Abstract
Purinergic signaling plays a major role in the regulation of phagocytosis in microglia. Interplay between P2 and P1 receptor activation is controlled by a cascade of extracellular enzymes which dephosphorylate purines resulting in the formation of adenosine. The ATP- and ADP-degrading capacity of cultured microglia depends on the expression of ecto-nucleoside triphosphate diphosphohydrolase 1 (CD39) and is several times higher when compared to astrocytes which lack this enzyme. In brain slices, deletion of CD39 resulted in a 50 % decrease of ADP-degrading ability, while the degradation of ATP was decreased to about 75 % of the values measured in wild-type brain tissue. Microglia in acute slices from cd39−/− animals had increased constitutive phagocytic activity which could not be further enhanced by ATP in contrast to control animals. Pharmacological blockage of P2 receptors decreased the constitutive phagocytic activity to a similar base level in wild-type and cd39−/− microglia. Activation of P1 receptors by non-hydrolysable adenosine analog significantly decreased phagocytic activity. Deletion of CD73, an enzyme expressed by microglia which converts AMP to adenosine did not affect phagocytic activity. Taken together, these data show that CD39 plays a prominent role in controlling ATP levels and thereby microglial phagocytosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-012-9339-y) contains supplementary material, which is available to authorized users.
Keywords: Phagocytosis, Microglia, Purinergic signaling, cd39, cd73
Introduction
Microglial cells are the resident immune cells and the professional phagocytes of the brain. They are able to clear cell debris, apoptotic cells, microbial pathogens, and other potentially harmful inclusions, such as β-amyloid deposits associated with Alzheimer’s disease [1]. Microglial phagocytosis is regulated by purinergic signaling. Activation of microglial P2Y6 receptor markedly increases microglial phagocytosis both in vitro and in vivo [2], whereas activation of P2X7 receptor has the opposite effect [3]. In peripheral macrophages, activation of adenosine (P1) receptors impairs phagocytosis [4, 5].
Nucleotides, such as ATP and ADP, are released from injured cells under pathological conditions and can thereby activate purinergic receptors. Also in the healthy brain, ATP is released as a neurotransmitter from neurons and from astrocytes [6]. ATP is an important component to mediate the propagation of calcium signals among populations of astrocytes termed astrocyte calcium waves [7]. Purinoreceptor-mediated signals are negatively regulated by the ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases)—extracellular enzymes hydrolyzing ATP and ADP to AMP. Three members of this enzyme family are expressed in the brain—E-NTPDase1, 2, and 3. Expression in the brain of E-NTPDase1 (also called CD39) is restricted to microglial cells and vascular endothelium of the blood vessels [8]. E-NTPDase1 rapidly converts both ATP and ADP to AMP, thereby depleting the extracellular space of ligands to P2X and P2Y receptors. E-NTPDase1 has an at least three times lower Michaelis constant than the other members of this enzyme family [9], making it an ideal candidate to terminate P2-receptor-mediated signaling or prevent the inactivation of purinergic receptors (for review, see [10]). E-NTPDase2, expressed by astrocytes, converts preferentially ATP into ADP but has a much lower hydrolysis rate for ADP [11], which can lead to ADP accumulation. The cellular localization of the third member of the family—E-NTPDase3—has not yet been characterized. It has substrate preferences intermediate between E-NTPDase1 and 2, resulting in slower removal of ADP from extracellular space as compared to E-NTPDase1. Interestingly, all E-NTPDases, including CD39, can hydrolyze UTP to UDP which is a potent activator of P2Y6 purinoreceptors and promotes microglial phagocytosis [2].
AMP is further hydrolyzed to adenosine by ecto-5-nucleotidase CD73, expressed by a variety of cells including microglia, and feed-forward inhibition was shown for this enzyme, where its activity is inhibited by ATP and ADP (for review, see [12]). In addition to conversion from ATP through the cd39-cd73 enzymatic cascade, adenosine can also be released into extracellular space directly from cells through the activity of specific transporters [13]. In the present study, we assayed microglial phagocytosis activity in acute brain slices and determined the involvement of P1 purinoreceptors and cd39 and cd73 enzymes in the regulation of microglial phagocytosis.
Materials and methods
Cell culture
Primary microglial and astrocyte cultures were prepared from cerebral cortex of newborn C57BL/6 mice as described previously [14].
Transgenic mice
cd39−/− mice on the C57BL/6x129svj background were described previously [15]. As controls, we used wild-type C57BL/6x129svj mice. cd73 −/− mice were described previously [16] and were kindly provided by Dr. Jurgen Schnermann, NIDDK, Bethesda. Wild-type C57BL/6 were used as controls for experiments with these mice.
All animal experiments were conducted in accordance with the German guidelines for animal care and approved by the local legal authorities.
Preparation of acute brain slices
Mice were decapitated and brains were dissected. Two hundred fifty micrometer-thick cortical slices were cut in ice-cold artificial cerebrospinal fluid solution (ACSF) consisting of 134 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, 26 mM NaHCO3, 1.25 mM K2HPO4, and 10 mM glucose, using a vibratome (HM 650 V, Microm; Walldorf, Germany) as described in [17].
Phosphate measurements by Malachite Green assay
To compare the enzymatic activities of extracellular ATP- and ADP-degrading enzymes in microglial cell and astrocyte cultures and acutely isolated brain slices, we incubated cultures or tissue slices with solutions containing 1 mM ATP or ADP and measured the free phosphate in the supernatant by Malachite Green assay (BioAssay Systems, Hayward, CA) as described in [18]. Briefly, 100 μl HEPES buffered salt solution (phosphate-free, pH 7.4) with 1 mM ATP or ADP was added to cell cultures (5 × 104 cells/well for astrocytes or microglia) or tissue slices in 96-well plates and incubated for 10 min at 37 °C. Then, a 90-μl aliquot of the supernatant was collected and transferred into pre-chilled 96-well plate containing 20 μl of 10 % trichloroacetic acid/well to stop the reaction. Remaining cells or tissues were homogenized correspondingly in 50 or 500 μl of 0.02 % sodium dodecyl sulfate in phosphate-buffered saline (PBS), centrifuged at 14,000 × g, and protein concentrations were measured in the extracts using Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). For free phosphate determination, supernatants were diluted 1:5 with fresh reaction buffer, and a colorimetric reaction was performed according to manufacturer’s instructions. Optical absorbance of the reaction product was measured at 620 nm using Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland), and free phosphate concentrations were calculated from the calibration curve. To determine the nonenzymatic production of free phosphate from ATP or ADP or direct release of phosphate from cells or tissues, we incubated ATP and ADP solutions without cells or incubated cells or tissues in the reaction solution without ATP/ADP correspondingly. These values were subtracted from the reported values to correct for the contribution of nonenzymatic production or direct phosphate release.
In situ phagocytosis assay with opsonized latex microspheres
Preparation of acute brain slices was performed as previously described. Briefly, the forebrain hemispheres of freshly isolated brain of 6–18-week-old mice were cut into 140-μm-thin coronal slices with a vibratome in ice-cold ACSF buffer. Slices were transferred onto a nylon grid incubated in ACSF, continuously bubbled with carbogen (95 % CO2, 5 % O2) for 2 h. Yellow-green fluorescent carboxylated microspheres (Fluoresbrite, 3 μm diameter, Polysciences Europe GmbH, Eppelheim, Germany) were coated with fetal calf serum by shaking at 1,000 rpm for 30 min at room temperature. After centrifugation for 2 min at 3,000 rpm, supernatant was discarded and microspheres were washed and resuspended in ACSF. On each acute brain slice, 500 μl of the suspension containing 8.4 × 106 microspheres were applied and incubated for 60 min at 37 ° C in an incubator. For experiments with pharmacological compounds, they were diluted in microspheres suspensions before addition to the slices. Pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid tetrasodium salt (PPADS), N,N''-1,4-Butanediylbis[N'-(3-isothiocyanatophenyl)thiourea (MRS 2578) and 1-(6-Amino-9H-purin-9-yl)-1-deoxy-N‐ethyl‐β‐D‐ribofuranuronamide (NECA) were from Tocris (Bristol, UK). After incubation, slices were washed three times with ice-cold PBS, fixed with 4 % paraformaldehyde and immunostained for microglia with anti-Iba 1 antibody as described below.
Immunohistochemistry
Fixed and washed brain slices were incubated for 4 h in permeabilization buffer (2 % Triton X-100, 2 % BSA, 10 % NGS in 0.1 M PB, pH 7,4), followed by a 48-h incubation with 0.75 μg/ml Iba1 antibody (Wako Pure Chemicals, Japan) in dilution buffer (1:10 of permeabilization buffer in 0.1 M PB, pH 7.4) at 4 °C, followed by incubation with Alexa Fluor 633 labeled goat anti-rabbit antibodies (Invitrogen, Karlsruhe, Germany) (4 μg/ml in dilution buffer) for 2 h at room temperature. After washing, slices were mounted in Aqua-Poly/Mount (Polysciences Europe GmbH, Eppelheim, Germany).
Confocal microscopy and statistical analysis
Confocal laser scanning microscopy was performed on Leica TCS SP5 with ×40 oil immersion objective (Leica, Wetzlar, Germany). We acquired 20-μm-thick z-stacks at 0.5 μm interval beginning from the slice surface in the cortex area of each slice. Total amount of engulfed beads per cell, number of phagocytic microglia, and total number of microglia cells were counted in each z-stack using ImageJ software [19]. Only beads colocalized in individual optical section with Iba1-positive microglia cells were counted as phagocytosed. The data were presented as phagocytic index, calculated for each confocal stack by multiplying the percentage of phagocytic cells with the average number of beads per phagocytic cell. One-way ANOVA test, followed by post hoc paired t tests with Bonferroni correction or t test were used for statistical analysis.
Results
Microglial cells have higher extracellular ATP-degrading activity than astrocytes
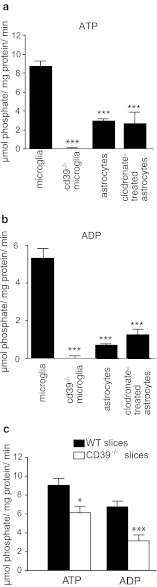
We compared the activity of extracellular enzymes degrading purine-phosphates in purified cell cultures of mouse astrocytes or microglia. Cell cultures were incubated with 1 mM ATP or ADP for 10 min, and free phosphate was measured in the supernatant using Malachite Green assay [18]. To exclude the contribution of nonenzymatic degradation of ATP and ADP, and direct release of phosphate by cultured cells, we incubated ATP and ADP in the absence of cells and cells without ATP and ADP. The levels of phosphate measured in these controls were subtracted from the experimental values. Both astrocyte and microglia cultures were able to generate free phosphate from ATP and ADP. Phosphate production from ATP was nearly three times higher in microglia than in astrocytes, namely 8.62 ± 0.43 (n = 74) versus 2.96 ± 0.18 (n = 34) μmol phosphate/mg protein/min (protein content was used as a reference) correspondingly, and an even higher difference in phosphate production was observed for ADP—5.31 ± 0.30 (n = 63) and 0.74 ± 0.07 (n = 26) μM phosphate/mg protein/min, respectively (Fig. 1a, b). To exclude a contamination by microglia in the astrocyte cultures [20], we also measured phosphate release by astrocyte cultures pretreated for 72 h with clodronate, which selectively kills microglia [21]. Enzymatic release of phosphate from ATP and ADP was not different as compared to untreated cultures, namely 2.96 ± 0.18 (n = 34) and 2.66 ± 0.08 μmol phosphate/mg protein/min (n = 12) for ATP and 0.74 ± 0.07 (n = 27) and 1.25 ± 0.36 μmol phosphate/mg protein/min (n = 12) for ADP for untreated and clodronate treated astrocytes, respectively (Fig. 1a, b).
Fig. 1.
Specific enzymatic activities of extracellular ATP- (a) and ADP-degrading enzymes(b) are virtually absent in primary microglia cultures from cd39−/− mice, while in primary cultures of astrocytes (either treated or untreated with clodronate to remove contaminating microglia in the astrocyte cultures), these enzymatic activities are substantially lower. c Genetic knockout of cd39 affects ATP and ADP degradation by brain tissue. Data shown as mean ± standard error of the mean, Significant p values are as follows: single asterisk indicates, p < 0.05; double asterisks indicate p < 0.01; triple asterisks indicate p < 0.001
Impact of CD39 expression on ATP and ADP dephosphorylation in microglia cultures and brain tissue
To test for the importance of CD39 for the dephosphorylation of extracellular ATP and ADP, we compared the microglial activity from wild-type and cd39 −/− animals. In microglia cultured from cd39−/− animals, generation of phosphate from ATP and ADP was below the detection limit of the phosphate measurement method used in this study (n = 49 for each substance). This indicates that CD39 is the major enzymatic component of microglia for the degradation of ATP and ADP.
To test for possible differences in dephosphorylation between ATP/ADP and uridine di- and triphosphates, we also examined the dephosphorylation of UTP and UDP by cultured microglia. The dephosphorylation capacity of microglia degrading UTP and UDP was remarkably similar (8.76 ± 0.52, n = 25 and 4.37 ± 0.26, n = 27) to values measured for ATP and ADP as substrates. UTP and UDP dephosphorylation was below detection in the microglia cultures from cd39−/− animals.
Comparison of ADP-derived phosphate production by acute slices from wild-type and cd39−/− animals also showed that absence of cd39 leads to significantly lower ADP degradation by the brain tissue (6.12 ± 0.54 vs. 3.14 ± 0.55 μmol phosphate/mg protein/min; Fig. 1c). Phosphate release from ATP was also significantly lower in cd39−/− slices (9.06 ± 0.67, n = 30 vs. 6.78 ± 0.69 μmol phosphate/mg protein/min, n = 20, from five independent slice preparations for each group, p = 0.013).
Dependence of microglial phagocytic activity on CD39 expression
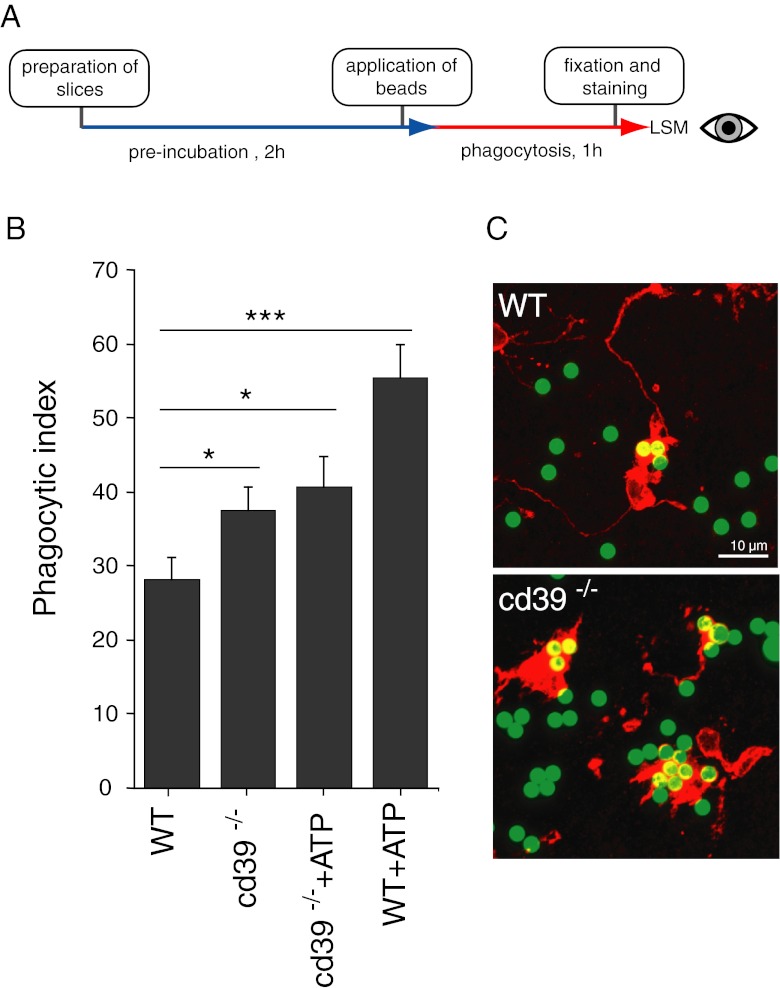
We have studied the effect of cd39 deficiency on microglial phagocytic activity in acute brain slices from adult animals using an in situ phagocytosis assay (Fig. 2a, see “Materials and methods” for details). Comparison of microglial phagocytic activity in situ in adult cd39−/− mice with wild-type animals, obtained from five independent experiments, revealed a significant elevation of constitutive phagocytic activity in cd39−/− animals. Phagocytic index was correspondingly 37.5 ± 3.2 and 28.3 ± 2.8, n = 84 for each group (n represents the number of confocal stacks used for analysis).
Fig. 2.
a Flow diagram of the in situ phagocytosis assay using acute brain slices. b Microglial phagocytosis was higher in cd39−/− slices compared to slices from wild-type adult mice. Application of 100 μM ATP led to increased phagocytosis in WT slices but had no effect in cd39−/− slices. Data presented as mean ± standard error of the mean. c Representative examples of fluorescent beads (green) phagocytosed by microglia (red) in acute brain slices from wild-type and cd39−/− mice, scale bar 10 μm. Significant p values are as follows: single asterisk indicates p < 0.05; double asterisks indicate p < 0.01; triple asterisks indicate p < 0.001
Microglial phagocytosis was increased from 28.3 ± 2.8 (n = 84) to 55.5 ± 4.5 (n = 73) by 100 μM ATP (Fig. 2b). However, when the effect of ATP was tested in cd39−/− animals, no significant change in the phagocytic index was observed (phagocytic indexes were correspondingly 37.5 ± 3.2 (n = 84) without and 40.7 ± 4.1 (n = 75) with ATP).
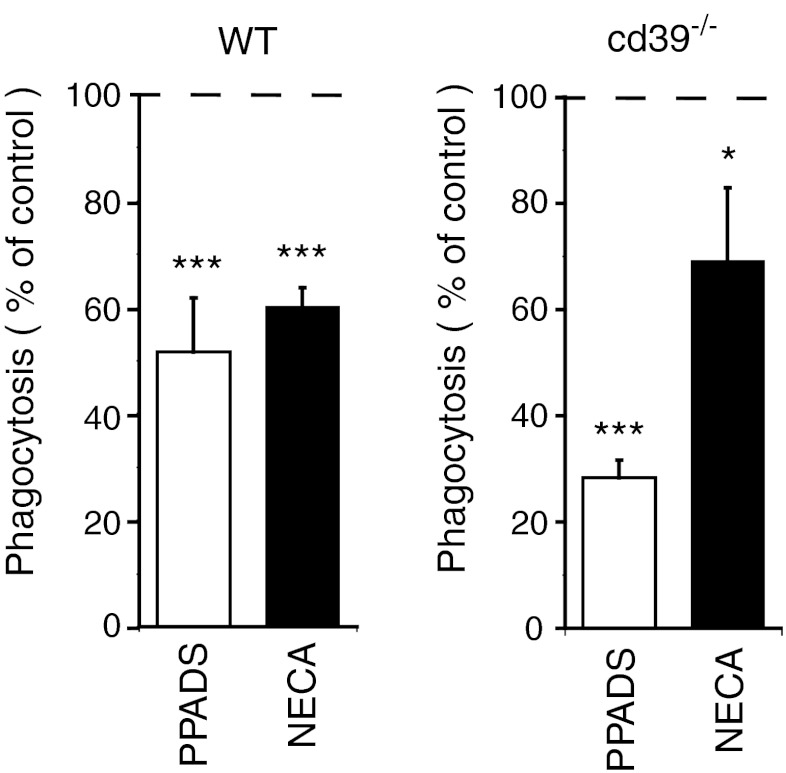
Absence of CD39 in cd39−/− animals can lead to chronically increased extracellular ATP and ADP concentrations. This elevated ATP/ADP tone can in its turn induce either chronical activation of P2 receptors or their inactivation, and both mechanisms can be responsible for the observed loss of stimulatory action of ATP on microglial phagocytosis in cd39−/− animals. To test whether phagocytosis activity is chronically stimulated by endogenous ATP, we blocked P2 receptors in wild-type and cd39−/− brain tissue. When the broad-spectrum P2-purinoreceptor blocker PPADS (100 μM) was added, microglial phagocytic activity was significantly reduced in both wild-type and cd39−/− slices to 51.8 ± 10.4 and 28.2 ± 3.5 % of phagocytic activities measured in non-treated slices from the same animals (Fig. 3, n = 39 for both groups). Since the effect of PPADS is more effective in cd39−/− versus control slices in relative terms, the remaining phagocytic activity after PPADS blockade is at a similar base level in cd39−/− versus control slices. Microglial phagocytic activity was also in a similar manner attenuated by the subtype-specific P2Y6 antagonist MRS 2578 (See Online Resource 1).
Fig. 3.
a Pharmacological blockage of P2 purinoreceptors by nonspecific P2 blocker PPADS (100 μM) significantly decreased microglial phagocytosis in brain slices from wild-type and cd39−/− mice. b Activation of P1 receptors by non-hydrolysable adenosine analog NECA (10 μM) also decreased phagocytosis in brain slices from wild-type and cd39−/− animals. Data presented as mean ± standard error of the mean percentages of phagocytosis measured in non-treated slices, represented by the dashed line. Significant p values are as follows: single asterisk indicates p < 0.05; double asterisks indicate p < 0.01; triple asterisks indicate p < 0.001
Activation of P1 receptors by non-hydrolysable analog of adenosine decreases microglial phagocytosis
In addition to the well-established activation of phagocytosis through P2 receptor signaling, we tested whether P1 receptor activation can influence this activity in microglia. We tested for the involvement of P1 receptors by activating the receptors with the non-hydrolysable analog of adenosine, NECA (10 μM). The phagocytic activity was reduced in the presence of NECA to 60.1 ± 3.5 % of control (n = 30; Fig. 3). The same effect of NECA was observed in slices from cd39−/− animals, where phagocytosis decreased to 69.0 ± 13.8 % as compared to control.
Influence of cd73 expression on AMP degradation and microglial phagocytosis
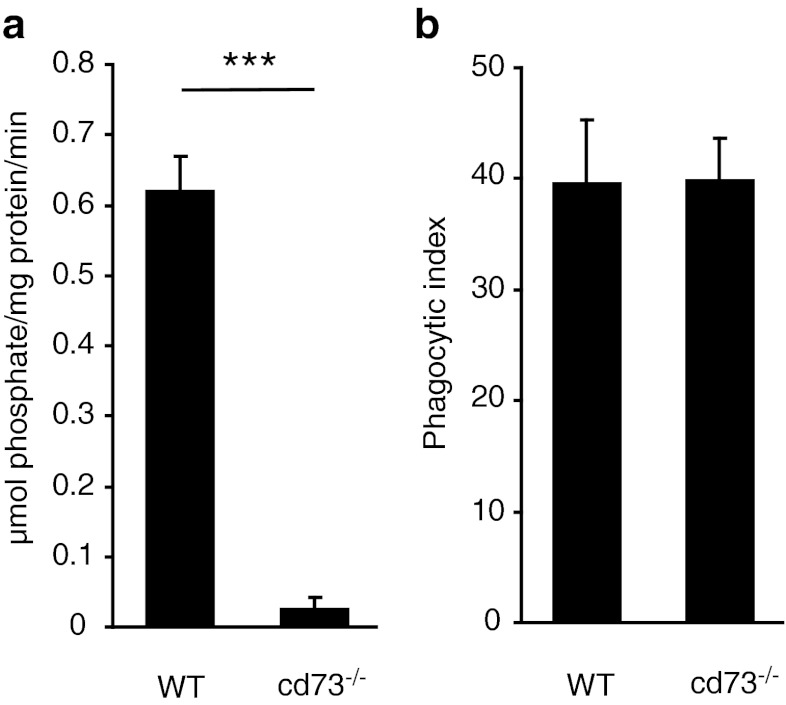
AMP produced by dephosphorylation of ATP and ADP is further degraded to adenosine by the extracellular ecto-nucleotidase CD73 which is the only extracellular enzyme with such activity known up to date. Phosphate production from AMP by brain tissue from cd73−/− animals revealed a strong decrease compared to the activity of brain tissue from wild-type animals (0.62 ± 0.04 and 0.02 ± 0.01 μmol/ mg protein/ min, n = 10, Fig. 4, a). Application of AMP to brain slices from wild-type and cd73−/− animals also showed a strong impact of cd73 for the degradation of AMP. In contrast, we found no difference in phagocytic activity in wild-type compared to cd73−/− microglia. Phagocytic indexes (39.7 ± 5.6 in wild-type and 39.9 ± 3.7 in cd73−/−) were not significantly different (n = 10 for each group, Fig. 4b).
Fig. 4.
a Dephosphorylation of extracellular AMP is virtually eliminated in the brain tissue from cd73−/− mice. b Phagocytosis of fluorescent beads by microglia is not different comparing the brain slices from wild-type and cd73−/− mice. Data presented as mean ± standard error of the mean. Significant p values are as follows: single asterisk indicates p < 0.05; double asterisks indicate p < 0.01; triple asterisks indicate p < 0.001
Discussion
Phagocytosis by brain microglia is tightly controlled through activation of purinoreceptors [2]. Accessibility of ligands for this receptors, nucleoside tri- and diphosphates, depends on the balance between their release from cells and enzymatic degradation [10]. Enzymes, responsible for the degradation differ in their kinetic properties and expression patterns between different cell types. E-NTPDase1 (CD39) is specifically expressed by microglia in the brain parenchyma [22] and is responsible for the high ATP/ADP degradation capacity of these cells. In comparison to astrocytes which express E-NTPDase2, we found that microglia have about threefold higher ATP-degrading capacity, and even higher for ADP due to difference in substrate specificity of these enzymes. While the microglial E-NTPDase1 can equally well convert ATP and ADP to AMP, astrocytic E-NTPDase2 is rather inefficient for metabolizing ADP [11]. Cd39 is responsible for nearly all ATP/ADP-degrading activity in cultured microglia as was revealed by comparison of specific enzymatic activities in microglia from wild-type and cd39−/− animals. In brain tissue, loss of CD39 activity had a much stronger effect on the ADP- than on ATP- degrading activity, leading to a loss of about 50 and 25 % of the ability of the tissue to dephosphorylate these substances, correspondingly. This can reflect the high redundancy of ATP-converting extracellular enzymes in the tissue, while efficient ADP removal strongly depends on CD39 function.
The majority of phagocytosis studies in microglia were performed in cell cultures [23], where cells were removed from their natural tissue surroundings and often treated with growth factors to increase cell yields. Such treatments can irrevocably change the cellular phenotype, and extrapolation of the obtained data to the microglia in tissue surroundings should be treated with caution. In vivo studies, where fluorescent particles were injected in the tissue and phagocytosis was evaluated using morphological methods are difficult to quantify [24]. We have therefore studied phagocytosis in acute brain slices. This assay combines the benefits of using microglial cells in their tissue context milieu with the advantage of reliable quantification of phagocytosis activity.
The phagocytic activity in cd39-deficient animals was higher as compared to control. We assume that due to the lack of cd39 activity, the basal ATP (or ADP) level is elevated which leads to a chronic stimulation of the microglial phagocytic activity. This is supported by the observation that (1) the blocker of P2 signaling, PPADS, reduced phagocytic activity in control and cd39-deficient animals to a common basal level and that (2) ATP did not increase phagocytic activity in cd39-deficient animals.
Similar as reported for microglial migratory activity, phagocytic activity is controlled by both P1 and P2 receptors [25]. It is well established that phagocytosis is facilitated by P2Y6 receptor activation [2]. We found that activation of P1 receptors by the non-hydrolysable analog of adenosine, NECA, led to a moderate inhibition of phagocytic activity. Indeed, a similar mechanism, namely negatively regulated phagocytosis by P1 receptor stimulation, has been described for mouse macrophages and neutrophils [5].
CD73 is expressed by microglia and converts AMP to adenosine. Indeed, dephosphorylation of extracellular AMP by slices from cd73−/− mice was drastically reduced as compared to control animals indicating virtual absence of other enzymes with similar activity in the mouse brain tissue. However, phagocytosis in slices from cd73−/− mice was undistinguishable from those in wild-type slices. This discrepancy can be explained by the ability of brain cells to use alternative mechanisms of maintaining extracellular adenosine concentration, such as equilibrative nucleoside transporters, which can directly release intracellular adenosine into extracellular space (see [26] for review).
Electronic supplementary material
(DOC 34 kb)
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft. We thank Regina Piske, Irene Haupt, and the staff of microscopy core facility at the Max Delbrueck Center for Molecular Medicine for technical assistance.
Abbreviations
- E-NTPDase
Ecto-nucleoside triphosphate diphosphohydrolase
- ACSF
Artificial cerebrospinal fluid
- PPADS
Pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid tetrasodium salt
- NECA
1-(6-Amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-D-ribofuranuronamide
Footnotes
Vitali Matyash and Helmut Kettenmann contributed equally to this paper.
References
- 1.Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain J Neurol. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang K-M, Yang C-S, Sun SH, Tzeng S-F. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem. 2009;111:1225–1237. doi: 10.1111/j.1471-4159.2009.06409.x. [DOI] [PubMed] [Google Scholar]
- 4.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Ja B, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186:2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haskó G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-U. [DOI] [PubMed] [Google Scholar]
- 8.Braun N, Sévigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000;12:4357–4366. [PubMed] [Google Scholar]
- 9.Kukulski F, Komoszyński M. Purification and characterization of NTPDase1 (ecto-apyrase) and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes. Eur J Biochem FEBS. 2003;270:3447–3454. doi: 10.1046/j.1432-1033.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 10.Kukulski F, Lévesque SA, Sévigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signalling. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis Foundation symposium 276:113–128 [PubMed]
- 13.Ja T, Jarvis SM. Adenosine transporters. Gen Pharmacol. 1996;27:613–620. doi: 10.1016/0306-3623(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 14.Prinz M, Kann O, Draheim HJ, Schumann RR, Kettenmann H, Weber JR, Hanisch UK. Microglial activation by components of gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J Neuropathol Exp Neurol. 1999;58:1078–1089. doi: 10.1097/00005072-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 16.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Investig. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb Cortex. 2008;18:2450–2459. doi: 10.1093/cercor/bhn009. [DOI] [PubMed] [Google Scholar]
- 18.Geladopoulos TP, Sotiroudis TG, Evangelopoulos AE. A malachite green colorimetric assay for protein phosphatase activity. Anal Biochem. 1991;192:112–116. doi: 10.1016/0003-2697(91)90194-X. [DOI] [PubMed] [Google Scholar]
- 19.Rasband WS (1997–2011) Image J. U. S. National Institutes of Health. http://imagej.nih.gov/ij/
- 20.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 22.Murabe Y, Sano Y. Morphological studies on neuroglia. VI. Postnatal development of microglial cells. Cell Tissue Res. 1982;225:469–485. doi: 10.1007/BF00214798. [DOI] [PubMed] [Google Scholar]
- 23.Smith ME. Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc Res Tech. 2001;54:81–94. doi: 10.1002/jemt.1123. [DOI] [PubMed] [Google Scholar]
- 24.Hughes MM, Field RH, Perry VH, Murray CL, Cunningham C. Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon LPS stimulation. Glia. 2010;58:2017–2030. doi: 10.1002/glia.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Färber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, Gertz K, Endres M, Bechmann I, Enjyoji K, Robson SC, Kettenmann H. The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia. 2008;56:331–341. doi: 10.1002/glia.20606. [DOI] [PubMed] [Google Scholar]
- 26.Parkinson FE, Damaraju VL, Graham K, Yao SYM, Baldwin SA, Cass CE, Young JD. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem. 2011;11:948–972. doi: 10.2174/156802611795347582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 34 kb)