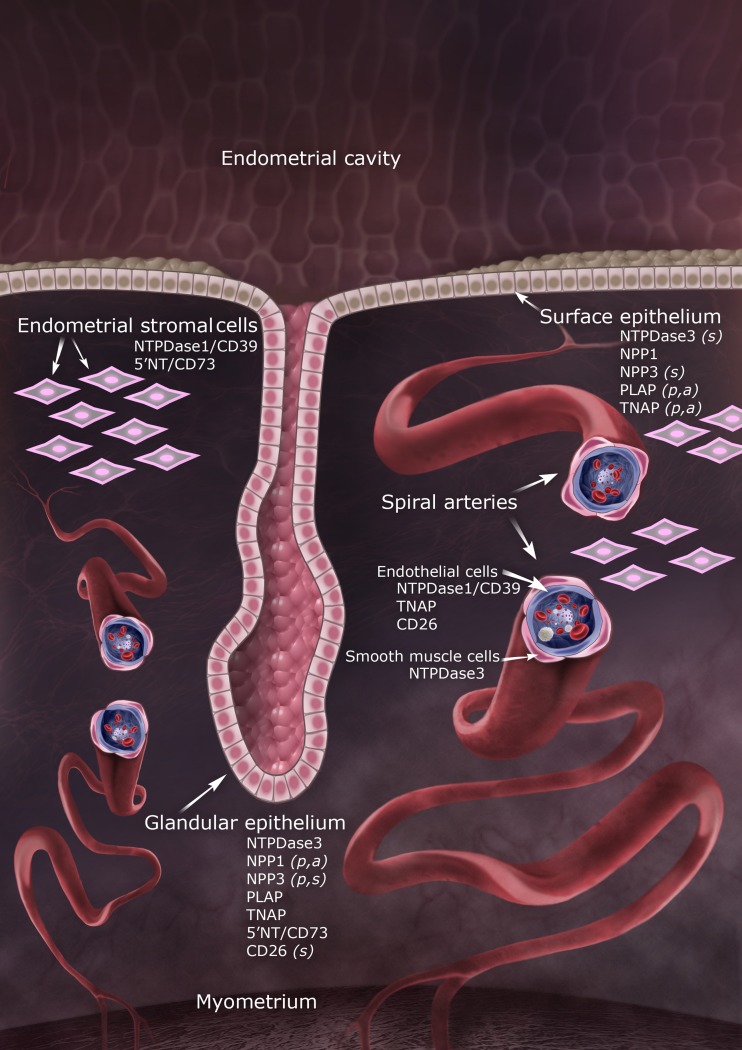
Figure 7 illustrates an endometrium showing the location of all the ecto-enzymes studied here.
Fig. 7.
Model of the human endometrium showing differential distribution of ecto-enzymes in surface and glandular epithelia, stromal cells and spiral arteries. Letters in parentheses indicate that the enzyme was only detected in proliferative (p), secretory (s) or atrophic (a) endometria
Discussion
Extracellular ATP and adenosine, acting through purinergic receptors, are signaling molecules playing a role in reproduction. Purinergic receptors have already been identified in endometrium with a variety of roles, such as ion transport, mucus secretion, cell proliferation and innate mucosal immunity. However, to date little has been known about the ecto-enzymes that regulate their ligand concentrations in human endometrium. In the present work, we have extensively characterized the expression of different families of ecto-nucleotidases in cyclic and postmenopausic endometria. Our results show that different enzymes, operating in concert or consecutively, are able to metabolize extracellular ATP to adenosine. These enzymes thus have the potential to modulate ligand availability for both nucleotide and nucleoside receptors, making them key molecules in the purinergic signaling of endometrium. In this section, we discuss in detail our findings for each family of enzymes.
Mammalian APs are ubiquitous enzymes that display broad substrate specificity towards phosphate compounds. In the rat, AP activity has been already described in uterine luminal and glandular epithelium, establishing a correlation between the luminal activity and endometrial sensitivity [33]. Moreover, a local increase in AP activity has been shown to occur at the site of blastocyst implantation, as part of the early decidual response [34]. These enzymes are thought to be involved in the attachment of blastocyst to the endometrium and in the maintenance of the composition and volume of luminal secretion essential for embryo development [35]. Studies of APs in relation to fertility have also been conducted in women showing up-regulation of the PLAP-2 gene as a marker of ongoing pregnancy after in vitro fertilization treatment [36]. In the present work, we localize the expression and activity of two AP enzymes, PLAP and TNAP, at the luminal and glandular epithelium of human endometrium. Our results coincide with previous studies [37, 38], and we add new data by comparing the expression along the cycle and in postmenopausic endometrium. We did not detect any significant quantitative variations in protein expression in glands, but changes in the distribution of PLAP and TNAP expression were consistently found in the luminal part of secretory endometria, where both enzymes were absent. Moreover, in these endometria, a new location for TNAP was seen at the stroma subjacent to the lumen. These variations might be related with changes needed for appropriate embryo attachment and implantation occurring mainly at the luminal part of the endometrium.
Ecto-5′-nucleotidase, an enzyme efficiently hydrolyzing AMP to adenosine, has already been identified in the mouse female reproductive tract, with marked changes in endometrial expression along the estrous cycle [29], and in pregnancy [28]. Besides a function in the regulation of uterine fluid composition, a role for this enzyme in the generation of extracellular adenosine needed for sperm capacitation has been postulated [39–41]. We show here that in human endometrium ecto-5′-nucleotidase is expressed in glands, with more intensity in the basal layer, and in the stroma, but not in luminal epithelium. The stroma displayed changes in the expression along the cycle, being maximal at the secretory phase. In situ AMPase activity, in the presence or absence of the specific inhibitor α,β-meADP, confirmed that the immunodetected protein was active in the above mentioned structures. Moreover, it is highly probable that the adenosine generated by this AMPase activity and accumulated in the stroma is involved in the regulation of cyclical inflammation physiologically occurring in endometrium [42]. Ecto-5′-nucleotidase might well act sequentially, after NTPDase1, an ecto-nucleotidase also present in the stroma.
NTPDase3 is expressed in luminal and glandular epithelia. NTPDase3 was already identified in other secretory epithelial cells from mouse reproductive organs such as epididymis, prostate and oviducts [27, 43]. We report here for the first time the expression of NTPDase3 in relation to blood vessels. This expression, however, is limited to the muscle layer of spiral arteries, without expression in the myometrial arteries, a fact that enhances the importance of this finding since NTPDase3 can be considered as a new marker of human spiral arteries. Spiral artery remodeling plays a central role in establishing and maintaining a normal pregnancy, and impaired remodeling is involved in common pregnancy disorders such as recurrent pregnancy loss and pre-eclampsia, a major complication of pregnancy and one of the leading causes of maternal and perinatal morbidity and mortality. In spite of the obvious importance, very little is known of the mechanisms responsible for this remodeling, and characterizing these arteries phenotypically has important implications for this understanding [44, 45].
The NPP family of enzymes has already been identified in epithelial cells, in relation with ion transport, amongst other functions [24]. Here we see that NPP1 and NPP3 are expressed in glandular epithelia with changes among endometrium types. Interestingly, the expression of both enzymes seems to be coordinated along the cycle; when there is less expression of one enzyme, there is greater expression of the other. Furthermore, NPP3 is exclusively expressed in glandular and luminal epithelia of cyclic endometria showing maximal expression in secretory endometria; NPP3 therefore becomes a biological marker of this type of endometrium. These marked differences between NPP3 expression in cyclic and post-menopausic endometria point to a relation with fertility and further studies would be of interest for human fertility. Moreover, our results demonstrate that NPP3 is a new marker of endometrial tubal metaplasia. This finding is clinically relevant for the diagnostic of this adaptive phenomenon, usually overlapped with pathological changes, and frequently overlooked and misdiagnosed [46].
CD26 has already been identified in female reproductive organs such as the placenta, ovary and endometrium, and a possible role as adhesion molecule in human blastocyst implantation has been proposed [47]. The fact that ecto-ADA is often associated in larger complexes with CD26 leads us to include the study of CD26 expression in the present work. Here we show that CD26 is highly expressed in secretory endometria and that is almost absent in atrophic endometria, also pointing to its possible implication in women fertility.
A simplified overview of our findings, including the different endometrial structures studied, is presented in Fig. 7. This study provides important new information about the regulation of purinergic signaling by ecto-nucleotidases in human endometria, and opens up the field for further investigation of their role in human fertility and in endometrial pathology.
Acknowledgments
We thank the Tumour Bank of Hospital Universitari de Bellvitge (IDIBELL's Biobank) and the Centres Científics i Tecnològics, Universitat de Barcelona, Campus de Bellvitge, Barcelona, Spain, for their technical assistance. We thank the Xarxa de Bancs de Tumors de Catalunya (XBTC) sponsored by Pla Director d'Oncologia de Catalunya for their contribution. We also thank Gloria Ganaway for her help with English editing. We are indebted to Oscar Frigola Morencia for the illustration of the endometrium. This study was supported by Instituto de Salud Carlos III grant FIS-PI10/00305 to M. Martín-Satué.
Abbreviations
- AP
Alkaline phosphatase
- α,β-meADP
alpha, beta-Methylene adenosine 5′-diphosphate
- NPP
Nucleotide pyrophosphatase/phosphodiesterase
- 5′-NT
Ecto-5′-nucleotidase
- NTPDase
Nucleoside triphosphate diphosphohydrolase
- PLAP
Placental AP
- PPi
Pyrophosphate
- SMA
Smooth muscle actin
- TNAP
Tissue nonspecific AP
References
- 1.Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3–4):229–2362. doi: 10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 3.Hutchings G, Gevaert T, Deprest J, Nilius B, Williams O, De Ridder D. The effect of extracellular adenosine triphosphate on the spontaneous contractility of human myometrial strips. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):79–83. doi: 10.1016/j.ejogrb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Chan HC, Liu CQ, Fong SK, Law SH, Wu LJ, So E, Chung YW, Ko WH, Wong PY. Regulation of Cl- secretion by extracellular ATP in cultured mouse endometrial epithelium. J Membr Biol. 1997;156(1):45–52. doi: 10.1007/s002329900186. [DOI] [PubMed] [Google Scholar]
- 5.Wang XF, Chan HC. Adenosine triphosphate induces inhibition of Na(+) absorption in mouse endometrial epithelium: a Ca(2+)-dependent mechanism. Biol Reprod. 2000;63(6):1918–1924. doi: 10.1095/biolreprod63.6.1918. [DOI] [PubMed] [Google Scholar]
- 6.Gorodeski GI, Hopfer U. Regulation of the paracellular permeability of cultured human cervical epithelium by a nucleotide receptor. J Soc Gynecol Investig. 1995;2(5):716–720. doi: 10.1016/1071-5576(95)00020-F. [DOI] [PubMed] [Google Scholar]
- 7.Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, Asada H, Yoshimura Y, Maruyama T. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol. 2009;182(11):7074–7084. doi: 10.4049/jimmunol.0900001. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi H, Yamaoka K, Urabe S, Kodama M, Kudo Y. Functional expression of purinergic P2X7 receptors in pregnant rat myometrium. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1117–R1124. doi: 10.1152/ajpregu.00507.2009. [DOI] [PubMed] [Google Scholar]
- 9.Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20(7):1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Bardini M, Lee HY, Burnstock G. Distribution of P2X receptor subtypes in the rat female reproductive tract at late pro-oestrus/early oestrus. Cell Tissue Res. 2000;299(1):105–113. doi: 10.1007/s004410050010. [DOI] [PubMed] [Google Scholar]
- 11.Slater M, Murphy CR, Barden JA. Purinergic receptor expression in the apical plasma membrane of rat uterine epithelial cells during implantation. Cell Calcium. 2002;31(5):201–207. doi: 10.1016/S0143-4160(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 12.Vasudevan K, Sztein JM. Treatment of sperm with extracellular adenosine 5′-triphosphate improves the in vitro fertility rate of inbred and genetically modified mice with low fertility. Theriogenology. 2011;76(4):729–736. doi: 10.1016/j.theriogenology.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Miranda E, Buffone MG, Edwards SE, Ord TS, Lin K, Sammel MD, Gerton GL, Moss SB, Williams CJ. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod. 2008;79(1):164–171. doi: 10.1095/biolreprod.107.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards SE, Buffone MG, Knee GR, Rossato M, Bonanni G, Masiero S, Ferasin S, Gerton GL, Moss SB, Williams CJ. Effects of extracellular adenosine 5′-triphosphate on human sperm motility. Reprod Sci. 2007;14(7):655–666. doi: 10.1177/1933719107306227. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn MR, Gao X, Airhart MJ, Skalko RG, Thompson LF, Knudsen TB. Adenosine levels in the postimplantation mouse uterus: quantitation by HPLC-fluorometric detection and spatiotemporal regulation by 5′-nucleotidase and adenosine deaminase. Dev Dyn. 1992;194(2):155–168. doi: 10.1002/aja.1001940208. [DOI] [PubMed] [Google Scholar]
- 16.Gillman TA, Pennefather JN. Evidence for the presence of both P1 and P2 purinoceptors in the rat myometrium. Clin Exp Pharmacol Physiol. 1998;25(7–8):592–599. doi: 10.1111/j.1440-1681.1998.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 17.Minelli A, Liguori L, Bellazza I, Mannucci R, Johansson B, Fredholm BB. Involvement of A1 adenosine receptors in the acquisition of fertilizing capacity. J Androl. 2004;25(2):286–292. doi: 10.1002/j.1939-4640.2004.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 18.Fraser LR. The role of small molecules in sperm capacitation. Theriogenology. 2008;70(8):1356–1359. doi: 10.1016/j.theriogenology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Schuh SM, Hille B, Babcock DF. Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism. Biol Reprod. 2007;77(6):960–969. doi: 10.1095/biolreprod.107.062562. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukulski F, Lévesque SA, Sévigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 22.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2006;2(2):361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2(2):351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millán JL. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2(2):335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Satué M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sévigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol. 2009;131(5):615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucci M, Murphy CR. Differential alterations in the distribution of three phosphatase enzymes during the plasma membrane transformation of uterine epithelial cells in the rat. Cell Biol Int. 1999;23(1):21–30. doi: 10.1006/cbir.1998.0317. [DOI] [PubMed] [Google Scholar]
- 29.Aliagas E, Torrejón-Escribano B, Lavoie EG, de Aranda IG, Sévigny J, Solsona C, Martín-Satué M. Changes in expression and activity levels of ecto-5′-nucleotidase/CD73 along the mouse female estrous cycle. Acta Physiol (Oxf) 2010;199(2):191–197. doi: 10.1111/j.1748-1716.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- 30.Wachstein M, Meisel E, Niedzwiedz A. Histochemical demonstration of mitochondrial adenosine triphosphatase with the lead–adenosine triphosphate technique. J Histochem Cytochem. 1960;8:387–388. doi: 10.1177/8.5.387. [DOI] [PubMed] [Google Scholar]
- 31.Schelstraete K, Deman J, Vermeulen FL, Strijckmans K, Vandecasteele C, Slegers G, De Schryver A. Kinetics of 13 N-ammonia incorporation in human tumours. Nucl Med Commun. 1985;6(8):461–470. doi: 10.1097/00006231-198508000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Kajiyama H, Kikkawa F, Ino K, Shibata K, Mizutani S. Expression of CD26/dipeptidyl peptidase IV in endometrial adenocarcinoma and its negative correlation with tumor grade. Adv Exp Med Biol. 2003;524:245–248. doi: 10.1007/0-306-47920-6_29. [DOI] [PubMed] [Google Scholar]
- 33.Bansode FW, Chauhan SC, Makker A, Singh MM. Uterine luminal epithelial alkaline phosphatase activity and pinopod development in relation to endometrial sensitivity in the rat. Contraception. 1998;58(1):61–68. doi: 10.1016/S0010-7824(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 34.Weitlauf H. Biology of implantation. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 391–440. [Google Scholar]
- 35.Emadi SM, Salehnia M. Localization and activity of mouse endometrial alkaline phosphatase after hyperstimulation and progesterone injection at the implantation time. Iran Biomed J. 2004;8(3):6. [Google Scholar]
- 36.Bersinger NA, Wunder DM, Birkhauser MH, Mueller MD. Gene expression in cultured endometrium from women with different outcomes following IVF. Mol Hum Reprod. 2008;14(8):475–484. doi: 10.1093/molehr/gan036. [DOI] [PubMed] [Google Scholar]
- 37.Davies JO, Howe K, Stirrat GM, Sunderland CA. Placental alkaline phosphatase in benign and malignant endometrium. Histochem J. 1985;17(5):605–612. doi: 10.1007/BF01003200. [DOI] [PubMed] [Google Scholar]
- 38.Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Orgel M, Treml S, Cerabona F, de Zwart P, Ochs U, Muller CA, Gargett CE, Kalbacher H, Buhring HJ. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cell Dev. 2010;19(5):669–677. doi: 10.1089/scd.2009.0290. [DOI] [PubMed] [Google Scholar]
- 39.Monks NJ, Fraser LR. Inhibition of adenosine-metabolizing enzymes modulates mouse sperm fertilizing ability: a changing role for endogenously generated adenosine during capacitation. Gamete Res. 1988;21(3):267–276. doi: 10.1002/mrd.1120210308. [DOI] [PubMed] [Google Scholar]
- 40.Monks NJ, Fraser LR. Enzymes of adenosine metabolism in mouse sperm suspensions. J Reprod Fertil. 1988;83(1):389–399. doi: 10.1530/jrf.0.0830389. [DOI] [PubMed] [Google Scholar]
- 41.Takayama T, Matsubara S, Shibahara H, Minakami H, Takizawa T, Sato I. Ultracytochemical localization of 5′-nucleotidase activity in human ejaculated spermatozoa. Int J Androl. 2000;23(2):106–108. doi: 10.1046/j.1365-2605.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 42.Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011;335(1):42–51. doi: 10.1016/j.mce.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Martín-Satué M, Lavoie EG, Fausther M, Lecka J, Aliagas E, Kukulski F, Sévigny J. High expression and activity of ecto-5′-nucleotidase/CD73 in the male murine reproductive tract. Histochem Cell Biol. 2010;133(6):659–668. doi: 10.1007/s00418-010-0704-z. [DOI] [PubMed] [Google Scholar]
- 44.Elia A, Charalambous F, Georgiades P. New phenotypic aspects of the decidual spiral artery wall during early post-implantation mouse pregnancy. Biochem Biophys Res Commun. 2011;416(1–2):211–216. doi: 10.1016/j.bbrc.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465–474. doi: 10.1016/j.placenta.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolae A, Preda O, Nogales FF. Endometrial metaplasias and reactive changes: a spectrum of altered differentiation. J Clin Pathol. 2010;64(2):97–106. doi: 10.1136/jcp.2010.085555. [DOI] [PubMed] [Google Scholar]
- 47.Shimomura Y, Ando H, Furugori K, Kajiyama H, Suzuki M, Iwase A, Mizutani S, Kikkawa F. Possible involvement of crosstalk cell-adhesion mechanism by endometrial CD26/dipeptidyl peptidase IV and embryonal fibronectin in human blastocyst implantation. Mol Hum Reprod. 2006;12(8):491–495. doi: 10.1093/molehr/gal019. [DOI] [PubMed] [Google Scholar]