Abstract
Schwann cells (SCs) are peripheral myelinating glial cells that express the neuronal Ca2+-dependent cell adhesion molecule, neural cadherin (N-cadherin). N-cadherin is involved in glia–glia and axon–glia interactions and participates in many key events, which range from the control of axonal growth and guidance to synapse formation and plasticity. Extracellular UTP activates P2Y purinergic receptors and exerts short- and long-term effects on several tissues to promote wound healing. Nevertheless, the contribution of P2Y receptors in peripheral nervous system functions is not completely understood. The current study demonstrated that UTP induced a dose- and time-dependent increase in N-cadherin expression in SCs. Furthermore, N-cadherin expression was blocked by the P2 purinoceptor antagonist suramin. The increased N-cadherin expression induced by UTP was mediated by phosphorylation of mitogen-activated protein kinases (MAPKs), such as Jun N-terminal kinase, extracellular-regulated kinase and p38 kinase. Moreover, the Rho kinase inhibitor Y27632, the phospholipase C inhibitor U73122 and the protein kinase C inhibitor calphostin C attenuated the UTP-induced activation of MAPKs significantly. Extracellular UTP also modulated increased in the expression of the early transcription factors c-Fos and c-Jun. We also demonstrated that the region of the N-cadherin promoter between nucleotide positions −3698 and −2620, which contained one activator protein-1-binding site, was necessary for UTP-induced gene expression. These results suggest a novel role for P2Y purinergic receptors in the regulation of N-cadherin expression in SCs.
Keywords: N-cadherin, Schwann cells, UTP, P2Y receptors, Mitogen-activated protein kinases, Peripheral nervous system
Introduction
Schwann cells (SCs) are the myelinating glia of the peripheral nervous system (PNS) and are essential for the production and maintenance of myelin after nerve injury. Axonal sprouts must reach the distal nerve stump by switching from a transmitting mode to a growth mode, while SCs undergo proliferation and phenotypic changes to prepare the local environment for correct axonal growth. An intricate signalling network that involves between neurons and SCs controls nerve regeneration via signalling molecules such as neurotrophic factors and adhesion molecules [1, 2]. Some studies have demonstrated the important role of the Ca2+-dependent glycoprotein neural cadherin (N-cadherin) in the growth and guidance of axons, as well as in cell adhesion (SC–SC and SC–axon contacts) and myelination [3–9]. These interactions are essential for SCs to form the bands of Bungner that bridge the injury site to provide an adhesive substrate for regrowing axons [1]. Investigation of the expression of N-cadherin in SC precursors during prenatal development has revealed that the expression of N-cadherin, which is focused in areas in which the cells associate with axonal growth cones, decreases as the precursors develop into mature SCs [4, 5, 10, 11].
Understanding the effects of trauma on the expression of N-cadherin in SCs will be useful for the design of strategies to promote axonal regeneration. The growth factor-like serum phospholipid lysophosphatidic acid (LPA) induces N-cadherin expression through activation of the LPA1 receptor and downstream pathways, which include Gi, phosphatidylinositol-3-kinase and Akt [12, 13]. Neuregulin-1 is another growth factor that up-regulates expression of N-cadherin on the surfaces of SCs. Neuregulin-1 activates the epidermal growth factor receptor and mitogen-activated protein kinases (MAPKs) [12]. Given that nucleotide receptors are attractive pharmacological targets for the enhancement of wound repair, UTP is a promising candidate for the enhancement of N-cadherin expression in SCs. After nerve injury, nucleotides are released into the extracellular medium, where they are able to interact with the neighbouring cells to induce differentiation, neurite outgrowth, survival or cell death, in order to promote wound healing and tissue regeneration [14, 15]. Activation of the UTP-sensitive P2Y receptor has been implicated in the increased expression of adhesion molecules, such as vascular cell adhesion molecule (VCAM) in endothelial cells [16, 17] and N-cadherin in astrocytes [18]. Nevertheless, nothing is known about the mediation of the expression of cell adhesion molecules by UTP in SCs.
To the best of our knowledge, the present study demonstrates for the first time that treatment with UTP increases the expression of N-cadherin in SCs, thereby promoting SC–SC adhesion. We also demonstrated that N-cadherin expression was increased after the activation of P2Y receptors, the subsequent phosphorylation of the MAPKs extracellular signal-regulated kinase (ERK) 1/2, p38 and c-Jun terminal kinase (JNK) and the ultimate up-regulation of the early transcription factors c-Jun and c-Fos. Finally, we showed that the region of the N-cadherin promoter that responded to UTP was localised between −3681 and −2620 nucleotides upstream of the transcription start site and contained one binding site for activator protein (AP)-1, which probably binds to heterodimers that contain c-Jun and c-Fos. Thus, extracellular UTP is an important inducer of morphological changes in SCs.
Materials and methods
Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin, streptomycin and glutamine were purchased from PAA (Linz, Austria). Donor bovine serum (DBS) was purchased from Gibco (Rockville, MD, USA). The enhanced chemiluminescence (ECL) detection kit was from GE Healthcare (Waukesha, WI, USA). Suramin, PBS, Hoechst 33342, trypan blue, protease and phosphatase inhibitor cocktails, SB202190, SP600125, U0126, Y27632, U73122, calphostin C, EGTA and UTP were from Sigma–Aldrich (St. Louis, MO, USA). Anti-mouse and anti-rabbit IgG peroxidase-conjugated secondary antibody and anti-mouse Alexa Fluor 405 were from Merck (Darmstadt, Germany). All other reagents used but not specified here were of analytical grade.
Schwann cell culture
The rat schwannoma cell line RT4-D6P2T was purchased from the European Collection of Cell Cultures (#93011415; ECACC, Salisbury, UK) and maintained in DMEM High glucose medium supplemented with 2 mM l-glutamine, 50 U/mL penicillin, 50 mg/L of 100 U/mL penicillin and 100 U/mL streptomycin and 10 % (v/v) DBS. Cultures were incubated in a 5 % CO2 humidified atmosphere at 37 °C. Cells were seeded at a density of 1.2 × 105 cells/cm2 and starved in 1 % (v/v) DBS for 24 h before nucleotide treatment.
Primary SC culture
Primary SCs were isolated from sciatic nerves of Sprague–Dawley rats on postnatal days 8–10, as described previously [19]. After chemical and mechanical dissociation, the dissociated cells were cultured on dishes in DMEM (high glucose) supplemented with 10 % (v/v) DBS, 5 μM forskolin, 20 μg/mL pituitary extract, 2 mM glutamine, 100 U/mL penicillin and 100 U/mL streptomycin for 3 h, and then non-adherent cells were plated on poly-l-lysine and laminin-coated culture dishes or wells. Cells were subjected to another round of differential adhesion to achieve >83 % SCs purity as determined by S100 and galactocerebroside immunoreactivity. Cells at 7–10 days in culture were used for experiments.
Cell line transfection
Constructs that contained either complete or truncated versions of the human N-cadherin promoter were a generous gift from Dr. P.J. Marie (University Paris Diderot, France). Plasmid DNAs were purified using the DNA Purification System (Promega, Madison, WI, USA). For the reporter assay, on the day before transfection, RT4-D6P2T cells were plated at a density of 105 cells/mL. Plasmids (0.7 μg for the reporter) were transfected using Lipofectamine in accordance with the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). To correct for variations in transfection efficiency in the reporter assay, cells were co-transfected in parallel with 15 ng of pGL3-Renilla (Promega). Luciferase activities were determined in cell lysates using the Dual Luciferase Reporter Assay Kit (Promega), in accordance with the manufacturer’s instructions, and are expressed in relative luciferase units.
Quantitative real-time PCR
Total RNA from 106 SCs was isolated using TRIzol reagent (Invitrogen, Paisley, UK). Total RNA was reverse transcribed using an iScript cDNA Synthesis Kit (Bio-Rad, Berkeley, CA, USA). The cDNA template was analysed by quantitative analysis of cDNA amplification, which was assessed by using the SYBR Green Kit (Bio-Rad). Standard PCRs were performed with the following primers: N-cadherin (NM_031333.1), forward, 5′-GCA CCA GGT TTG GAA TGG G-3′, reverse, 5′-CAT GTT GGG AGA AGG GGT G-3′; c-Jun (NM_021835.3), forward, 5′-GCG GCT GAA GTT GGG CGA GT-3′, reverse, 5′-GGG TTA GCC TGG GCT GTG CG-3′; c-Fos (NM_022197.2), forward, 5′-GGT CTC CTC CGT GGC CCC AT-3′, reverse, 5′-CTT GCA GGC AGG TCG GTG GG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NM_017008.3), forward, 5′-TGG GAA GCT GGT CAT CAA C-3′, reverse, 5′-GCA TCA CCC CAT TTG ATG TT-3′. The NM number indicates the accession number of each gene in the NCBI nucleotide database. All amplified PCR products were verified by analysis using melting curves. Quantification was performed by the ∆∆Ct method, and levels of mRNA expression were normalised relative to the housekeeping gene that encodes GAPDH.
Western blotting
After treatment with UTP, the cells were washed with cold PBS and lysed in buffer that contained 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 % (v/v) NP-40 and protease and phosphatase inhibitors. The lysates were incubated on ice for 10 min and sonicated. Protein concentrations were measured by the Bradford method. Thereafter, 30 μg of cell lysate was denatured with sample buffer [50 mM Tris–HCl, 2 % (v/v) SDS, 100 mM DTT, 10 % (v/v) glycerol, pH 6.8], subjected to 10 % SDS/PAGE and transferred to Immobilon-P membranes (Millipore, Billerica, MA, USA). The membranes were blocked for 1 h with 5 % (w/v) dried skimmed milk in TBS-T buffer [50 mM Tris, 150 mM NaCl, 100 mM KCl and 0.1 % (v/v) Tween-20, pH 7.4] and incubated with the following primary antibodies: rabbit anti-phospho-ERK1/2 (9101; Cell Signaling Technology, Beverly, MA, USA; dilution 1:1,000), rabbit anti-phospho-p38 (ab4822; Abcam, Cambridge, UK; dilution 1:1,000), rabbit anti-phospho-JNK (9251; Cell Signaling Technology; dilution 1:1,000), rabbit anti-ERK1/2 (9102; Cell Signaling Technology; dilution 1:1,000), rabbit anti-p38 (ab27986; Abcam; dilution 1:1,000), rabbit anti-JNK (9252; Cell Signaling Technology; dilution 1:1,000), mouse anti-E-cadherin (6101811; BD Biosciences, Franklin Lakes, NJ, USA; dilution 1:500), mouse anti-N-cadherin (610921; BD; dilution 1:1,000), mouse anti-neural cell adhesion molecule (NCAM) (71652; Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:1,000), mouse anti-VCAM (53548; Santa Cruz Biotechnology; dilution 1:1,000) and mouse anti-GAPDH (AM4300; Applied Biosystems, Carlsbad, CA, USA; dilution 1:20,000). Antibody binding was detected with the corresponding HRP-coupled secondary antibody, and the bands were visualised using the ECL detection system. The immunoreactive bands were quantified using Image J software (National Institutes of Health, USA).
Immunofluorescence
Schwann cells cultured on coverslips were fixed in 4 % (v/v) paraformaldehyde in PBS, permeabilized by incubation for 20 min in a solution that comprised PBS–Triton (0.2 %; v/v), 0.5 % (w/v) BSA and 20 mM glycine, and then labelled with Hoechst 33342 (1 μg/mL) for 30 min at 25 °C in the dark. For N-cadherin staining, coverslips were incubated for 1 h with mouse anti-N-cadherin (610921; BD; dilution 1:250) and with corresponding fluorescent secondary antibody (1:2,000) for 1 h. Finally, coverslips were mounted with the anti-fading medium Mowiol (Sigma), and fluorescence microscopic images were obtained using a laser confocal microscope (Leika DM IRB, Wetzlar, Germany).
Statistical analysis
Results are expressed as the mean ± SD of at least three independent experiments. Statistical analyses were carried out using the GraphPad Prism package (ANOVA analysis plus Newman–Keuls post-test: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Results
UTP increases N-cadherin expression in SCs
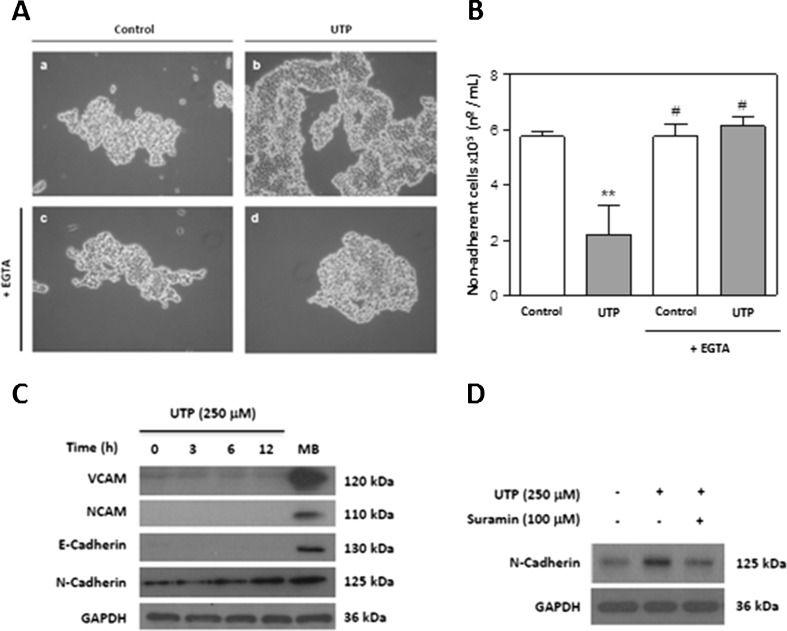
We first analysed the effects of UTP on SCs to investigate the potential to use UTP to increase cell–cell contacts. Treatment of the RT4-D6P2T schwannoma cell line with 250 μM UTP for 5 h increased cell clustering (Fig. 1a, (a, b)). To investigate the dependence of cell adhesion on Ca2+, cells were pretreated in the presence or absence of the extracellular Ca2+-chelating compound EGTA before UTP treatment. As shown in Fig. 1a (c, d), EGTA pretreatment blocked the increase in SC clustering that was induced by UTP. Quantitative adhesion analysis corroborated the conclusions that UTP diminished significantly the number of non-adherent cells (P ≤ 0.01) and that this effect was reversed by EGTA (Fig. 1b). To characterise further which cell adhesion molecules (CAMs) were implicated in this adherent effect, cells were treated with UTP, and the expression of different CAMs was analysed by Western blotting. No signals were found for the NCAM and E-cadherin proteins, and there was only a faint signal for VCAM, which was not regulated by UTP. However, UTP treatment increased the expression of N-cadherin, a Ca2+-dependent CAM (Fig. 1c). The results suggest that N-cadherin could mediate the Ca2+-dependent cell–cell adhesion that is induced by UTP. We confirmed that UTP regulated N-cadherin expression in primary cultures of SCs from postnatal rats. Figure 1d shows that UTP increased the level of N-cadherin protein after 24 h treatment, and this effect was blocked by the P2 receptor antagonist, suramin.
Fig. 1.
Extracellular UTP increases N-cadherin expression in Schwann cells. a Photomicrographs prepared using an inverted microscope (×20), showing adhesion after the “shaking off” method involving trypsin treatment. Cells were treated with UTP (250 μM) for 5 h with (c, d) or without (b) EGTA pretreatment (1 mM, 20 min), and their appearance was compared with that of controls (a). b Non-adherent viable cells were counted after trypsin treatment using trypan blue exclusion staining. Values are expressed as the mean ± SD (n = 3). Statistical significance: **P ≤ 0.01 compared to control; #P ≤ 0.05 compared to UTP treatment. c Schwann cell protein extracts (30 μg) treated for different time periods with 250 μM UTP were resolved by SDS-PAGE and blotted with specific antibodies against VCAM and NCAM (Ca2+-independent adhesion proteins) and against N-cadherin and E-cadherin (Ca2+-independent adhesion proteins). Immunoblots are representative of three independent experiments. Mouse brain (MB) protein lysate was included as a positive control. d The P2Y-receptor antagonist suramin inhibited UTP-induced N-cadherin expression. Primary cultures of SCs were pretreated with suramin (100 μM) for 20 min before UTP (250 μM) treatment and lysates were blotted with antibodies against N-cadherin and GAPDH as a control. Immunoblots are representative of three independent experiments
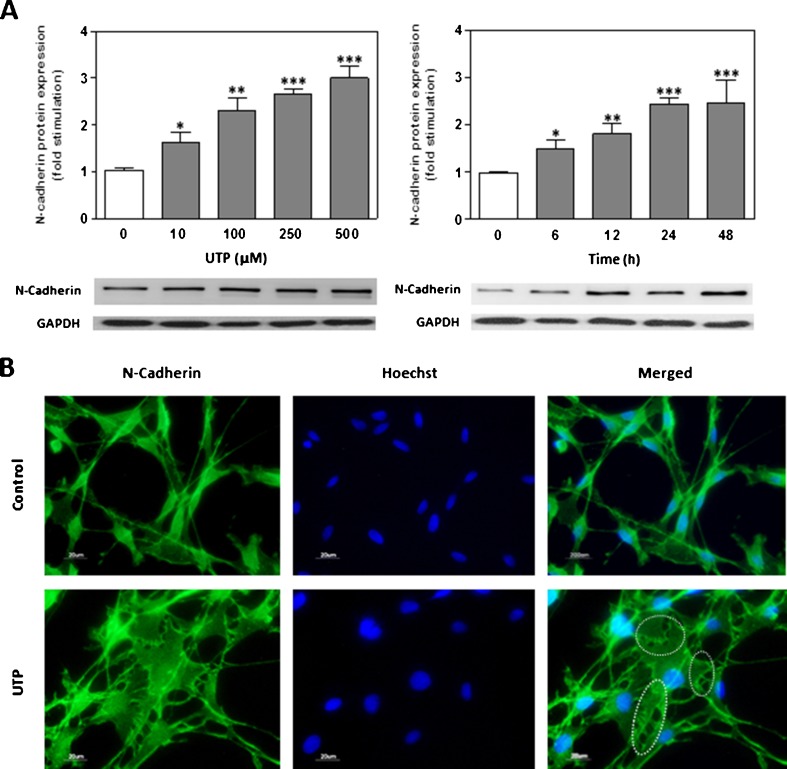
To further characterise the effects of extracellular UTP on N-cadherin expression, dose–response and time-course studies were performed by Western blot analysis on RT4-D6P2T cells that expresses functionally active P2Y receptors (Fig. 2a). Levels of N-cadherin protein were significantly increased in a dose-dependent manner after UTP treatment (24 h, 10–500 μM). Time-dependence experiments were performed using 250 μM. Western blot analysis showed that N-cadherin protein expression significantly increased after 6–48 h of treatment in a time-dependent way. These results demonstrated that UTP induces N-cadherin protein expression on RT4-D6P2T cells following a dose and time dependency. Cells that were treated with UTP (250 μM) for 24 h and stained with N-cadherin antibody showed stronger signal in the cytoplasm, as well as occasional formation of bands of attachment (Fig. 2b, dotted lines). In contrast, control cells showed a characteristic bipolar morphology, with a predominantly perinuclear distribution of N-cadherin.
Fig. 2.
Extracellular UTP increases N-cadherin expression in a schwannoma cell line. a Extracellular UTP increased N-cadherin expression in a dose- and time-dependent manner. Schwann cell protein extracts (30 μg) treated for different time periods manner with UTP (250 μM) or with increasing concentrations of UTP for 24 h were resolved by SDS-PAGE, blotted, and probed with an N-cadherin antibody. Fold stimulation in N-cadherin expression is expressed as the mean ± SD (six independent experiments). Statistical significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. b Schwann cells treated with UTP (250 μM) for 12 h were mounted on coverslips and subjected to immunocytochemistry against N-cadherin (green). Up-regulation of N-cadherin in cell–cell contacts (dotted lines) was visualised by fluorescence microscopy. Nuclei were stained with Hoechst 33342 (blue). Scale bars: 20 μm
Phosphorylation of MAPK mediates N-cadherin expression
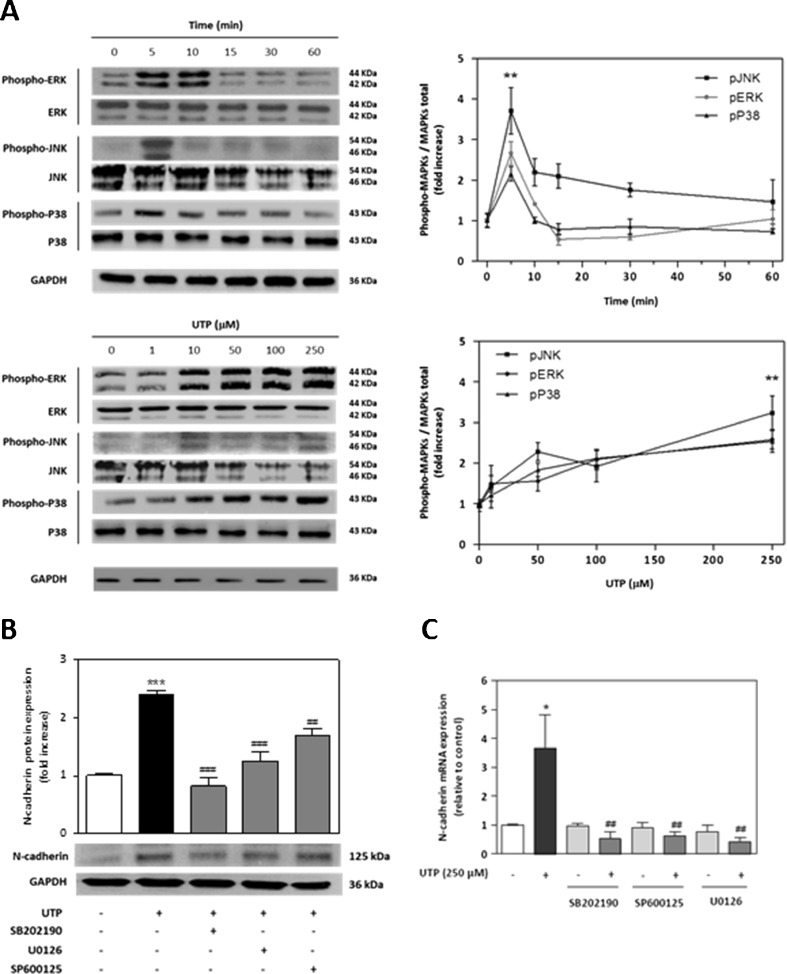
Given that P2 receptors have been reported to signal to MAPK [20, 21], we next determined whether UTP-induced N-cadherin expression in SCs is mediated by the MAPK pathway. We treated RT4-D6P2T cells with UTP at different times and concentrations and analysed the lysates by Western blotting for three phosphorylated MAPKs: mitogenic MAPK ERK1/2, and the two stress-related MAPKs JNK and p38. All three of these kinases were phosphorylated in a dose-dependent manner, with a significant increase (P ≤ 0.01) in rates of phosphorylation at 250 μM UTP (Fig. 3a). Moreover, a time-course experiment with 250 μM UTP showed maximum phosphorylation of the three kinases within 5–10 min after treatment with UTP (Fig. 3a). We analysed whether MAPK signalling cascades were involved in the UTP-induced increase of N-cadherin expression. Cells were pretreated with different selective inhibitors for each MAPK: U0126, which is an inhibitor of MEK1/2 (upstream ERK1/2 kinase); SB202190, an inhibitor of p38; or SP600125, an inhibitor of JNK. We found a significant decrease in levels of N-cadherin protein after UTP treatment when cells were preincubated with inhibitors against p38 (P ≤ 0.01), ERK1/2 and JNK (P ≤ 0.05), as compared with UTP-treated cells (Fig. 3b). We also found that UTP treatment increased levels of N-cadherin mRNA, with a maximum value at 1 h after initial exposure to exogenous UTP (Fig. 3c). Quantitative real-time PCR amplification demonstrated that MAPK inhibitors blocked completely the UTP-induced increase in N-cadherin mRNA (P ≤ 0.01), which confirmed the role of MAPK phosphorylation in the regulation of N-cadherin expression (Fig. 3c). Interestingly, at the protein level, only SB202190 blocks completely the N-cadherin expression. This discrepancy between mRNA and protein expressions for ERK, JNK and MAPKs suggests a possible effect of p38 MAPK on post-transcriptional regulation or conformational N-cadherin protein stabilisation.
Fig. 3.
N-cadherin expression depends on MAPK phosphorylation. a Western blot analysis of MAPK activation by UTP treatment. Schwannoma cells were treated with UTP (250 μM) for different times or were treated with UTP (5 min) at several concentrations. Western blots were performed using antibodies against phosphorylated and total MAPK (ERK1/2, JNK and p38). Fold increases in MAPK activities are expressed as the mean ± SD of the ratio of phosphorylated MAPK/total MAPK (n = 3, **P ≤ 0.01). Representative Western blots for each kinase are shown above the graphs. b Western blotting and c real-time PCR analysis of UTP-induced N-cadherin expression in the presence of different MAPK inhibitors. Cells were preincubated (30 min) with 10-μM selective inhibitor for each MAPK: U0126 (selective inhibitor of MEK/ERK), SP600125 (selective inhibitor of JNK); and SB202190 (selective inhibitor of p38) before UTP treatment. Values are the mean ± SD (three independent experiments). Statistical significance: *P ≤ 0.05, ***P ≤ 0.001, compared to controls; ##P ≤ 0.01, ###P ≤ 0.001 compared to UTP treatment
Phosphorylation of MAPK by UTP depends on P2Y receptors
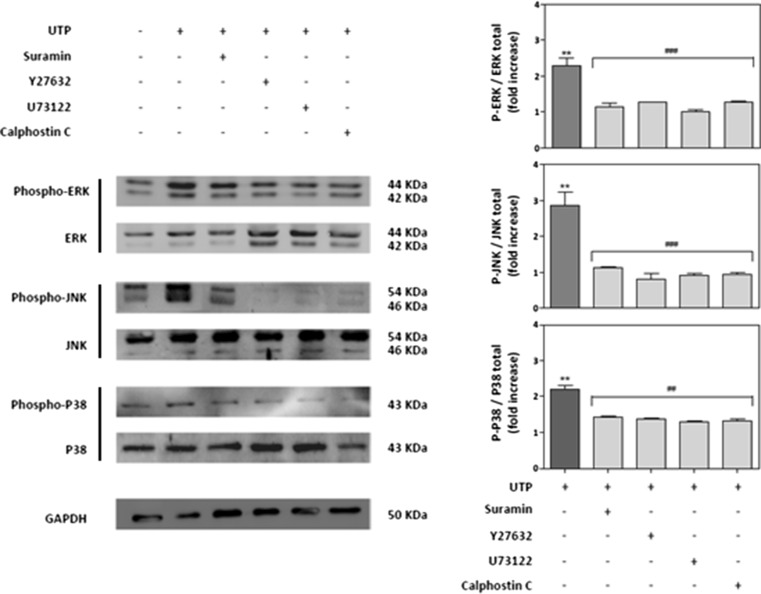
To determine whether phosphorylation of the three MAPKs by extracellular UTP was mediated by P2Y receptors, cells were pretreated with suramin before the addition of UTP. Suramin, which is a non-selective antagonist of P2 receptors, abolished the UTP-induced increase in MAPK phosphorylation (Fig. 4). It is noteworthy that P2Y receptors that are coupled with heterodimeric G proteins regulate effector systems, including Rho-associated protein kinase (ROCK), phospholipase C (PLC) and protein kinase C (PKC). Hence, we pretreated RT4-D6P2T cells with an inhibitor of either ROCK (Y27632), PLC (U73122) or PKC (calphostin C) for 5 min before UTP treatment. As shown in Fig. 4, all three inhibitors blocked UTP-induced phosphorylation of the three MAPKs studied. This suggests that the ROCK, PLC and PKC signalling pathways promote JNK, ERK and p38 phosphorylation after UTP treatment.
Fig. 4.
MAPK activation depends on the P2Y receptor signalling pathway. Schwannoma cells were pretreated for 30 min before UTP treatment with selective inhibitors of different proteins involved in the P2Y receptor signalling pathway: suramin (100 μM; P2Y receptor antagonist), Y27632 (10 μM; ROCK inhibitor), U73122 (10 μM; PLC inhibitor) or calphostin C (10 μM; PKC inhibitor). After extracellular UTP treatment (5 min), proteins from cell lysates were resolved by SDS-PAGE and blotted against each phosphorylated or total MAPK. Representative Western blots for each kinase are shown. Fold increases in MAPK activity are expressed as the mean ± SD of the ratio of phosphorylated MAPK/total MAPK (n = 4, *P ≤ 0.05; **P ≤ 0.01)
Extracellular UTP increases early expression of the genes that encode c-Fos and c-Jun
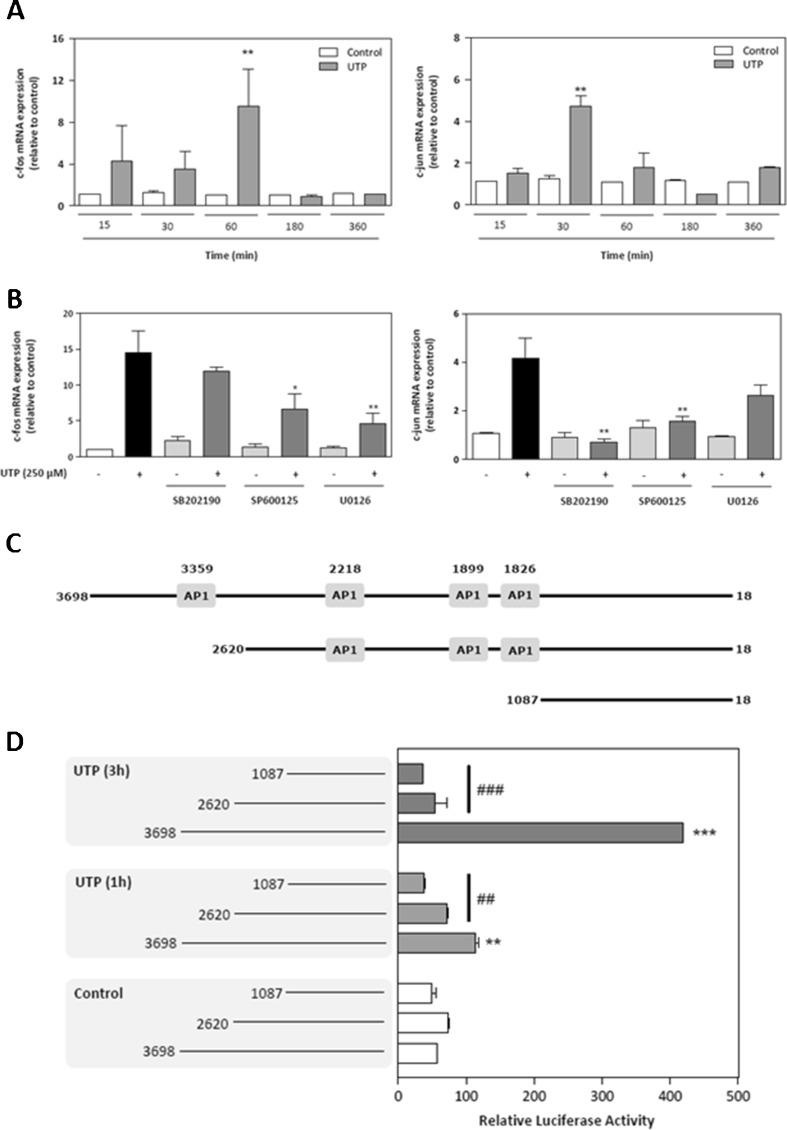
We investigated expression of the genes that encode c-Fos and c-Jun, which are two important early genes regulated by MAPKs. Both c-Fos and c-Jun down effectors of MAPK signalling pathways and regulate formation of the heterodimer transcription factor AP-1 [22, 23]. We performed quantitative real-time PCR expression analysis of the early genes c-Fos and c-Jun between 15 min and 6 h after UTP treatment. SCs had significantly increased levels of the mRNA for c-Fos (ninefold; P ≤ 0.01) at 60 min, and significantly increased levels of the mRNA for c-Jun (fivefold; P ≤ 0.01) at 30 min (Fig. 5a). To investigate whether the MAPK pathway was involved in this up-regulation, cells were pretreated with different selective inhibitors of each MAPK for 30 min and then with UTP for 1 h (Fig. 5b). The inhibitors U0126 or SP600125 caused a significant decrease in c-Fos expression, which indicated a role for ERK and p38 kinases. In contrast, as shown in Fig. 5b, the fourfold up-regulation of c-Jun mRNA levels after UTP treatment was reversed substantially by exposure to inhibitors of JNK (SP600125) and p38 (SB202190), which was consistent with the fact that JNK is the major kinase related to c-Jun expression. Taken together, the results suggest that the three MAPKs regulate c-Fos and c-Jun levels in SCs.
Fig. 5.
Extracellular UTP induces expression of the early genes c-Fos and c-Jun through MAPK activation. a Schwannoma cells were treated with UTP (250 μM) for five different time periods. Total RNA was subjected to real-time PCR analysis and expression of c-Fos (left) or c-Jun (right) mRNA was evaluated. Early gene expression is expressed as the fold stimulation compared to housekeeping gene GAPDH. Values are mean ± SD (n = 3, **P ≤ 0.01). b Schwannoma cells were pretreated (30 min) with 10 μM of each of the selective MAPK inhibitors SB202190, SP600125 and U0126. After UTP treatment (250 μM for 1 h), total RNA was prepared and subjected to real-time PCR analysis to determine the expression of transcripts that encoded c-Fos (left) and c-Jun (right). Early gene expression is expressed as fold stimulation compared to housekeeping gene GAPDH. Values are the mean ± SD (n = 3, *P ≤ 0.05, **P ≤ 0.01). c Schematic representation of the human N-cadherin promoter (AY512658) and two 5′-deletion mutants used, all of which were cloned in a pGL3 plasmid linked to luciferase. AP-1: AP-1 binding sites. d Schwannoma cells were either co-transfected with the complete sequence of the human N-cadherin promoter or with one of two 5′-deletion mutants that contained fewer AP-1 binding sites linked to firefly luciferase and with pGL3 that contained the coding sequence for Renilla luciferase. After 48 h, transfected RT4 cells were treated with 250 μM UTP for 1 h (grey boxes) or 3 h (black boxes), and luciferase activity was analysed. Firefly luciferase activity was normalised by comparison to levels of Renilla luciferase activity. Data represent transcriptional activities relative to the empty basic pGL3 plasmid. The results are the mean ± SD of two independent experiments performed in triplicate (n = 6, **P ≤ 0.01, ***P ≤ 0.001)
An AP-1 binding site seems to be necessary for N-cadherin gene expression
The basal promoter of human N-cadherin was described recently [23]. To characterise the promoter elements that are necessary for UTP-induced transcription of N-cadherin in SCs, we analysed the effect of various 5′ deletions in the promoter region that spans the 3,681-bp region upstream of the start codon of the human N-cadherin gene using the vector pGL3 and the luciferase reporter assay. Two deletions of 1078 and 2611 nucleotides from the end distal to the transcription start site were performed to generate shorter promoters of 2,620 and 1,087 bp, respectively (Fig. 5c). We transfected RT4-D6P2T cells with the pGL3-3698/-18 (complete 3,681-bp sequence with four AP-1 sites), pGL3-2620/-18 (2,620 bp with three AP-1 sites), or pGL3-1087/-18 (1,087 bp with no AP-1 sites) construct, and then treated the cells with UTP (250 μM) for 1 or 3 h. Transfection of RT4-D6P2T cells with the pGL3698/-18 construct (complete promoter) resulted in a 55-fold increase in luciferase activity as compared with the empty pGL3 vector. After the 1- and 3-h UTP treatments, there was an increase in promoter activity (twofold and eightfold, respectively, compared with untreated cells) for the pGL3-3698/-18 construct (Fig. 5d). The UTP response was absent in cells that were transfected with any of the deleted promoter constructs. The results indicate that the region from −3698 to −2610 is crucial for the response to UTP treatment, which suggests that the absence of only one AP-1 binding site in the human N-cadherin promoter is sufficient to block the UTP-induced promoter activity.
Discussion
Growing evidence suggests that nucleotides that are released upon injury or under physiological conditions stimulate nucleotide P2 receptors and serve as endogenous signals to induce a rapid response in glial cells [24–26]. Neurons and SCs in the PNS express purinergic receptors that are linked to the release of intracellular Ca2+ stores and the regulation of several pathways [27–30]. Moreover, the actions of extracellular UTP nucleotide in SCs remain to be clarified. Liu et al. demonstrated that the activation of P2Y2 purinergic receptors by UTP stimulated ATP release by exocytosis, providing a positive purinergic feedback [31]. On the other hand, in the rat RT4-D6P2T schwannoma cell line, UTP induces a transient elevation in concentrations of intracellular Ca2+ and a subsequent cytoskeletal reorganisation [32]. In this context, N-cadherin, a member of the Ca2+-dependent CAM family, is related to multiple cytoskeletal elements and has an important role in SC migration, neurite outgrowth, synaptic rearrangement, creation of SC–SC junctions, and the alignment of SCs with axons [4, 5, 33–39].
To analyse these signalling pathways further, we investigated whether extracellular UTP increased N-cadherin-mediated cell–cell adhesion. We found that UTP, through P2Y receptors, increased the transcription and protein expression of N-cadherin in a time- and dose-dependent manner, which resulted in process retraction, cell flattening and spreading and cell clustering. In addition, we observed that N-cadherin was the only cadherin for which expression was modulated by UTP. Thus, increased N-cadherin expression induced by UTP might possibly mediate SC–SC interaction and the initial growth of SC processes that are aligned with axons [4].
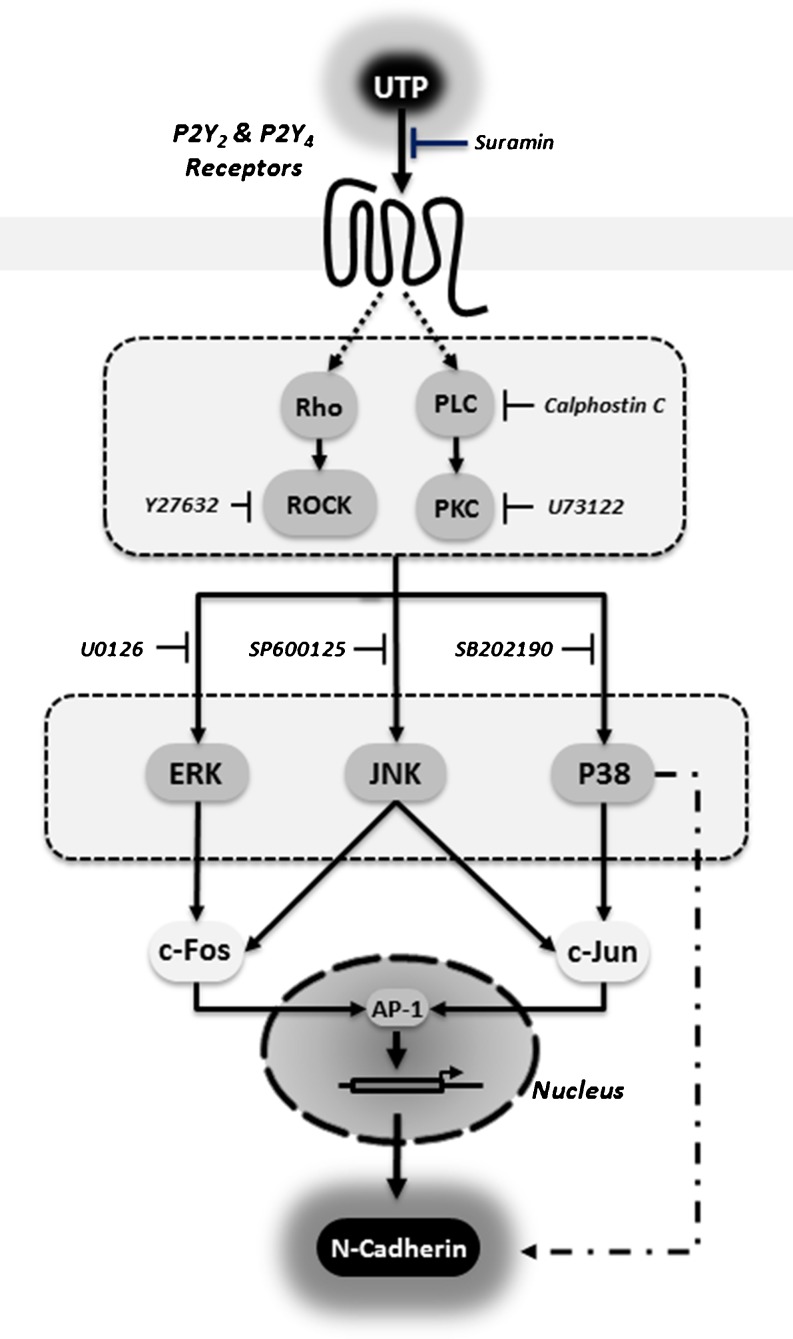
The G protein-coupled P2Y2 receptor is known to activate several heterodimeric G proteins that can also initiate chemotactic signalling events. These events include the activation of Rho and integrins, formation of stress fibres and directional cell migration [40, 41]. In the present study, by using specific inhibitors of ROCK, PLC and PKC, we demonstrated that the up-regulation of N-cadherin expression was mediated by the P2Y2 receptor through the activation of the downstream dependent pathway, which involves protein G, ROCK, PLC, Ca2+ and PKC (Fig. 6). Recent studies have demonstrated that the P2Y2 receptor needs to interact with integrins to access and activate G proteins [42, 43]. Although the mechanism of this interaction is unclear, it is possible that N-cadherin might be involved in the association of complexes that contain integrins and G proteins, because G proteins interact with the cytoplasmic tails of several cadherins [44, 45].
Fig. 6.
Schematic overview of the purinergic signalling pathway involved in P2Y receptor activation leading to N-cadherin expression in Schwannoma cell line
We also demonstrated that UTP-induced N-cadherin expression depended on early phosphorylation of JNK, ERKs and p38 MAPK (Fig. 6). Indeed, after nerve injury, increased activity in multiple pathways—including the ERK/MAPK, JNK/c-Jun, Notch and JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathways—can be detected in SCs [46, 47]. Furthermore, some authors suggest that ERK and JNK activity defines the state of SC differentiation. Whereas basal levels are necessary for differentiation of precursors, high ERK and JNK activities drive dedifferentiation and migration [48–52]. In contrast, the role of the p38 MAPK in SCs is controversial. Some authors have suggested that it is associated with regulation of myelination [53, 54], and others that it is a negative regulator of SC differentiation and myelination [55]. Thus, activation of the MAPK pathway by extracellular UTP seems to mediate the increase of N-cadherin expression and could explain the relationship of MAPKs with cell adhesion, migration and dedifferentiation. On the other hand, activation of the MAPK pathway modulates several transcription factors that regulate the SC phenotype. Transcription factors have been classified either as positive regulators that promote differentiation (e.g. Krox-20, OCT-6, Sox-10 and nuclear factor-κB) or as negative regulators that promote dedifferentiation (e.g. c-Jun, Sox-2, Pax-3 and Id2) [56–58]. Nuclear accumulation of both the c-Fos and c-Jun transcription factors in astrocytes and microglial cells has been described after exposure to nucleotides [59, 60]. We demonstrated that the increase in c-Fos and c-Jun after UTP treatment is mediated by MAPKs (Fig. 6). c-Jun and c-Fos interact to form the activated heterodimeric AP-1 transcriptional factor, which can bind to specific sequences in the promoter regions of many genes, such as N-cadherin [61]. In addition, it was found that both c-Jun and c-Fos occupy the AP-1 site in the N-cadherin promoter in response to UTP, and that the absence of only one AP-1 binding site in the human N-cadherin promoter is sufficient to block the UTP-induced promoter activity of N-cadherin (Fig. 6). The parallel signalling pathways involved include the JNK/c-Jun and ERK/c-Fos signalling cascades, which might explain the SC dedifferentiation phenotype observed.
We conclude that in schwannoma cell line, activation of the G protein-coupled receptor P2Y by UTP leads to increased expression of N-cadherin, an adhesion molecule that is related to SC migration, neurite outgrowth, synaptic rearrangement, formation and maintenance of SC–SC junctions and the alignment of SCs with axons. Selective P2Y receptor agonists might provide new opportunities for the enhancement of cell–cell adhesion.
Acknowledgments
This research was supported by an unrestricted research grant from Ferrer S.A. (Barcelona, Spain). The authors acknowledge the Medical Department of Ferrer S.A. (Lorenzo JL, Manso P and Lopez C) for their support in the preparation of this paper. We are grateful to Dr. Irene Bolea for critical reading of the manuscript.
Footnotes
Tania Martianez and Aloa Lamarca contributed equally to this work.
Contributor Information
Nuria Casals, Phone: +34-93-5042000, FAX: +34-93-5042001, Email: ncasals@uic.es.
Alejandro Gella, Phone: +34-93-5042000, FAX: +34-93-5042001, Email: alexgella@gmail.com.
References
- 1.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14(1–2):67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 2.Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous System. Trends Neurosci. 2012;35(2):123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL, Jr, Colman DR. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol. 1995;129(1):189–202. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J Neurosci. 2002;22(10):4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner IB, Guerra NK, Mahoney J, Kumar A, Wood PM, Mirsky R, Jessen KR. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006;54(5):439–459. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- 6.Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mège RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98(4):534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31(8):3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Takeda Y, Murakami Y, Asou H, Uyemura K. The roles of cell adhesion molecules on the formation of peripheral myelin. Keio J Med. 2001;50(4):240–248. doi: 10.2302/kjm.50.240. [DOI] [PubMed] [Google Scholar]
- 10.Corell M, Wicher G, Limbach C, Kilimann MW, Colman DR, Fex Svenningsen Å. Spatiotemporal distribution and function of N-cadherin in postnatal Schwann cells: a matter of adhesion? J Neurosci Res. 2010;88(11):2338–2349. doi: 10.1002/jnr.22398. [DOI] [PubMed] [Google Scholar]
- 11.Kanemaru K, Okubo Y, Hirose K, Iino M. Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J Neurosci. 2007;27(33):8957–8966. doi: 10.1523/JNEUROSCI.2276-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner JA, Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci USA. 1999;96(9):5233–5238. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21(18):7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- 15.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, González FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278(27):24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 17.Seye CI, Yu N, González FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279(34):35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 18.Tran MD, Wanner IB, Neary JT. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J Neurochem. 2008;105(1):272–286. doi: 10.1111/j.1471-4159.2008.05214.x. [DOI] [PubMed] [Google Scholar]
- 19.Kreider BQ, Messing A, Doan H, Kim SU, Lisak RP, Pleasure DE. Enrichment of Schwann cell cultures from neonatal rat sciatic nerve by differential adhesion. Brain Res. 1981;207(2):433–444. doi: 10.1016/0006-8993(81)90375-9. [DOI] [PubMed] [Google Scholar]
- 20.Belcheva MM, Coscia CJ. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals. 2002;11(1):34–44. doi: 10.1159/000057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004;1(1):23–34. doi: 10.1017/S1740925X04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho BY, Wu YM, Chang KJ, Pan TM. Dimerumic acid inhibits SW620 cell invasion by attenuating H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol Sci. 2011;7(6):869–880. doi: 10.7150/ijbs.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Mée S, Fromigué O, Marie PJ. Sp1/Sp3 and the myeloid zinc finger gene MZF1 regulate the human N-cadherin promoter in osteoblasts. Exp Cell Res. 2005;302(1):129–142. doi: 10.1016/j.yexcr.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy C, Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessel. 1985;22(3):145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- 25.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23(12):625–633. doi: 10.1016/S0166-2236(00)01674-X. [DOI] [PubMed] [Google Scholar]
- 26.Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3–10. doi: 10.1007/s11302-004-4740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995;483(Pt 1):41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berti-Mattera LN, Wilkins PL, Madhun Z, Suchovsky D. P2-purigenic receptors regulate phospholipase C and adenylate cyclase activities in immortalized Schwann cells. Biochem J. 1996;314(Pt 2):555–561. doi: 10.1042/bj3140555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15(9):813–827. doi: 10.1016/S0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 30.Corriden R, Insel PA. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 2012;8:587–598. doi: 10.1007/s11302-012-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci. 2005;21(1):151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 32.Martiáñez T, Carrascal M, Lamarca A, Segura M, Durany N, Masgrau R, Abian J, Gella A. UTP affects the Schwannoma cell line proteome through P2Y receptors leading to cytoskeletal reorganisation. Proteomics. 2012;12(1):145–156. doi: 10.1002/pmic.201100187. [DOI] [PubMed] [Google Scholar]
- 33.Gess B, Halfter H, Kleffner I, Monje P, Athauda G, Wood PM, Young P, Wanner IB. Inhibition of N-cadherin and beta-catenin function reduces axon-induced Schwann cell proliferation. J Neurosci Res. 2008;86(4):797–812. doi: 10.1002/jnr.21528. [DOI] [PubMed] [Google Scholar]
- 34.Bozdagi O, Wang XB, Nikitczuk JS, Anderson TR, Bloss EB, Radice GL, Zhou Q, Benson DL, Huntley GW. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30(30):9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez P, De Roo M, Poglia L, Klauser P, Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J Cell Biol. 2010;189(3):589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci USA. 2010;107(21):9873–9878. doi: 10.1073/pnas.1003480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arikkath J. N-cadherin: stabilizing synapses. J Cell Biol. 2010;189(3):397–398. doi: 10.1083/jcb.201004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiga M, Levinson JN, Bamji SX. N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J Biol Chem. 2011;286(1):851–858. doi: 10.1074/jbc.M110.176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka H, Takafuji K, Taguchi A, Wiriyasermkul P, Ohgaki R, Nagamori S, Suh PG, Kanai Y. Linkage of N-cadherin to multiple cytoskeletal elements revealed by a proteomic approach in hippocampal neurons. Neurochem Int. 2012;61(2):240–250. doi: 10.1016/j.neuint.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Van Kolen K, Slegers H. Integration of P2Y receptor-activated signal transduction pathways in G protein-dependent signalling networks. Purinergic Signal. 2006;2(3):451–469. doi: 10.1007/s11302-006-9008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A, Boyer JL, Der CJ, Zohn IE. Transformation by a nucleotide-activated P2Y receptor is mediated by activation of Gαi, Gαq and Rho-dependent signaling pathways. J Mol Signal. 2010;5:11. doi: 10.1186/1750-2187-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Z, Seye CI, Weisman GA, Laurie E. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci. 2007;120(Pt9):1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J Cell Sci. 2007;120(Pt 9):1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta -catenin release. Proc Natl Acad Sci USA. 2001;98(2):519–24. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Schlippe M, Marshall JF, Perry P, Stone M, Zhu AJ, Hart IR. Functional interaction between E-cadherin and alphav-containing integrins in carcinoma cells. J Cell Sci. 2000;113(Pt 3):425–437. doi: 10.1242/jcs.113.3.425. [DOI] [PubMed] [Google Scholar]
- 46.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166(2):392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 47.Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12(7):839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci USA. 2003;100(24):14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12(11):1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 50.Zine A, van de Water TR The MAPK/JNK signalling pathway offers potential therapeutic targets for the prevention of acquired deafness. Curr Drug Targets CNS Neurol Disord. 2004;3(4):325–332. doi: 10.2174/1568007043337166. [DOI] [PubMed] [Google Scholar]
- 51.Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69(1):91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newbern JM, Snider WD. Bers-ERK Schwann cells coordinate nerve regeneration. Neuron. 2012;73(4):623–626. doi: 10.1016/j.neuron.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. J Mol Neurosci. 2008;35(1):23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- 54.Hossain S, de la Cruz-Morcillo MA, Sanchez-Prieto R, Almazan G. Mitogen-activated protein kinase p38 regulates krox-20 to direct Schwann cell differentiation and peripheral myelination. Glia. 2012;60(7):1130–1144. doi: 10.1002/glia.22340. [DOI] [PubMed] [Google Scholar]
- 55.Yang DP, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen KR, Maurel P, Parkinson DB, Kim HA. p38 MAPK activation promotes denervated schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32(21):7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bremer M, Fröb F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59(7):1022–1032. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]
- 57.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 59.Bolego C, Ceruti S, Brambilla R, Puglisi L, Cattabeni F, Burnstock G, Abbracchio MP. Characterization of the signalling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br J Pharmacol. 1997;121(8):1692–1699. doi: 10.1038/sj.bjp.0701294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priller J, Reddington M, Haas CA, Kreutzberg GW. Stimulation of P2Y-purinoceptors on astrocytes results in immediate early gene expression and potentiation of neuropeptide action. Neuroscience. 1998;85(2):521–525. doi: 10.1016/S0306-4522(97)00653-2. [DOI] [PubMed] [Google Scholar]
- 61.Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, Kwon KI, Jeong TC, Jeong HG. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55(4):594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]